Abstract
Composite films with Aloe vera (A), chitosan (Ch) and essential oils (EOs) were formulated. Six of the twelve combinations tested formed films: A70Ch30, A70Ch30-15, A60Ch40, A60Ch40-15, A50Ch50, and A50Ch50-15. The A60Ch40-15 film showed the best physicochemical characteristics as well as the greatest in vitro antifungal activity. Although the A90Ch10 and A80Ch20-15 mixtures did not form films, their solutions showed high antifungal activity in vitro. Based on multivariate analysis of the data, A60Ch40-15, A90Ch10 and A80Ch20-15 films were selected as coating treatments for papaya during storage at 30 ± 2 °C and 80% RH. Uncoated fruits (control 1) and treated with synthetic fungicide (control 2) were used as control. Coated fruits showed lower respiration rate, greater firmness and fewer changes in external coloration compared to control. Furthermore, these coatings reduced the incidence and severity of fungal disease by 40–50% compared to control 2. Aloe vera-chitosan films (A90Ch10 and A60Ch40-15), enriched with the EOs of cinnamon (10 mL L−1) and thyme (10 mL L−1), improved quality of the fruit (higher firmness, lower CO2 content, less internal color change) with 50% less disease incidence during storage at room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Papaya (Carica papaya L.) is a plant native to the tropical areas of Mexico and Central America. The fruit has high nutritional and medicinal value as well as attractive sensory properties (Lakshmi et al. 2011). Mexico produced more than 836,000 tons of papaya in 2014 (FAO 2016), making it the 5th largest producer of papaya globally. The main production region is located in the states of Chiapas, Oaxaca and Colima; however, the quality and safety of the fruits are threatened by several postharvest diseases that are mainly caused by fungi (Bosquez-Molina et al. 2010).
Currently, there is increasing consumer demand for natural products (Benitez et al. 2015); nonetheless, the first choice to minimize the presence and damage caused by pathogens is the use of synthetic fungicides, which have adverse effects on the human health and the environment and promote the emergence of resistance (Ramos-García et al. 2012). Thus, one strategy used to replace synthetic fungicides is the use of edible films, which could be elaborated from a single biopolymer or the combination of more than one (lipids, polysaccharides, proteins or others) (Benítez et al. 2015). These films could reduce water loss and respiration rate and show antioxidant effects, and the films may decrease the presence and damage caused pathogens in fruits and vegetables (Vásconez et al. 2009).
Recently, Aloe vera gel has been used for film formulation (Cheng-Pei et al. 2010; Benítez et al. 2015). This gel is mainly composed of polysaccharides, glycoproteins, vitamins, enzymes and phenolic compounds (Alvarado-González et al. 2012). When used as a coating, it allows the maintenance of physicochemical characteristics and the extension of shelf life for different fruits, such as plum (Martínez-Romero et al. 2017), grape (Chauhan et al. 2014) and kiwi (Benítez et al. 2015). This coating gel is also effective against pathogenic bacteria harmful to fruits and vegetables (Cheng-Pei et al. 2010; Benitez et al. 2015), but in papaya, it shows little antifungal activity (Lakshmi et al. 2011).
On the other hand, some reports have shown that combining two polymers can increase the antifungal effect of the coating. Chitosan–gelatin (Poverenov et al. 2014) and chitosan-A. vera (Manoj et al. 2016, Vieira et al. 2016) combinations are used for bell pepper and blueberry. In addition, other reports have shown the antifungal effects of films can be increased by the addition of essential oils (EOs) (Bakkali et al. 2008). The EOs of clove, cinnamon and star anise have been incorporated into chitosan films for the control of the fungi Aspergillus oryzae (Wang et al. 2011).
In papaya fruit, mesquite gum has been combined with the EOs of thyme and Mexican lime (Bosquez-Molina et al. 2010) and chitosan with the EOs of cinnamon and thyme (Salvador-Figueroa et al. 2017) to control Colletotrichum gloeosporioides and Rhizopus stolonifer. Although the results obtained in papaya are encouraging, the reduction in the presence of pathogens does not reach the same levels as those achieved when using synthetic fungicides. Therefore, the incorporation of EOs into edible coatings composed of two types of polymers can be a useful strategy for controlling postharvest diseases.
