Abstract
Kisra, a fermented sorghum flat bread, was prepared from two sorghum genotypes termed Wad-Ahmed (high tannin) and Tabat (low tannin) in Sudan that has been fermented with different starter levels [20, 50, 75 and 100 g of fermented baobab fruit pulp flour (FBFPF)/100 g flour]. Chemical composition, antinutritional factors, mineral extractability, ascorbic acid, in vitro protein (IVPD) and starch digestibilities (IVSD) of Kisra were determined. Preparation of Kisra with the sorghum genotypes fermented with higher levels of FBFPF enhanced the protein, fiber, fat, ash, and minerals contents and their extractability (P ≤ 0.05). Maximum amino acids contents were found in Kisra prepared from Tabat sorghum flour fermented with 100 g FBFPF/100 g flour. Ascorbic acid, IVPD and IVSD of Kisra from both genotypes increased with FBFPF levels, with a concomitant decrease in phytate and tannin contents (P ≤ 0.05). Sensory attributes of the Kisra were enhanced in Tabat and Wad-Ahmed sorghum genotypes prepared with 50 and 100 g FBFPF/100 g flour, respectively. Application of FBFPF is known to be an effective traditional starter, and it could improve the nutritional quality of Kisra bread.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sorghum (Sorghum bicolor L. Moench) is the fifth most important cereal crops in the world preceded by maize, wheat, rice and barley and its total annual production quantity was 64 million tonnes in 2016 (FAOSTAT 2018, http://www.fao.org/faostat/en/#data/QC accessed on 17/10/2018). In arid and semi-arid regions of the world, the majority of people depend mainly on sorghum grains as the staple food due to high cost of animal-based foods and low income of the people (Elkhalifa et al. 2017). Recently, there has been an increase in sorghum production, consumption and utilization due to several factors such as genetic improvement and introduction of new varieties that are highly productive and tolerant to adverse environmental conditions and has great nutritional and health potentials of its grains (Taylor et al. 2006). With the annual production of 6.5 million tonnes in 2016, Sudan is ranked as the third highest sorghum producer in the world (FAOSTAT 2018). Sorghum is widely consumed in Sudan after processing to a variety product such as thick porridge (aceda), thin fermented gruel (Nasha), boiled grain (balela), pancake-like fermented flat bread (Kisra), dough-like fermented food (Hussuwa), non-alcoholic beverages prepared from germinated grains and fermented flours (Abreh and Hulumur), and alcoholic beverage (Merissa) (Mohamed Ahmed et al. 2018). Among these products, Kisra by far, is the most widely produced and consumed product throughout the whole country (Mahgoub et al. 1999).
Kisra is thin pancake-like flat bread prepared traditionally from naturally fermented sorghum flour and the quality of the product depends on sorghum genotype, quality and the age of the starter, temperature, time and consistency. Kisra fermentation is mainly considered as lactic acid and yeast fermentation and the production of organic acids and alcohols is desirable because of their role in taste and flavor of the final product (Osman et al. 2010). Kisra has a long history as staple food for people in Sudan and neighboring countries and nowadays it is regarded as home-based industry in the Sudan (AwadElkareem and Taylor 2011). Globally, due to the incidence of celiac and gluten intolerance health problems, there is increasing interest in gluten-free products. In this regard, Kisra could potentially be used as the basis for the development of gluten-free sandwich wrap (AwadElkareem and Taylor 2011). However, Kisra produced mainly from sorghum have been reported to have low protein quality, especially lysine (Mohammed et al. 2007). Studies have shown that supplementation of sorghum flour with 10% whey protein (Ibrahim et al. 2005) and fenugreek seed flour (Suliman et al. 2003) greatly increased their protein content and sensory attributes. Other readily available sources like by-products from tree fruits can also be utilized to improve the quality attributes of sorghum Kisra.
