Abstract
This investigation was carried out to evaluate the effect of active and passive modified atmosphere packaging on quality and shelf life of yellow bell pepper fruits. Yellow bell pepper fruits were packaged in 150 gauge LDPE packages with oxygen absorbers for active modification and without oxygen absorber for passive modification of headspace and were stored at different temperatures i.e. 5, 10 and 15 °C and RH of 85 ± 5%. Headspace gas concentration within the packages was monitored regularly. The quality of packaged fruits was studied in terms of physiological loss in weight, firmness, total colour difference antioxidant capacity and total phenolic content. The actively modified packages attained steady state levels of 4.8% O2 and 7.1% CO2 on 4th day of storage as compared to passively modified packages in which steady state was not attained even at end of storage period of 12 days. The retention of quality attributes was observed to be higher in active packages than in passive packages. Moreover, the shelf life of actively packaged fruits was enhanced to 28 days as compared to 12 days for passively packaged fruits. The in-pack atmosphere attained in active packages hence proved beneficial in retarding the senescence thereby extending the shelf life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bell pepper (Capsicum annuum L.) is one of the most popular fruit which is rich in bioactive compounds and also have high antioxidant capacity. India produces about one-fourth of the world production of bell pepper with an annual production of 0.9 million tons from an area of 0.885 million hectare with a productivity of 1266 kg per hectare (Sreedhara et al. 2013). Presently, many varieties of bell pepper are available in the market, majority of which change their colour from green to yellow, orange or red, when they are completely ripe (Mendoza et al. 2015). Bell pepper usage in India is increasing now-a-days due to increase in the demand by the urban consumers. There is also a high demand for export. It is produced throughout the world for fresh market consumption. Its consumption is growing popularity mainly due to its availability in wide variety of colors, shapes and sizes and its characteristics flavor (Frank et al. 2001; Lucier and Lin 2001).
Yellow bell pepper (Capsicum annuum L.) is rich in bioactive compounds, especially, carotenoids and vitamin C (Howard et al. 2000; Antoniali et al. 2007) and also contains moderate to high level of phenolics and flavonoids contents (Howard et al. 2000) and is low in calories. The presence of these bioactive compounds provide numerous health benefits such as protection against the oxidative damage to cells and thus prevent the development of common degenerative diseases such as cancer, cardiovascular diseases, cataracts, diabetes etc. Green peppers are harvested before they ripen whereas yellow bell pepper are harvested when they are completely ripe. Hence, yellow bell pepper is a highly perishable and needs appropriate handling and adequate care to maintain shelf-life and quality (Singh et al. 2014).
Packaging of fresh produce is a dynamic field. Fresh fruits and vegetables are biologically active even after harvest. Freshly harvested produce is perishable, hence deteriorate in a shorter period of time. Thus a good and effective packaging is needed for enhancing the shelf life of freshly harvested produce. The conventional food packaging serves basic purpose of storage, preservation and protection of food (Galić et al. 2011; Kumar et al. 2017). Continuous attempts are being made in fresh fruits and vegetables preservation so as to provide superior quality products to the consumers. At present, preservation techniques being used are controlled atmosphere (CA) and modified atmosphere packaging (MAP) which can be combined with low temperature in order to obtain excellent results with respect to food quality (Almenar et al. 2009). These conventional packaging techniques have contributed significantly towards the preservation and shelf life enhancement of food but they are not sufficient enough to cope with the increasing consumer requirements for innovative and better packaging (Yam et al. 2005). The various challenges in food packaging industry are extension of shelf life, convenience, safe and healthy food with lesser food wastage (Kerry 2014). As the challenges in the food protection and preservation are continuously increasing, food packaging technology is also continuously developing (Realini and Marcos 2014). The conventional solutions to solve this problem are passive packaging techniques that can retard the adverse effect of environment on the packaged products but are not efficient in keeping the sensory quality of fresh and sensitive foods concomitantly with a sustained shelf life (Lopez et al. 2004). Advanced packaging techniques having additional functions are always looked for in response to the consumer requirements for minimal processed foods with lower amount of preservatives, raised regulatory requirements and increasing concern for the food safety (Yam et al. 2005). In order to meet the needs and challenges of food packaging technology the area of active and intelligent packaging is being researched.