However, combining two or more polymers can change the physicochemical characteristics of the films. The chitosan-tapioca starch (Chillo et al. 2008) and Aloe vera-gelatin (Cheng-Pei et al. 2010) combinations improved the physicochemical and mechanical characteristics compared to the pure films. The film solubilities of Aloe vera-gellan gum (Alvarado-González et al. 2012), Aloe vera-chitosan gel (Khoshgozaran-Abras et al. 2012) and chitosan-starch (Vásconez et al. 2009) were lower then those of their corresponding pure films.
Based on the above results, the objective of this work was to determine the physicochemical and antifungal characteristics of Aloe vera-chitosan films doped with EOs and their effects on the shelf life of papaya Maradol stored at room temperature under humid tropical conditions.
Materials and methods
Reagents
Chitosan (85% deacetylated, MW = 340.33), glacial acetic acid, Tween 20 (Sigma-Aldrich®) and glycerol (Meyer®) were used. The EOs of cinnamon (Cinnamomum zeylanicum) and thyme (Thymus vulgaris) were from Meyer® reagents. The potato dextrose agar (PDA) used was from Sigma-Aldrich®. All chemicals used were of reagent grade.
Obtaining the Aloe vera gel
Mature leaves of Aloe vera plants (SST = 1.33%) with no visual damage were harvested in Tapachula Chiapas, Mexico (14°49′8”N, 92°20′7″W) and transported on ice to the laboratory. Then, the samples were washed with water, disinfected by immersion in sodium hypochlorite (1% v v−1) for 15 min and dried by exposure to flowing air. Aloe vera gel was then obtained according to the method of Vieira et al. (2016). For this, the Aloe vera gel matrix was separated from the outer cortex leaf, and the colorless hydroparenchyma was ground (Osterizer® blender) at a low speed for 5 min. The resulting mixture was filtered through Tyler 20 mesh (841-μm screen) to remove the fibrous fraction. The liquid obtained constituted fresh Aloe vera gel, which was stored at 4 °C in amber containers to prevent oxidation.
Preparation of the film
To make the films, several proportions of Aloe vera gel were combined with chitosan solutions to obtain two groups of treatments, and each group included five specific treatments (ten in total). For the first group of treatments, a stock solution of chitosan (15 g L−1) dissolved by stirring for 24 h at 30 °C in aqueous acetic acid solution (10 mL L−1) was used. The treatments in this group were based on combinations of Aloe vera gel:chitosan solution in proportions 90:10 (A90Ch10), 80:20 (A80Ch20), 70:30 (A70Ch30), 60:40 (A60Ch40) and 50:50 (A50Ch50). In the second group of treatments, the same proportions of Aloe vera:chitosan solution were used, but in this case, the chitosan solutions were of different concentrations to afford the same final concentrations of chitosan (15 g L−1) in each treatment. The treatments in this group were labeled as follows: A90Ch10-15, A80Ch20-15, A70Ch30-15, A60Ch40-15, and A50Ch50-15. Additionally, two control treatments were used; one was formulated with Aloe vera gel alone and the other with 15 g L−1 solution of chitosan alone. For all treatments, the pH was adjusted to 4. Subsequently, all mixtures were doped to give final concentrations of 10 mL L−1 cinnamon EO, 10 mL L−1 thyme EO, 5 mL L−1 Tween 20 and 10 mL L−1 glycerol. Immediately, the mixtures were subjected to ultrasound (Ultrasonic Processor® VCX 750, amplitude 60%) for 5 min (50 s sonication and 10 s inactivity) to form microemulsions. After homogenization, 7 mL of each solution was poured into sterile Petri dishes (60 mm diameter) and allowed to stand (30 °C, 48 h) until formation of the film. The films were stored at 4 °C in hermetic polyethylene bottles until analysis.