Baobab tree (Adansonia Digitata L) is one of the most widely used trees and has been employed for centuries in many African countries to provide food, medicine and fodder (Sidibe and Williams 2002). In Sudan, the dry pulp of the fruit is consumed fresh or dissolved in water to be used as fermenting agent in local brewing or as a sauce in food (Abdalla et al. 2010). The fruit pulp is regarded as good source of vitamin C, pectin and major minerals such as calcium, magnesium and phosphorous (Kabore et al. 2011). However, there is insufficient information on the utilization of fermented baobab fruit pulp as starter culture for fermentation of cereal-based products. In addition, research into improving the quality of Kisra by using new sorghum genotypes and fermented baobab fruit pulp has not been performed. Therefore, the aim of this study was to evaluate the quality attributes of Kisra prepared from high and low tannin sorghum genotypes that have been fermented with different levels of fermented baobab fruit pulp flour (FBFPF).
Materials and methods
Sample collection
Two sorghum genotypes, locally known as Wad-Ahmed (high tannin) and Tabat (low tannin) were collected from Sinnar Research Station, Agricultural Research Corporation, Sinnar, Sudan. Baobab fruit was obtained from local market in Khartoum, Sudan. The grains and fruits were carefully cleaned to remove all foreign materials. The grains were then decorticated to extraction rate of 90%. Seeds of dried baobab fruit were detached from its pulp by using a special high rate mechanical extracting machine. Baobab fruit pulp flour (BFPF) was prepared by grinding the dried pulp to pass through a 0.4-mm mesh screen and kept at 4 °C until used. All reagents used in this study were of analytical grade.
Fermentation process
Fermented BFPF
Natural fermentation of BFPF by microflora presented in the grain was carried out by mixing the flour with water (1:2 w/v). The mixture was incubated at 37 °C for 24 h in a sterile covered flask, and then stored at 4 °C in tightly closed containers until used for the fermentation of sorghum flour.
Fermented sorghum batter
The method of El Tinay et al. (1985) was employed in the traditional fermentation (lactic acid fermentation) of the sorghum flour of both genotypes (low and high tannin) as practiced in most Sudanese households. Initially, the sorghum flour was naturally fermented by the original microorganisms presented in the grain. This involved addition of flour to water at a ratio of 1:2 (flour: water, 40 g flour: 80 mL water) and the batter was kept in the incubator at 37 °C for 24 h. The obtained sorghum starter was kept in the refrigerator until used for fermentation of the flour samples. Sorghum flour (180 g) was mixed with 360 mL of water and then 60 mL starter obtained from previously fermented batter was added to 540 mL of the flour paste and mixed thoroughly. This was followed by addition of FBFPF starter at different levels (0, 25, 50, 75, and 100% of FBFPF/60 mL of sorghum starter) to 540 mL batter and the volume of the sorghum starter was decreased accordingly. The mixture was incubated at 37 °C for 24 h in 1 L sterile conical flask. The fermented sorghum batters with and without FBFPF starter were used for preparing Kisra.
Preparation of Kisra
The Kisra was prepared traditionally as described by Mallasy et al. (2002). Fermented batters were baked as Kisra sheets on a hot plate (Balady model, Sudanese) at 160–170 °C for 30–60 s. Part of the produced Kisra was used for sensory evaluation. The remaining Kisra samples were dried in hot air oven (Heraeus UT 5042, Germany) at 65 °C for 16 h. The samples were then milled to pass through 0.4 mm mesh screen and stored at 4 °C in tightly closed containers until analyzed. The control samples were Tabat and Wad-Ahmed sorghum-based Kisras fermented with only sorghum starter (60 mL), without addition of BFPF starter.
Chemical composition
The amount of chemical composition of the Kisra samples were determined as described in AOAC (2000) method. The official methods; AOAC 925.40, 950.48, 923.03, 935.38, and 920.86 were used for the determination of moisture, protein, ash, fat, and fiber contents, respectively. Carbohydrate content was calculated by subtracting the sum of moisture, protein, ash, fat, and fiber from 100.