Active packaging involves addition/insertion of a supplementary constituent have been purposely included inside the package or either on the packaging material in order to improve the functioning of the packaging system (Robertson 2006). The active packaging system interacts with the internal atmosphere of the food package and thus helps in maintaining the quality and extending shelf life of the packaged food. As oxygen is considered the chief cause of the food spoilage so the most commonly used active packaging systems remove oxygen from the food package. Thus present research was undertaken in order to develop a packaging system in which the gas concentration could be reduced to safe level which is beneficial for extension of shelf life. Active packaging technology is desired for prevention or reduction of the food spoilage. Considering the continuously growing demand for high quality safe food, efficient and effective food packaging is required. In the light of the above considerations, efforts were made to develop active packaging for extending the shelf life of fresh yellow pepper.
Materials and methods
Respiration and transpiration rate
Respiration rate for bell-pepper was determined using closed system technique at different temperatures (5, 10, and 15 °C) with RH of 85% (Singh et al. 2014; Kaur et al. 2011). Respiration rate is measured in terms of oxygen consumption rate and carbon dioxide evolution rate. For measuring the transpiration rate, fresh samples were placed in a petri dish and then kept inside a desiccator which was then kept in environmental chamber maintained at experimental temperature and constant relative humidity. The samples were weighed after a regular interval using an electronic weighing balance until weight loss became consistent. Total weight loss was used to calculate the transpiration rate of the sample which was further used to evaluate the transpiration coefficient of freshly harvested bell pepper.
Sample preparation for packaging and storage
Fresh yellow bell pepper (Capsicum annuum L. var. Oribelli) was procured from farms of Punjab Agricultural University, Ludhiana grown under poly-house conditions. The freshly harvested bell pepper fruits were washed with water in order to remove any dirt, surface dried and then sorted for uniform size. Samples were kept inside cold rooms at 10 °C and 90% RH for 2 h for equilibration. Equal weight of fruits were then placed in packages (8 cm × 10 cm) made of 150 gauge of low density polyethylene (LDPE). The oxygen absorber sachets (O-Busters FT-20CC), made of finely powdered ferrous iron oxide (Iron II Oxide; FeO), natural zeolite and salt (acts as a catalyst) which are vacuum sealed in polyester or polyethylene material were procured from Sorbead India, Gujarat, India. Ferrous iron oxide powder reacts with moisture and oxygen inside the packages and is oxidised to ferric iron oxide thereby reducing the oxygen concentration within the headspace. These food grade and non-toxic oxygen absorber sachets each weighing 2 g, were placed inside the packages just before sealing using an impact sealer. The samples packaged without oxygen absorber were taken as control. All the packages thus prepared were stored in walk-in cold rooms maintained at different temperature of 5, 10 and 15 °C and relative humidity of 90 ± 2%. Headspace gas concentration within the packages was measured at regular intervals. Quality analysis of bell pepper fruits was done at regular interval of 4 days in terms of physical parameters i.e. physiological loss in weight (PLW), firmness and total colour difference and Bioactive compounds i.e. total phenols and antioxidant capacity.
Headspace analysis
The gas composition inside the packages was analysed regularly with the help of gas analyser (Systech Instruments; Model: Gaspace Advance, UK). It is an oxygen and carbon dioxide analyser used for the measurement of O2 and CO2 in food packages. The components of this instrument are oxygen, carbon dioxide sensors, LCD readout, internal sampling pump and sampling probe. The probe attached to the instrument, had a filter placed on the end and then a needle was inserted on it. The instrument was then calibrated to check the accuracy. The probe was kept in air and start button was pressed. The calibrated readings were checked to be 20.3–21.1% O2 and 0–0.3% CO2. Once it had been calibrated then the probe was entered into the package by piercing. Each package had a septum pasted on it through which the probe needle was inserted.