Physicochemical properties of the films
Considering each film as a replicate, ten films of each treatment were subjected to the following analyses: the tensile strength with a penetrometer (Tr® Italy, equipped with an 8 mm diameter tip) and estimated according to the equation TS (MPa) = F (N)/area (mm2); solubility according to the method proposed by Wang et al. (2011); water sorption (WS) and moisture content following the method described by Binsi et al. (2013); the water vapor transmission rate (WVTR) using the method suggested by Chillo et al. (2008), and from this, the water vapor permeability (WVP) was calculated using the formula WVP (g mm m−2 s−1 kPa−1) = (WVTR L)/ΔP where L is the film thickness (mm), and ΔP is the differential of the partial pressures between the two sides of the film (3.179 kPa); the thickness (μm) was measured with a digital micrometer (Fowler®); and color was measured with a MiniScan EZ colorimeter, and the L*, a*, b* values of the CIE Lab scale are reported.
Spore production of phytopathogenic fungi
The fungi Colletotrichum gloeosporioides and Rhizopus stolonifer were isolated from infected papaya fruit. Infected portions of papaya were placed in the center of Petri dishes containing PDA agar and incubated at 30 ± 2 °C until mycelial growth. Then, portions of mycelium were regrown in Petri dishes with fresh PDA. Once the mycelium and reproductive parts were developed, visual identification of the fungi with the aid of an optical microscope (Carl Zeiss® Model Axiolab) was carried out using lactophenol blue preparations and using the dichotomous keys described by Barnett and Hunter (1972).
Monosporic cultures were prepared in Petri dishes with PDA, and discs with mycelium (10 mm diameter of PDA) were taken and placed in Roux bottles containing PDA. These samples were incubated at 30 ± 2 °C for 14 days to obtain spores in Ringer solution. Aliquots (0.5 mL) of each solution were placed in a Neubauer chamber, and the spores were counted with a microscope (Carl Zeiss®) using 40X magnification. For both fungi, the solutions were adjusted by dilution to a concentration of 106 spores mL−1.
Antifungal activity in vitro
Because the A90Ch10, A90Ch10-15, A80Ch20, A80Ch20-15 and control Aloe vera (A) treatments did not form films, in vitro evaluation of the antifungal activities was performed by two procedures. a) For solutions (all treatments), in Petri dishes (100 mm diameter) containing PDA, 100 μL of spore suspension (106 spores mL−1) was dispersed, and then 12 holes (8 mm diameter each) were drilled into the agar, and the solution of the treatment (100 μL) was added to the holes. b) For the treatments that formed films, disks of the films (8 mm diameter) were placed on the surface of Petri dishes with PDA that had been inoculated with 100 μL of spore suspension (106 spores mL−1). Four different treatments were randomly combined and placed on each plate. Plates from both methods were incubated at 30 °C. Inhibition of fungal growth was determined from the diameter of the halos present around the well or film (Du et al. 2009). For Rhizopus stolonifer, solution treatments were evaluated at 48 h and films at 24 h, whereas with Colletotrichum gloeosporioides, determinations for solutions were made at 96 h and for films at 240 h. A visual inspection of the plates was carried out, and they were photographed. The dimensions of the halos were then determined using digital Vernier Stainless® calipers. For each treatment (film or solution), three replicates were conducted.
Shelf life of coated fruit
To evaluate the shelf life of coated papaya fruit, the best treatments were chosen based on the results of the growth inhibition (halos) of fungi and the physicochemical characteristics of the films. Thus, four treatments were selected, (1) A100, (2) A90Ch10, (3) A80Ch20-15 and (4) A60Ch40-15, and they were applied to Maradol papaya fruit.