Minerals composition
Mineral contents of the Kisra samples were determined according to Pearson (1970) with slight modification. Exactly 2 g of sample was heated at 550 °C for 3 h and cooled. The ash obtained was treated with 10 mL concentrated hydrochloric acid (50% HCl) with the addition of 5 mL of nitric acid (33%), and then placed in a water bath for (100 °C) 1 h. Then, 10 mL of HCl was added and the sample was returned to the water bath again for 15 min. The mixture was then transferred to 100 mL volumetric flask containing distilled water with a final volume of 100 mL, and well shaken. After sample preparation, mineral concentration was determined. Sodium (Na) and potassium (K) were determined by flame photometer (PFP7, JENWAY, Ltd., England). Different concentrations (5–80 μg/mL) of Na (R2 = 0.998) and K (R2 = 0.991) were used to construct the standard curve for quantification of Na and K in Kisra samples. Calcium (Ca), magnesium (Mg) and iron (Fe) were determined by atomic absorption spectrophotometry (AAS). After calibration of the AAS with standard solutions, the quantity of each element was calculated from its standard curve; Ca (0.25–8.0 μg/mL, R2 = 0.999), Mg (0.13–4.0 μg/mL, R2 = 0.990), and Fe (0.06–2.0 μg/mL, R2 = 0.994). Phosphorus (P) was determined by ultraviolet–visible (UV–Vis) spectrophotometry using molybdate–vanadate method (Chapman and Pratt 1982). Briefly, 5 mL of mineral extract was mixed with 10 mL ammonium molybdate–vanadate reagent and after 30 min incubation at room the absorbance was measured at 440 nm and the amount of P in the samples were calculated from the standard curve treated on the same way.
Mineral extractability
The HCl extraction of minerals of the Kisra samples was performed as described by Chauhan and Mahjan (1988). Briefly, 1 g sample was added to 10 mL of 0.03 M HCl and shaken for 3 h at 37 °C. The mixture was then filtered and the clear extract obtained was oven dried at 100 °C and acid-digested. The content of the extractable minerals was analyzed as described above. The percentage HCl extractability was calculated using the formula:
Amino acid composition
Amino acid contents of Kisra samples were determined as described in the official method (AOAC 2000). Briefly, 5 mL of 6 N HCl was added to 500 mg of powdered Kisra samples in a tube and then the mixture was hydrolyzed at 100 °C for 24 h. Thereafter, the samples were cooled and the pH was adjusted to 2.2 with 1 N NaOH. The volume was made to 100 mL with buffer (pH 2.2) and then was filtered through membrane filter. The liberated amino acids in the hydrolysate were subjected to automated online derivatization with o-phthalaldehyde and were then separated using an automated amino acid analyzer (JLC-500/V2, Amino Tac; JEOL, Japan) based on ion-exchange chromatography. The amino acids were detected at 570 nm except proline that was detected at 440 nm from a separate detector channel. The amino acids were expressed as μg of amino acid per mg of protein.
Ascorbic acid (AA), Antinutritional factors, IVPD and IVSD
Ascorbic acid content was determined using 2,6-dicholoro-phenol indophenol dye reagent according to the method described by Ruucke (1963). The determination of phytic acid of the samples was carried out as described by Wheeler and Ferrel (1971). Briefly, 2 g sample was extracted with 50 mL of 3% trichloroacetic acid (TCA) for 3 h with mechanical shaking. The mixture was centrifuged and 10 mL aliquot of supernatant was added to 4 mL of FeCl3 solution (containing 2 mg Fe+3 per mL 3% TCA) and heated in boiling water for 45 min. Different Fe(NO3)3 concentrations (0.1–3.2 mg/mL) was used as standard and phytate phosphorous was calculated from the standard curve (R2 = 0.989) and was expressed as Fe(NO3)3 equivalents, by assuming a Fe:P molar ratio of 4:6. The vanillin-HCl method (Price et al. 1978) was used to determine the content of tannins. Briefly, extraction was carried out by adding 0.2 g sample to 10 mL of 1% HCl in methanol (v/v). The mixture was shaken for 20 min and centrifuged at 2500 rpm for 5 min. Exactly 1 mL of supernatant was added to 5 mL of vanillin HCl reagent (equal volumes of 8% HCl in methanol and 4% vanillin in methanol). A standard catechin solution (0.16–5.0 mg/mL) was prepared and used as reference standard (R2 = 0.992). The absorbance of sample and standard solutions were read using spectrophotometer (Jenway V6300) at 500 nm after 20 min incubation at 30 °C. Tannin concentration was expressed as catechin equivalent percentage.