Physiological loss in weight (PLW)
The weight of fresh bell pepper was measured at the onset of storage. The change in weight of the samples during storage was observed at regular interval of 4 days. The weighing was done with a digital balance having least count of 0.01 g (Singh et al. 2014). The PLW was calculated with respect to initial weight of sample as:
Firmness
Texture measurement of bell pepper was done using Texture analyser (Make: Stable Micro Systems, Model: TA.TXT. Plus). The texture analysis of bell pepper was done at regular interval of 4 days during storage. The texture analyser was calibrated for force and distance before starting the experiments. The probe P/2 N aluminium needle and force load cell of 5 kg were used. The test speed was kept 2 mm/s (Castro et al. 2007). Firmness was measured by taking the average of the firmness value obtained by keeping the bell pepper in different position with respect to the probe.
Total colour difference
The colour of the bell pepper samples was evaluated by using Color Reader CR-10 (Konica Minolta Sensing Inc.). For color measurements, the petri dish was filled with bell pepper so that no light could pass through it during the measurement. The ‘L’, ‘a’ and ‘b’ values were noted and then they were compared with the values of fresh bell pepper. The change in colour was calculated using the following equation given by Gnanasekharan et al. (1992)
where L0, a0 and b0 are the respective readings of fresh sample.
Antioxidant capacity
Antioxidant capacity of bell pepper was measured by the evaluating the free radical-scavenging effect on the 2, 2-diphenyl-l-picrylhydrazyl (DPPH) radical (HiMedia Laboratories, India) (De Ancos et al. 2002). 1 g of yellow bell pepper was crushed and mixed with the 5 ml of methanol. The solution was then centrifuged using a cold centrifuge (MP 400-R, Eltek Limited, India) at 6000 rpm for about 5 min at 4 °C. Aliquots of 0.01 ml of supernatant were mixed with 3.9 ml of methanolic DPPH (0.025 g/l) and 0.090 ml of distilled water. The solution was then shaken in vortex shaker (Labco, New Delhi, India) and then kept in the dark for about 30 min. Absorbance of solution was measured against blank at 515 nm by using UV–Vis spectrophotometer (Spectroscan 80DV, Biotech Engineering Management Company Limited, UK)
where ODcontrol = optical density of control; ODsample = optical density of sample.
Total phenolic content
The total phenols of bell pepper were measured by A765 with Folin–Ciocalteau reagent method (McDonald et al. 2001). Each 1 g of bell pepper was crushed and mixed with 10 ml of methanol: water which was prepared by mixing methanol and water in equal proportions. The extract was further mixed with 5 ml of Folin Ciocalteu reagent (1:10 diluted with distilled water) and 4 ml of aqueous Na2CO3 (1 M). The mixture was kept for some time until a colour change was observed and the absorbance of the mixtures was measured against the blank at 765 nm by using UV–Vis spectrophotometer (Model Spectroscan 80DV, Biotech Engineering Management Company Limited, UK).
Statistical analysis
All the experiments were performed in duplicates. The observed data was subjected to ANOVA using SAS software (SAS Institute India Pvt. Ltd.) and significance was determined at p < 0.05. Least square means were computed and adjustments for multiple comparison was done using Tukey–Kramer test.
Results and discussion
Respiration and transpiration studies
True density of freshly harvested bell pepper fruits was measured in triplicate and the average value was found to be 0.987 g/ml. From steady state respiration rates, the respiration quotients (RQ) were determined. Steady state RQ is given in Table 1. Transpiration rate is the rate of transfer of water vapour from surface of fresh produce to the surrounding atmosphere. Transpiration coefficient of fresh bell pepper were determined by replacing the experimental values of the weight loss of fresh bell pepper according to the equation
dm = weight loss (g), dt = time (h), W = weight of crop taken (g), \( p_{{{\text{H}}_{ 2} {\text{O}}}}^{sat} \) = saturated vapour pressure (kPa), \( p_{a} \) = vapour pressure around crop surface (kPa).