Application of Aloe vera-chitosan coatings to papaya fruit
Fruits of C. papaya var. Maradol (N = 270) were acquired from Agro Pacífico S.A. of C.V. company (14°54′37.8″N 92°20′13.3″W) in the city of Tapachula, Chiapas, Mexico. The fruits were harvested at the mature green stage, and they were of similar size and free of damage and decay. Under a completely randomized design, the fruits were divided into six groups of 45 fruits each. Four groups were assigned for each of the previously selected treatments, one absolute control (uncoated) and one group that was treated with the fungicide Mancozeb®. All fruit were washed with water, immersed in 200 ppm sodium hypochlorite solution for 3 min and allowed to dry at room temperature. The film solutions were applied manually with a polyurethane foam brush. Subsequently, they were dried at room temperature under flowing air. The solution of synthetic fungicide (6 g L−1 of active ingredient) was also applied with a polyurethane brush. All fruits were randomly placed in a closed storage room at ambient temperature (30 ± 2 °C) and RH of 80% for 12 days.
Postharvest quality characteristics of fruit
From the day the coatings were applied (day 0), every 48 h, five fruits per treatment were evaluated based on weight loss (Adventurer™ Pro, model AV264C), firmness (Tr® Italy penetrometer) and external color (MiniScanEZ colorimeter). Three measurements per fruit were performed in the apical, middle and peduncle regions. Titratable acidity (TA) (AOAC 2010) and total soluble solids (TSS) (ATAGO digital refractometer, model PAL−1) were also quantified. Likewise, every 24 h, the CO2 production was measured in triplicate by placing a fruit in a sealed vessel (7 L) for 2 h, after which time the amount of CO2 produced using an IAQ-CALC probe (TSI®) was recorded and is reported as mg CO2 kg−1 s−1 (Salvador-Figueroa et al. 2017).
Presence of fungi
The incidence of disease (%) and severity index were estimated using procedures suggested by Bosquez-Molina et al. (2010). For the severity index, we used a scale from 0 to 4, where 0 = 0% damage to the fruit surface, 1 = 1–5% (initial damage), 2 = 6–15% (slightly damaged), 3 = 16–30% (moderately damaged), and 4 = greater than 31% (severely damaged) to the fruit.
Statistical analysis
The data of all the variables evaluated in films and fruits were subjected to analysis of variance and Duncan’s multiple range test (α = 0.05). To identify the treatments (films) with the most appropriate characteristics for use as coatings and the treatments (solutions and films) with more significant antifungal activity, all variables were analyzed using multivariate statistics. Using the hierarchical ascendant classification (HAC) method, two dendrograms were constructed based on Euclidean distance dissimilarity using the Ward agglomeration method. One dendrogram was constructed with the films physicochemical characteristics and the other with data on the inhibition of mycelial growth of C. gloeosporioides and R. stolonifer from films and solutions. All analyses were processed using the statistical software XLSTAT © v2012.
Results and discussion
Physicochemical properties of the films
The combinations of Aloe vera-chitosan (A90Ch10, A90Ch10-15, A80Ch20, and A80Ch20-15) as well as the control solution of Aloe vera (A) did not form films. Table 1 shows the values (average ± standard deviation) of the tensile strength and color parameters (L*, a* and b*) measured for the treatments that did form films. The tensile strength values ranged from 0.50 to 35.13 MPa. The incorporation of chitosan positively affected (P < 0.05) the firmness of A. vera films. Thus, the firmer films were those of treatments that were prepared with smaller volumes of Aloe vera. This agreed with the results of Cheng-Pei et al. (2010) and Khoshgozaran-Abras et al. (2012), who mention that this behavior of the biofilms was related to the high water content of A. vera (98.6%) and to the addition of glycerol because of its hydrophilic nature that retained the water inside the polymer matrix, which reduced intermolecular interactions, making the film more flexible (Thakhiew et al. 2010). For the treatments A50Ch50 (14.33 ± 5.94 MPa) and A50Ch50-15 (8.68 ± 3.70 MPa), the tensile strengths were higher than those reported by Khoshgozaran-Abras et al. (2012). In addition to the concentration of chitosan, the organic acid (acetic acid) used to dissolve the polymer and the presence of EOs can contribute to the increase in the firmness of the films (Wang et al. 2011). Likewise, the tensile strength of the control (Ch) film was higher than that reported by others (Salvador-Figueroa et al. 2017; Khoshgozaran-Abras et al. 2012) but within the reported range for chitosan films made with EOs (10–51 MPa) (López-Mata et al. 2013).