The method of Monjula and John (1991) was used to determine the IVPD of the samples. Briefly, a known sample weight containing 16 mg nitrogen was digested with 1 mg pepsin (activity: 1:3000, Carolina) in 0.1 M HCl (15 ml) for 2 h at 37 °C. Fifteen milliliters of 10% trichloroacetic acid (TCA) was added to the mixture to stop the reaction. The volume was then filtered through Whatman No. 1 filter paper. The nitrogen content in the filtrate was determined using micro-Kjeldahl method and the digestibility was calculated as:
The procedure of Mouliswar et al. (1993) was used to determine the IVSD of the samples. In this assay, 2% of the sample slurry was heated in a water bath at 100 °C for 15 min, followed by the addition of 30 mL of 0.2 M glycine–HCl buffer (pH, 2.0) containing 10 mg pepsin in 50 mL of the slurry. The mixture was incubated at 37 °C for 2 h followed by the adjustment of the pH to 7 with 0.2 N NaOH. The final volume was adjusted to 100 mL with distilled water. Five milliliters of 0.5 M phosphate buffer (pH 8.0) containing 15 mg of pancreatin (Carolina) and 15 mg amyloglucosidase (specific gravity: > 1, Carolina) was added to 10 mL of the mixture, which was incubated for 2 h at 37 °C. After that, the reaction was terminated by heating the mixture in a water bath at 100 °C. Then, 2 mL of dinitrosalicylic acid reagent was added to 0.5 mL of the mixture to determine the amount of reducing sugar. The standard used was glucose and a conversion factor of 0.9 was used to calculate the starch equivalent.
Sensory evaluation
A panel of fifteen semi-trained panelists (males and females, age range 20–40 years) at Food Research Center, Khartoum, Sudan, was used to judge the quality of the different types of Kisra fermented with and without baobab starters, as well as the control Kisra. Although the panelists have long experiences in evaluating the sensory attributes of cereal-based products, they were given three preliminary sessions to familiarize them with the sensory properties to be assessed. Sensory evaluation was done on the same day that the Kisra breads were prepared. The panelists were asked to evaluate each sample for taste, color, texture, appearance and overall acceptability, using a 9 points hedonic scale (9 for like extremely down to 1 for dislike extremely) as described by Iwe (2002). The samples were coded with three-digit random numbers and placed in random order under normal light (600 lx) for panel analysis. During sensory evaluation, panelists were instructed to drink water or rinse their mouths to clear the palate after each evaluation.
Statistical analysis
Three fermentation batches were conducted and all measurements were done in triplicates. The experiments were designed using a completely randomized block design and the effects of the treatments on the parameters determined were statistically analyzed using SAS/STAT software (SAS Institute, Cary, NC, USA). Duncan multiple range test with a probability P ≤ 0.05 was used to separate the means.
Results and discussion
Effect of FBFPF starter levels on chemical composition of Tabat Kisra flour (TKF) and Wad-Ahmed Kisra flour (WKF)
As shown in Table 1, the moisture contents of TKF and WKF without FBFPF addition were 5.17 and 5.06%, respectively. Changes in moisture content were observed after incorporation of FBFPF in both TKF and WKF. Addition of FBFPF at 25 and 50% levels increased the moisture content of TKF as compared with control, while only 75% FBFPF addition had significantly higher moisture content than control sample in WKF. Also, the fat content of control TKF (1.66%) and WKF (1.89%) were significantly higher than those prepared with FBFPF. This effect was found to increase as the level of FBFPF increases with lowest value obtained in those fermented with 100% FBFPF (1.06%). These values were lower than previously reported values for sorghum flour (Osman et al. 2010). The decrease in fat contents may be due to FBFPF increasing the rate of lipolysis to fatty acids and glycerol during the fermentation process. This agrees with those found in pigeon pea where fermentation lowered their fat contents (Adebowale and Maliki 2011). Moreover, FBFPF at varying fermentation times has low fat (0.16–0.25%) content (data not shown) and this agrees with that reported by Shukla et al. (2001). Thus, increasing the level of FBFPF added to the sorghum flours could result in reducing the fat content of the product.