Headspace concentration
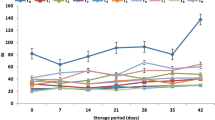
Headspace gas concentration (O2 and CO2) was measured at a regular interval using gas analyser (Systech Instruments; Model: Gaspace Advance, UK). It is clear from Table 2 that headspace gas concentration was significantly affected by the storage period (p ≤ 0.01). The headspace O2 concentration decreased and CO2 concentration increased with the increase in storage period under all conditions (Fig. 1). Similar trends were observed by Kaur et al. (2011) and Kirandeep et al. (2018). Oxygen absorber helped in reducing the headspace O2 concentration to below 2% (Table 3). Oxygen absorbers cause rapid decrease in the headspace oxygen concentration and also removed the oxygen that permeated through the packaging film (Rooney 1995; Gontard 2000). The amount of oxygen absorber also had significant effect. Higher amount of oxygen absorber lead to the development of anaerobic conditions. The headspace CO2 concentration was higher in samples packaged without oxygen absorber as compared to samples packed with oxygen absorber. High CO2 concentration damage the bell peppers causing discolouration, pitting and softening. The results are in accordance with Charles et al. (2003). The effect of storage temperature was also found to be significant (p ≤ 0.05). The actively modified packages attained steady state levels of 4.8% O2 and 7.1% CO2 on 4th day of storage as compared to passively modified packages in which steady state was not attained even at end of storage period of 12 days (Table 3). Active packaging helped in the quick establishment of the steady state which reduced the respiration and transpiration rates and thus helped in extension of shelf life where as in samples stored under passively modified packaging the steady state was not achieved and samples deteriorated rapidly.
Physical parameters
Physiological loss in weight (PLW)
The physiological loss in weight of samples was measured at regular interval of 4 days. The variation of PLW during storage under active and passive modified atmosphere is shown in Fig. 2. The bell pepper samples packaged without oxygen absorber were taken as control samples. The effect of storage period on PLW was significant at 1% level of significance (Table 2). PLW values of samples increased during storage under both active as well as passive modified atmosphere packages (Table 3). It was observed that loss in weight of control samples was almost two times more than that of samples stored with oxygen absorbers. Oxygen absorbers also significantly affected the PLW values of samples. The samples stored with oxygen absorbers recorded better quality and PLW was very low. It may be due to the reason that oxygen absorber sachets reacts with the moisture present inside the packages thereby reducing moisture accumulation and their reaction reduces oxygen concentration inside the packages which retards respiration hence senescence. However there was not much difference in PLW of samples stored under low oxygen i.e. with 2 g and 4 g oxygen absorber. Further analysis of the data revealed that the effect of temperature on PLW was significant (p ≤ 0.05). Higher values of PLW were observed for samples stored at 15 °C as compared to 5 and 10 °C. It may be due to the fact that respiration and transpiration rates are increased at elevated temperatures. With the increase in storage time, all the samples lost weight. Wills et al. 2007 suggested that 4–6% weight loss in fresh commodities led to shrivelled fruit and commercial value loss. Our PLW values were well within the limits.
Firmness
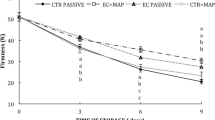
Firmness of fresh bell pepper was 567.09 gf. Firmness showed a decreasing trend with the increase in storage period under all the packaging treatments during storage (Fig. 2). Analysis of the data revealed that the effect of oxygen absorber on firmness was significant at 5% level of significance (Table 2). Firmness of control samples was quiet lower as compared to the firmness of active packaging samples (Table 3). This retention of firmness in samples packaged with oxygen absorber can be attributed to the delay in the progress of senescence of bell pepper due to reduced oxygen concentrations. Oxygen absorbers help in the removing dissolved oxygen and also removes the oxygen that permeates through the packages. It help in reducing the rate of respiration and transpiration thus preventing degradation of the samples which in turn help in maintaining the firmness. It was found that the samples packed with 2 g of oxygen absorber had better firmness as compared to the packages with 4 g oxygen absorber. This may be due to the reason that more oxygen absorber led to the development of anaerobic conditions which lead to spoilage of sample. Storage temperatures also had a significant effect on firmness of fresh bell pepper fruits (p ≤ 0.05). Samples stored at 5 and 10 °C recorded better firmness as compared to the samples stored at 15 °C. This is because at higher temperatures rate of transpiration increases which leads to the loss of moisture and loss in weight of the samples which causes reduction in the firmness. The interaction of oxygen absorber and storage period did not had any significant effect but the effect interaction of oxygen absorber and storage period was significant at 1% level of significance. Smith et al. (2003) reported that the mass loss and firmness are closely related to each other. Similar results of were observed by Singh et al. (2014).