Regarding color (L*, a* and b* values), although significant differences were found between treatments (Table 1), all films were transparent. This transparency is highly desirable in materials that are used as coatings for foods, as they do not affect the natural brightness of the fruit. There are no reports on this parameter for Aloe vera films combined with chitosan and EOs; however, the films had lower L* values that those of films based on polymers such as chitosan-starch (Chillo et al. 2008) or chitosan-coconut oil films (Binsi et al. 2013). The results were similar to those reported by Alvarado-González et al. (2012), who combined Aloe vera-gellan gum. The treatments A50Ch50 and A50Ch50-15 presented values of L* that were higher than those reported by Khoshgozaran-Abras et al. (2012), which could be due to the use of glycerol, as it is reported that glycerol tends to increase the value of L* (Chillo et al. 2008). The tonalities (evaluated based on a* and b*) showed variations that give a slightly yellowish tone to the films, probably due to the use of Tween 20 (Ziani et al. 2008) and the EOs, which are yellow–brown. Negative values of a* (which indicate a tendency towards green chroma) were recorded in the treatments A70Ch30-15 and A60Ch40-15, which also showed the highest values of b* (indicating a yellow tint). This behavior of both treatments may be a function of the concentration of polymers (Aloe vera polysaccharides and chitosan), as suggested by Chillo et al. (2008), but given the L* values obtained for these treatments (higher values), the films tend to be less colored, as reported by Ziani et al. (2008). The treatments A50Ch50 and A50Ch50-15 presented lower values of a* and b* compared with those reported by Khoshgozaran-Abras et al. (2012) for films with the same proportion of Aloe vera-chitosan. These authors reported values of a* = 2.43 and b* = 20.01. This result could be because during the extraction of the solution of A. vera, we sought to minimize the oxidation (air exposition) of phenolic compounds that result in the polymerization of anthraquinones, which are responsible for the brown coloration (Chillo et al. 2008). The color values obtained for the control film (Ch) were different from those reported by Khoshgozaran-Abras et al. (2012), which may be due to the concentration of chitosan used and the addition of glycerol, Tween 20, and the EOs.
Regarding the thickness, the control films (Ch) had an average thickness of 73.02 ± 24.09 μm (Table 1), which is similar to that of the other treatments and significantly different (P < 0.05) only from the A50Ch50-15 film. This shows that the thickness is a function of the concentration of chitosan, as reported by Cheng-Pei et al. (2010). Binsi et al. (2013) also mention that the variables with the greatest influence on the thickness of the film are the interactions, alignment, and compactness of the chitosan molecules during the formation and drying process of the resulting film, demonstrating that the inclusion of a higher concentration of chitosan in the films limits the water content and allows the formation of thicker films. However, our results show that the films of all treatments were thicker than those reported by other authors (Khoshgozaran-Abras et al. 2012, Binsi et al. 2013). Concerning the water sorption, solubility and moisture content of the films, the incorporation of chitosan had a significant effect. The films containing the highest concentration of Aloe vera (lower concentration of chitosan) exhibited the highest values for water sorption, for solubility and for moisture content; in contrast, the films where the concentration of chitosan was maintained at 15 g L−1 presented the lowest values, similar to those reported by Binsi et al. (2013). It has been reported that a higher concentration of chitosan reduces water–polymer interactions (Khoshgozaran-Abras et al. 2012) either because chitosan presents self-aggregation or because the interaction sites are occupied by residual molecules of Aloe vera gel, which are also hygroscopic. Alvarado-González et al. (2012) reported lower water absorption (13%) in films made of Aloe vera-gellan gum and attributed this to the interaction between the mannan present in the Aloe vera gel with the gellan gum, which creates intrinsic networks that restrict water sorption. This could explain the low values for these physicochemical parameters.