However, addition of FBFPF starter to TKF and WKF raised (P ≤ 0.05) their ash contents over that of control TKF and WKF with highest values obtained in samples treated with 100 g FBFPF/100 g flour. The increase in ash content following addition of FBFPF could be attributed to high ash contents (4.40–4.62%) observed in BFPF fermented for several periods (data not shown). Our findings agree with that found in wheat flour following supplementation with sorghum and chickpea flours and was attributed to increased ash contents of sorghum and chickpea (Gadallah et al. 2017). Incorporation of FBFPF may have improved the fermentation of the two sorghum genotypes and thus increased organic minerals’ utilization by microbes.
The protein and fiber contents of TKF (10.01%) and WKF (9.95%) without FBFPF addition were significantly (P ≤ 0.05) enhanced after fermentation with FBFPF and this increased with increase in FBFPF level. This could be attributed to the high fiber content (4.05–5.20%) of FBFPF as observed in our preliminary study on FBFPF (data not shown). The values found in this study were lower than those reported for sorghum flours (Osman et al. 2010; Afify et al. 2012). Previous study has found that crude fiber content decreases during fermentation and this was attributed to degradation by fermenting microbes (Babalola and Giwa 2012), which is contrary to our present findings. Also, the increment in the amount of protein after fermentation with BFPF could be due to the synthesis of protein by microorganisms from metabolic intermediates.
Effect of FBFPF starter levels on mineral contents and extractability of TKF and WKF
The results in Table 2 showed that the contents of major minerals particularly P and Mg and Fe of control TKF and WKF were significantly enhanced after incorporating FBFPF as the starter. The increment in P content during fermentation may have been due to the activity of the phytase microbial enzyme, which hydrolyzes phytate, thereby releasing more P from phenolic compounds. Increase in mineral contents of the Kisra was observed with increasing FBFPF levels with the exception of Fe, where no significant improvement was observed after fermentation of the Kisra with FBFPF. This could be attributed to high mineral content of baobab fruit pulp flour as reported in a previous study (Muthai et al. 2017) and in our preliminary investigation on FBFPF at different fermentation periods (data not shown). These values were high as compared to the values obtained with fermented sorghum genotypes flour (Afify et al. 2012).
Among the major minerals, the HCl extractability analysis revealed that Na was the most available mineral, while Mg was the least available mineral for both TKF and WKF (Table 3). HCl extractability of Fe of TKF and WKF without FBFPF addition was 49.50 and 50.58%, respectively. The HCl extractability of the minerals was enhanced after fermentation of the sorghum genotypes with FBFPF as starter. In a similar study, Sokrab et al. (2014) reported that fermentation of high and low phytate corn genotypes improved the HCl extractability of Na and Ca. The improvement in the HCl extractability of the minerals of the genotypes may be due to hydrolysis of phytate and tannin by the released phytase and tannase, respectively during fermentation. The dephosphorylation mechanism involves the removal of phosphate groups from the inositol ring of phytate, which decreases the strength of mineral binding to phytate, thereby enhancing the bioavailability of essential minerals (Sandberg et al. 1999). The mineral extractability of both Kisras increased progressively and significantly (P ≤ 0.05) with the starter (FBFPF) levels, except for K and P of WKF and K of TKF, which were extracted best in samples prepared with 25 and 50 g FBFPF/100 g flour for WKF and TKF, respectively. This increase could be attributed to the high mineral extractability of the FBFPF starter (Abdalla et al. 2010). Furthermore, the Fe extractability of TKF and WKF prepared with 100 g FBFPF/100 g flour were also enhanced (P ≤ 0.05) to 52.01 and 51.77%, respectively. The obtained values were high as compared to that of fermented sorghum genotypes flour reported by Idris et al. (2005).