Total colour difference
The values of total colour difference of bell pepper during storage are given in Table 3. The plot of total colour difference for fresh bell pepper fruits versus storage time are shown in Fig. 2. An overview of plots reveals that total colour difference showed an increasing trend with the increase in storage period under all conditions. The effect of oxygen absorber on total colour difference was significant (p ≤ 0.05) (Table 2). Total colour difference of samples in packages without oxygen absorber was higher as compared to the total colour difference of bell pepper stored in packages with oxygen absorber of varying amount. This retention of colour in packages with oxygen absorber is due to delay in the progress of senescence of bell pepper which is due to depletion of headspace O2 concentrations and establishment of the steady-state micro-environment inside the packages (Singh et al. 2013). Low oxygen concentration help in preserving the colour, texture and aroma of different fruits and vegetables. The bell pepper fruits packed with 2 g of oxygen absorber had better colour retention as compared to the packages with 4 g oxygen absorber. Further comparison of the plots at different storage temperatures revealed that temperature also had a significant (p ≤ 0.05) effect on total colour difference of bell pepper. Similar observation were reported by Weichmann (1986) for control atmospheric storage of fresh fruits and vegetables and Zhou et al. (1992) for pepper fruit.
Biochemical parameters
Antioxidant capacity
Yellow bell pepper is a rich source of antioxidants. Antioxidants can delay or prevent the oxidation or free radical mediated oxidation of a substrate when present in low concentrations, leading to the formation of stable radicals after scavenging (Singh et al. 2016b). Antioxidant compounds of fruits and vegetables reduces the risk of neurodegenerative disorders, retards ageing process and helps in lowering the incidence of degenerative diseases, such as heart disease, inflammation, arthritis, cancer and brain dysfunction (Singh et al. 2015). The value of antioxidant capacity in fresh samples was 49.48%. Antioxidant capacity of samples showed an increasing trend during initial days of storage however with the progress in senescence with increasing storage period it showed a decreasing trend (Fig. 3). The preservation of antioxidants in samples without oxygen absorber was very low as compared to the samples stored with oxygen absorber. The antioxidant capacity on 12th day at 10 °C was 25.63% for samples stored without absorber whereas for samples stored with absorber the value was 51.32% (Table 3). This retention of antioxidant capacity in packages with oxygen absorber is because of the fact that oxygen absorbers help in the removal of headspace oxygen concentration and also the oxygen absorber have an important role in the removal of dissolved oxygen. It helped in preserving the quality of bell pepper fruits by preventing the oxidative degradation of various vitamins and minerals (Cichello 2014). The statistical analysis indicates that effect of all the parameters i.e. Storage temperature, oxygen absorber and storage period on antioxidant capacity was significant at 1% level of significance (Table 2). Antioxidant capacity decreased rapidly at 15 °C as compared to 5 and 10 °C. This may be because of the fact that antioxidants are sensitive towards heat and are deteriorated at higher temperatures. The results were in good correspondence with the results reported by Singh et al. (2014) for chilli peppers at different ripening stages. Similar results were also found in bell pepper by Chitravathi et al. (2015).
Total phenols
Fresh fruits and vegetables are good sources of vitamins and minerals. These are also rich sources of several bioactive compounds (mainly polyphenols) which have many health promoting effects (Singh et al. 2016a, c). The phenolic compounds act as primary antioxidants or free radical terminators and are considered one of the main phytochemicals related to human health which are present in fruits and vegetables (Singh et al. 2015, 2016a). Total phenols of fresh bell pepper was 1.5 mg/100 g. The values of total phenols of the samples showed an increasing trend during storage for samples stored without oxygen absorber whereas for samples stored with oxygen absorber the total phenols first increased and then decreased (Fig. 3). Statistical analysis reveals that the effect of oxygen absorber and storage temperature on total phenols of samples was significant at 5% level of significance (Table 2).Total phenols in samples packaged without oxygen absorber was higher as compared to the samples stored with oxygen absorber of varying amount (Table 3). The several effects of oxygen on foods and beverages includes rancidity of unsaturated fats (i.e. ‘off-flavours’ and toxic end-products), darkening of fresh meat pigments by promoting the growth of aerobic bacteria and fungi, stale odour of soft bakery foods and phenolic browning of fruit/vegetables. Thus the oxygen absorber helped in lowering the amount of total phenols and better preservation of the quality of the crop. Zhuang et al. (2012) studied the phenolic content of different varieties of bell pepper. He revealed that phenolic content of bell pepper accumulated during the development stage and storage, so the fully coloured bell peppers had significantly higher phenolic contents than green bell pepper fruits.