The moisture content is directly associated with solubility. Low solubility (and moisture) values are desirable for films with higher strength and lower rates of mass transfer (Wang et al. 2011) for use as coatings on fruit, such as what was sought in the present work. The water vapor permeability (WVP) values of the films were in the range of 2.1–3.1 × 10−10 g m−1 s−1 Pa−1. The WVP of the A70Ch30 film was significantly different (P < 0.05) from that of the others and was the highest (3.1 × 10−10 g m−1 s−1 Pa−1), which could be because this treatment had a higher content of A. vera and consequently more hydrophilic components that retain water molecules through hydrogen bonds (Khoshgozaran-Abras et al. 2012). However, the other treatments demonstrated that the films presented lower permeability to water vapor as the volume of chitosan increased, which agrees with the results of Galus and Lenart (2013), who reported that when combining two polymers (alginate and pectin), the water vapor transmission was reduced. Our values are lower than those reported by others (Khoshgozaran-Abras et al. 2012; Wang et al. 2011; Vásconez et al. 2009; Chillo et al. 2008).
Effect of treatments on in vitro fungal growth
The area of in vitro growth inhibition of C. gloeosporioides and R. stolonifer caused by the solutions and films of Aloe vera-chitosan mixed with EOs are shown in Table 2. The highest inhibition areas were found for C. gloeosporioides (1803.44 ± 914.75 mm2) with the A90Ch10 treatment and for R. stolonifer with the A80Ch20-15 treatment solution (329.77 ± 30.63 mm2). The results suggest that the solutions were dispersed in the agar, increasing the contact area between the fungus and the active components; thereby reducing its growth (Aloui and Khwaldia 2016). Although the inhibitory effect of Aloe vera was strongest in combination with 15 g L−1 of chitosan and EOs, as mentioned by Ramos-García et al. (2012) and Bakkali et al. (2008), as the amount of Aloe vera decreased, the antifungal effect was reduced, probably due to the reduction in the concentration of bioactive molecules such as aloe-emodin and aleonin, which are responsible for the fungicidal activity of A. vera (Chauhan et al. 2014). For the films, the antifungal effect was improved by the addition of chitosan, which is similar to what was seen with the solutions; however, for the films, the treatments with higher proportions of chitosan visually exhibited greater antifungal capacities. Four treatments (A60Ch40-15, A50Ch50, A50Ch50-15, and synthetic fungicide) showed the highest areas of inhibition of the growth of the fungus R. stolonifer. Conversely, for C. gloeosporioides inhibition, all treatments except for the A70Ch30 treatment were efficient. Most likely, the chitosan (the major component) contains a greater number of amino groups, which have an affinity for the negative charges of the fungi, increasing the growth inhibition.
Selection of coatings to apply to papaya fruit
The individual analyses (ANOVA tests) of the parameters evaluated in the films made it difficult to develop a general interpretation to select the treatments with more promising results for use as coatings in the papaya fruit. Therefore, multivariate analyses were performed. The dendrogram constructed from the physicochemical data of the films (Suppl. Figure 1-a) grouped the seven treatments into four clusters (C1–C4) with a dissimilarity of ~ 3000. In turn, C1 grouped three treatments, C3 grouped two treatments, while C2 and C4 contained only one treatment each. Of the clusters that grouped a treatment, we selected the treatment A60Ch40-15 since the films of this treatment presented the best characteristics (lower solubility, water sorption, and humidity) unlike the treatment A70Ch30, which presented properties undesirable for use as a coating in fruits. However, in the dendrogram made with the antifungal capacity data (Suppl. Figure 1-b), five clusters (C1–C5) were obtained to group all treatments. C1 grouped six treatments, C2 contained one treatment, C3 grouped two treatments, while C4 and C5 grouped two treatments each. In this way, the treatments grouped in clusters C2 and C4 were chosen since they presented the highest antifungal activities, and these treatments were A90Ch10, A80Ch20-15, and A60Ch40-15. Coincidentally, the A60Ch40-15 treatment also showed the most appropriate physicochemical characteristics.