Effect of FBFPF starter levels on amino acid profile of TKF and WKF
Table 4 shows the changes in the amino acids profiles of Kisra fermented with different levels of FBFPF starter. The essential amino acid composition of TKF and WKF prepared without FBFPF addition ranged from 14.3–211.6 to 18.9–273.2 mg/100 g, with methionine and histidine having the lowest values, respectively. Leucine was the maximum essential amino acid found in both genotypes. Moreover, proline and tryptophan were the highest and lowest non-essential amino acids, respectively found in both Kisra types. Fermentation of the sorghum genotypes with FBFPF as starter increased the amino acids with the exception of glycine, tyrosine, glutamic acid, serine and prolein of TKF and cysteine and serine of WKF. Increase in FBFPF levels further enhance the amino acid content of the Kisra with the highest values being observed in those fermented with 100 g FBFPF/100 g flour. The increase in amino acids after fermentation may be due to the metabolic activity of microorganisms involved in fermentation through which some amino acids might be utilized and others might be produced. Similarly, increase in amino acids of baobab seeds following fermentation has been reported (Parkouda et al. 2015) and this may have contributed to the amino acids of the Kisra. Regardless of the fermentation treatment, all the essential amino acids of the samples were lower than those of recommended levels (FAO/WHO/UN 1985) except leucine and lysine of WKF fermented with 100 g FBFPF/100 g flour those have higher and comparable values to that of RDA.
Effect of FBFPF starter levels on antinutritional factors, IVPD, IVSD and AA of TKF and WKF
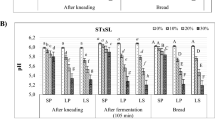
The effects of FBFPF levels on the antinutritional factors, IVPD and IVSD of Kisra breads prepared from fermented Tabat and Wad-Ahmed sorghum flours are shown in Fig. 1. Samples TKF and WKF without FBFPF addition displayed the highest contents of tannin (0.388 and 0.487%, respectively) and phytate (205.41 and 247.8 mg/100 g, respectively) (Fig. 1a, b). Also, the IVPD of the control TKF and WKF samples were 70.94 and 68.92%, respectively (Fig. 1c) while the IVSD of the samples were 52.59 and 23.92%, respectively (Fig. 1d). The values of IVPD were within the range (49.25–55.85%) reported by Awadelkareem et al. (2009) for sorghum flours.
Anti-nutrients contents, in vitro protein and starch digestibility and ascorbic acid content of Tabat and Wad-Ahmed sorghum-based Kisra prepared with and without FBFPF. TKF Tabat kisra flour; WKF Wad-Ahmed Kisra flour; FBFPF baobab fruit pulp flour; IVPD in vitro protein digestibility; IVSD in vitro starch digestibility; AA ascorbic acid
Figure 1 reveals that incorporation of FBFPF as starter in the fermentation of the sorghum genotypes significantly (P ≤ 0.05) reduced the phytate and tannin contents. Also, further significant reduction (P ≤ 0.05) in the antinutrients was observed with increase in levels of BFPF starter. However, sharp rise in IVPD and IVSD were evident following the incorporation of FBFPF, and this was more pronounced as FBFPF level increased. Previous study has reported decrease in the level of phytic acid of fermented sorghum grains (Idris et al. 2005) and this was attributed to the action of enzyme, phytase, released during fermentation. Also, the reduction in the level of tannin in Kisra prepared with FBFPF starter may be attributed to the action of tannase produced by microorganisms during fermentation. According to Hassan and El Tinay (1995), fermentation can cause changes in the fractions of endosperm protein, which renders starch more accessible to the digestive enzyme, which may result in an increased IVSD.
The results of the AA as shown in Fig. 1e revealed that both the control TKF and WKF samples and those of Kisra fermented with FBFPF starter had no AA value. However, fermentation of the sorghum genotypes with FBFPF starter at levels higher than 25 g FBFPF/100 g lead to significant rise in AA of both Kisras. This could be due to high ascorbic acid contents present in baobab fruit pulp as reported by Abdalla et al. (2010) and our preliminary results on the FBFPF subjected to different fermentation periods (data not shown).
Effect of FBFPF starter levels on sensory attributes of TKF and WKF
Table 5 shows the sensory evaluation of Kisra as influenced by fermentation with different levels of FBFPF. Fermentation of the sorghum genotypes with FBFPF improved the sensory traits of TKF with those treated with 50% FBFPF exhibiting the best attributes, although not significantly different from other treated samples. Similarly, higher sensory attributes was observed in WKF following the addition of 75 and 100% FBFPF levels. Baobab fruit pulp had better sensory attributes and is widely consumed by majority of people in Sudan and this could have contributed to the improvement in the overall sensory attributes of the Kisra.