Conclusion
On the basis of the conducted experiments it can be concluded that active modified atmosphere packaging of yellow bell pepper fruits helped in extending the shelf life up to 28 days whereas shelf life of bell pepper stored under passive modified atmosphere packaging was only 12 days. Retention of quality parameters i.e. firmness, antioxidant capacity was maximum in the samples stored under active packaging. Thus, packaging of yellow bell pepper fruits in 150 gauge LDPE films along with oxygen absorbers (2 g) can substantially extend the shelf life when stored under low temperature i.e. 10 °C. The amount of oxygen absorber should be optimized as higher amount of oxygen absorber can lead to the development of anaerobic conditions. Oxygen control is intended for retention of bioactive compounds. Active packaging is being examined as a possible source of remedy for deterioration of food. More studies need to be conducted on fresh produce using different types of active packaging so that postharvest losses are reduced and shelf life is enhanced with better quality retention.
References
Almenar E, Catala R, Hernandez MP, Gavara R (2009) Optimization of an active package for wild strawberries based on the release of 2-nonanone. LWT-Food Sci Technol 42:587–593
Antoniali S, Martins Leal PA, de Magalhães AM, Fuziki RT, Sanches J (2007) Physico chemical characterization of ‘Zarco HS’ yellow bell pepper for different ripeness stages. Sci Agric (Piracicaba, Brazil) 64(1):19–22
Castro SM, Saraiva JA, Lopesdasilva JA, Delgadillo AVL, Smout C, Hendrick M (2007) Effect of thermal blanching and of high pressure treatments on green and red bell pepper fruits. J Food Chem 107:1436–1449
Charles F, Sanchez J, Gontard N (2003) Active modified atmosphere packaging of fresh fruits and vegetables: modeling with tomatoes and oxygen absorber. J Food Sci 68(5):1736–1742
Chitravathi K, Chauhan OP, Raju PS (2015) Influence of modified atmosphere packaging on shelf-life of green chillies (Capsicum annuum L.). Food Pack Shelf Life. https://doi.org/10.1016/j.fpsl.2015.02.001
Cichello SA (2014) Oxygen absorbers in food preservation: a review. J Food Sci Technol. https://doi.org/10.1007/s13197-014-1265-2
De Ancos B, Sgroppo S, Plaza L, Cano MP (2002) Possible nutritional and health-related value promotion in orange juice preserved by high-pressure treatment. J Sci Food Agric 82:790–796
Frank CA, Nelson RG, Simonne EH, Behe BK, Simonne AH (2001) Consumer preferences for color, price, and vitamin C content of bell peppers. HortScience 36:795–800
Galić K, Sčetar M, Kurek M (2011) The benefits of processing and packaging Trends in Food. Sci Technol 22:127–137
Gnanasekharan V, Shewfelt RL, Chinnan MS (1992) Detection of colour changes in green vegetables. J Food Sci 57:149–154
Gontard N (ed) (2000) Les Emballages Actifs. Tec & Doc Editions, Lavoisier, Paris
Howard LR, Talcott ST, Brenes CH, Villalon B (2000) Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum Species) as influenced by maturity. J Agric Food Chem 48:1713–1720
Kaur P, Rai DR, Paul S (2011) Nonlinear estimation of respiratory dynamics of fresh-cut spinach (Spinacia Oleracea) based on enzyme kinetics. J Food Process Eng. https://doi.org/10.1111/j.17454530.2009.00508.x
Kerry JP (2014) New packaging technologies, materials and formats for fast-moving consumer products. In: Han JH (ed) Innovations in food packaging. Academic Press, San Diego, pp 549–584
Kirandeep D, Kaur P, Singh B, Kumar N (2018) Effect of temperature and headspace O2 and CO2 concentration on the respiratory behaviour of fresh yellow bell-pepper (Oribelli). Int J Chem Stud 6(2):3214–3420
Kumar N, Kaur P, Bhatia S (2017) Advances in bio-nanocomposite materials for food packaging: a review. Nutr Food Sci 47(4):591–606
Lopez RA, Almenar E, Hernandez MP, Lagaron JM, Catala R, Gavara R (2004) Overview of active polymer-based packaging technologies for food applications. Food Rev Int 20(4):357–387
Lucier G, Lin BH (2001) Sweet peppers: saved by the bell. Agricultural outlook. Economic Research Service, USDA12–15
McDonald S, Prenzler PD, Antolovich M, Robards K (2001) Phenolic content and anti-oxidant activity of olive extracts. Food Chem 73:73–84
Mendoza CC, Sanchez E, Marquez EM, Arreola JPS, Cordova MAF (2015) Bioactive compounds and antioxidant activity in different grafted varieties of bell pepper. Antioxidants 4:427–446
Realini CE, Marcos B (2014) Active and intelligent packaging systems for a modern society. Meat Sci 98:404–419
Robertson GL (2006) Active and intelligent packaging. In: Food packaging: principles and practice, 2nd edn, Chap 14. CRC Press, Boca Raton, p 545
Rooney ML (1995) Active packaging in polymer films. In: Active food packaging. Blackie Academic Professional, London
Singh R, Giri SK, Kotawaliwale N (2013) Shelf-life enhancement of green bell pepper (Capsicum annuum L.) under modified atmosphere storage. Food Pack Shelf Life 1:101–112
Singh M, Kumar A, Kaur P (2014) Respiratory dynamics of fresh baby corn (Zea mays L.) under modified atmospheres based on enzyme kinetics. J Food Sci Technol 51(9):1911–1919
Singh JP, Kaur A, Shevkani K, Singh N (2015) Influence of jambolan (Syzygium cumini) and xanthan gum incorporation on the physicochemical, antioxidant and sensory properties of gluten-free eggless rice muffins. Int J Food Sci Technol 50(1190):1197
Singh B, Singh JP, Kaur A, Singh N (2016a) Bioactive compounds in banana and their associated health benefits: a review. Food Chem 206:1–11
Singh JP, Kaur A, Singh N, Nim L, Shevkani K, Kaur H, Arora DS (2016b) In vitro anti- oxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT-Food Sci Technol 65:1025–1030
Singh JP, Kaur A, Shevkani K, Singh N (2016c) Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J Food Sci Technol 53:4056–4066
Smith AC, Waldron KW, Maness N, Perkins-Veazie P (2003) Vegetable texture: measurement and structural implications. In: Bartz JA, Brecht JK (eds) Postharvest physiology and pathology of vegetables, 2nd edn. Marcel Dekker, Inc., New York, pp 297–331
Sreedhara DS, Kerutagi MG, Basavaraja H, Kunnal LB, Dodamani MT (2013) Economics of capsicum production under protected conditions in Northern Karnataka. Karnataka J Agric Sci 26(2):217–219
Weichmann J (1986) The effect of controlled atmosphere storage on the sensory and nutritional quality of fruits and vegetables. Hort Rev 8:102–103
Wills RBH, McGlasson WB, Graham D, Joyce DC (2007) Postharvest: an introduction to physiology and handling of fruit, vegetables and ornamentals, 5th edn. University of New South Wales Press, Sydney, p 227
Yam KL, Takhistov PT, Miltz J (2005) Intelligent packaging: concepts and applications. J Food Sci 70:1–10
Zhou YF, Abe K, Iwata T (1992) Effect of shredding modes on the deterioration of the quality of partially processed pepper fruits. Nipp Shok Kogyo Gakkaishi 39:161–166
Zhuang Y, Chen L, Sun L, Cao J (2012) Bioactive characteristics and antioxidant activities of nine peppers. J Funct Foods 4:331–338
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Devgan, K., Kaur, P., Kumar, N. et al. Active modified atmosphere packaging of yellow bell pepper for retention of physico-chemical quality attributes. J Food Sci Technol 56, 878–888 (2019). https://doi.org/10.1007/s13197-018-3548-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3548-5