Conclusion
The use of fermented baobab fruit pulp flour as starter in the fermentation of sorghum flour used for Kisra preparation greatly lowered the antinutritional factors of the fermented sorghum dough product. Furthermore, fermentation with FBFPF starter is considered as a powerful method in improving the nutritional, total and extractable minerals and sensory attributes of sorghum products such as Kisra.
References
Abdalla AA, Mohammed MA, Mudawi HA (2010) Production and quality assessment of instant baobab (Adansonia digitata L.). Adv J Food Sci Technol 2(2):125–133
Adebowale OJ, Maliki K (2011) Effect of fermentation period on the chemical composition and functional properties of Pigeon pea (Cajanus cajan) seed flour. Int Food Res J 18(4):1329–1333
Afify AMR, El-Beltagi HS, Abd El-Salam SM, Omran AA (2012) Effect of soaking, cooking, germination and fermentation processing on proximate analysis and mineral content of three white sorghum varieties (Sorghum bicolor L. Moench). Not Bot Horti Agrobo 40(2):92–98
AOAC (2000) Official methods of analysis, 16th edn. Association of Official Analytical Chemists, Washington, DC
AwadElkareem AM, Taylor JRN (2011) Protein quality and physical characteristics of Kisra (fermented sorghum pancake-like flatbread) made from tannin and non-tannin sorghum cultivars. Cereal Chem 88(4):344–348
Awadelkareem AM, Muralikrishna G, El Tinay AH, Mustafa AI (2009) Characterization of tannin and study of in vitro protein digestibility and mineral profile of sudanese and Indian sorghum cultivars. Pak J Nutr 8:469–476
Babalola RO, Giwa OE (2012) Effect of fermentation on nutritional and anti-nutritional properties of fermenting Soy beans and the antagonistic effect of the fermenting organism on selected pathogens. Int Res J Microbiol 3(10):333–338
Chapman HD, Pratt PF (1982) Methods of analysis of soil, plant and water, 2nd edn. University of California, Agricultural Division, Davis
Chauhan BM, Mahjan H (1988) Effect of natural fermentation on the extractability of minerals from pearl millet flour. J Food Sci 53:1576–1577
El Tinay AH, El Mehdi ZM, El Soubki A (1985) Supplementation of fermented sorghum kisra bread with legume protein isolates. J Agric Food Chem 21:679–687
Elkhalifa AEO, Bernhardt R, Cardone G, Marti A, Iametti S, Marengo M (2017) Physicochemical properties of sorghum flour are selectively modified by combined germination-fermentation. J Food Sci Technol 54(10):3307–3313
FAO/WHO/UN (1985) Energy and protein requirements. WHO Tech. Series; No. 724. Wold Hold Health Organization, Geneva
Gadallah MGE, Rizk IRS, Elsheshetawy HE, Bedeir SH, Abouelazm AM (2017) Impact of partial replacement of wheat flour with sorghum or chickpea flours on rheological properties of composite blends. J Agric Vet Sci 10(1):83–98
Hassan IAG, El Tinay AH (1995) Effect of fermentation on tannin content and in vitro protein and starch digestibility of two sorghum cultivars. Food Chem 53(2):149–151
Ibrahim FS, Babiker EE, Yousif NE, El Tinay AH (2005) Effect of fermentation on biochemical and sensory characteristics of sorghum flour supplemented with whey protein. Food Chem 92:285–292
Idris WH, AbdelRahman SM, EL Maki HB, Babiker EE, EL Tinay AH (2005) Effect of germination, fermentation and cooking on phytic acid and tannin contents and HCl-extractability of minerals of Sorghum (Sorghum biocolor) cultivars. J Food Technol 3(3):410–416
Iwe MO (2002) Handbook of sensory methods and analysis, 1st edn. Rejoint Communication Services Ltd., Enugu
Kabore D, Sawadogo-Lingani H, Diawara B, Compaore CS, Dicko MH, Jakobsen M (2011) A review of baobab (Adansonia digitata) products: effect of processing techniques, medicinal properties and uses. Afr J Food Sci 5(16):833–844
Mahgoub SEO, Ahmed BM, Ahmed MMO, El Agib ENA (1999) Effect of traditional Sudanese processing of Kisra bread and hulu-mur drink on their thiamine, riboflavin and mineral contents. Food Chem 67:129–133
Mallasy OL, Eltinay AH, Ahmed AR (2002) Biochemical and sensory evaluation of wheat bran supplemented sorghum bread. Plant Foods Human Nutr 57:63–71
Mohamed Ahmed IA, Al-Juhaimi FY, Bekhit AEA (2018) Fermentation of grains. In: Aluko R, Birch EJ, Larsen D, Melton L, Rogers M, Shahidi F, Stadler R, Sun-Waterhouse D, Varelis P (eds) Reference module in food science, encyclopedia of food chemistry, vol 2. Elsevier, Amsterdam, pp 107–116
Mohammed SA, Ali AO, Elkhalifa EA (2007) Supplementation of fermented sorghum bread “kisra” with dehulled and defatted sesame flour. Gezira Agric Sci 5(2):181–188
Monjula S, John E (1991) Biochemical changes and in vitro protein digestibility of the endosperm of germinating Dolichos lablab. J Sci Food Agric 55:529–539
Mouliswar P, Kurien S, Daniel VA, Malleshi NG, Venkatarao S (1993) In-vitro digestibility of protein and starch as energy food and its bulk reduction. J Food Sci Technol 30:36–39
Muthai KU, Karori MS, Muchugi A, Indieka AS, Dembele C, Mng’omba S, Jamnadass R (2017) Nutritional variation in baobab (Adansonia digitate L.) fruit pulp and seeds based on Africa geographical regions. Food Sci Nutr 5(6):1116–1129
Osman MA, AbdelRahman IE, Hamad SH, Dirar HA (2010) Biochemical changes occurring during traditional Sudanese processing of Kisra bread. J Food Agric Environ 8(2):102–106
Parkouda C, Ba HF, Ouattara SL, Tano-Debrah K, Diawara B (2015) Biochemical changes associated with the fermentation of baobab seeds in Maari: an alkaline fermented seeds condiment from western Africa. J Ethnic Foods 2:58–63
Pearson DC (1970) The chemical analysis of foods. Churchill, London
Price ML, Van Scoyoc S, Butler LG (1978) A critical evaluation of the vanillin reactions as an assay for tannin in sorghum grain. J Agric Food Chem 26:1214–1218
Ruucke JA (1963) Chemical methods of analysis of fruits and vegetables. Publication 154. Department of Agriculture, Canada
Sandberg AS, Brune M, Carlsson NG, Hallberg L, Skoglund E, Rossander-Hulthen L (1999) Inositol phosphates with different number of phosphate groups influence iron absorption in humans. Am J Clin Nutr 70:240–246
Shukla YN, Dubey S, Jain SP, Kumar S (2001) Chemistry, biology and uses of Adansonia digitata—a review. J Med Aromat Plant Sci 23:429–434
Sidibe M, Williams JT (2002) Fruits for the future 4: Baobab (Adansonia digitate). International Centre for Underutilised Crops, Southampton
Sokrab AM, Mohamed Ahmed IA, Babiker EE (2014) Effect of fermentation on antinutrients, and total and extractable minerals of high and low phytate corn genotypes. J Food Sci Technol 51(10):2608–2615
Suliman AE, Ali OA, Elkhalifa EA (2003) Some characteristic of the Sudanese bread (kisra) supplemented with fenugreek (Trigonellafoenum-graecum L.). Gezira Agric Sci 1(2):15–25
Taylor JR, Schober TJ, Bean SR (2006) Novel food and non-food uses for sorghum and millets. J Cereal Sci 44:252–271
Wheeler EL, Ferrel RE (1971) A method for phytic acid determination in wheat and wheat fractions. Cereal Chem 28:313–320
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Makawi, A.B., Mustafa, A.I., Adiamo, O.Q. et al. Quality attributes of Kisra prepared from sorghum flour fermented with baobab fruit pulp flour as starter. J Food Sci Technol 56, 3754–3763 (2019). https://doi.org/10.1007/s13197-019-03848-w
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03848-w