Physiological characteristics of papaya fruits coated with Aloe vera-chitosan films mixed with EOs
The application of the different coatings based on Aloe vera-chitosan mixed with EOs on the fruit of C. papaya Maradol did not negatively impact the physiological parameters of the normal process of maturation. Uncoated fruit exhibited the highest weight loss values out of all treatments (Fig. 1a), which was significant (P < 0.05) on days two and four. In contrast, the fruit coated with the solution of A80Ch20-15 showed the lowest weight loss during storage at room temperature. The above results demonstrate that the combination of A. vera with chitosan in these proportions reduces the rate of water vapor transmission (which could not be assessed in the present study since this combination did not form a film); and this type of effect occurs when two polymers with different properties, such as alginate and pectin, are mixed (Galus and Lenart, 2013). The main factor responsible for the loss of firmness is the enzymatic degradation of the cell wall (Poverenov et al. 2014), meaning that the coatings of A. vera-chitosan probably reduced the action of these enzymes as a consequence of the decrease of the respiratory rate associated with the production of endogenous ethylene. Loss of water (loss of weight) also contributes to the decrease in firmness, which explains why the coated fruits remained firmer than the uncoated fruits; the coating provided a barrier to water diffusion, decreasing transpiration (Yulianingsih et al. 2013). The fruits did not present significant differences (P > 0.05) among treatments for the pH and SST (Fig. 1c, e), and the visual differences are probably due to the variability of fruit and the natural maturation process, which were similar in both coated and uncoated fruit. There were also no significant differences (P > 0.05) in titratable acidity during the first 6 days of storage, but from day eight, fruits coated with treatments A80Ch20-15 and A60Ch40-15 presented higher acidity contents (Fig. 1d) than the remaining four treatments. The degradation of organic acids during the maturation process (days of storage) is directly related to the production of sugars and their subsequent use in the respiratory process (Ochiki et al. 2015); thus, that the coatings that maintained the acidity function adequately as semipermeable barriers and reduce the respiratory rate.
Regarding respiration (amount of CO2 produced), there were no significant differences (P > 0.05) among treatments evaluated during the first 2 days of storage (Fig. 1f). The use of the coatings also had an effect on the appearance of the respiratory peak of the papaya fruit. The Aloe vera gel coating (A100) resulted in an unusually small increase in CO2 concentration produced on day 4 of storage (3.6 × 10−4 mg CO2 kg−1 s−1). Mancozeb®-treated fruit exhibited a respiratory peak on day 5 of storage, and the uncoated fruit (controls) reached their peak on day seven. Other treatments showed the same phenomenon on days nine (A60Ch40-15) and ten (A80Ch20-15 and A90Ch10) of storage. Thus, with the exception of the A100 treatment, all other coatings delayed the appearance of the climacteric respiration peak, demonstrating, as previously stated, that the coatings effectively decreased the gas exchange (Manoj et al. 2016). However, it is necessary to clarify that for treatment A80Ch20-15, on day ten, the respiration was measured with fruit contaminated with fungi because this was the treatment that presented the highest incidence of fungus; the same occurred on day nine with the fruit treated with synthetic fungicide and uncoated fruit. The presence of fungus could explain the appearance of an unusual second respiratory peak since the presence of fungi induces wounds in the tissue of the fruit and thus unusual production of CO2 (Ramos-García et al. 2012).
The color of the peel exhibited treatment-dependent behavior. Thus, during the first 4 days of storage at room temperature, all treatments showed similar and statistically equal (P > 0.05) values for parameter L*. From the second day (values of a* and b*) and day 6 (L*), the treatments that did not have a coating presented gradual changes in these parameters with significant differences from the other treatments (P < 0.05) towards yellow-orange hues (Fig. 2a–c), which is the characteristic color of the mature fruit. During the 12 days of storage, the peel color parameters remained almost unchanged in the fruits of the coated treatments, but the fruits treated with A60Ch40-15 and A90Ch10 showed a slight change in their coloration values towards those of the ripe fruit. Although visually the same phenomenon was observed with the internal color, the color parameters L*, a*, and b* of the pulp showed very similar values for all treatments, and no significant variations during storage were observed (Fig. 2d–f). The positive effect of the coatings on the parameters of weight, firmness, respiration, acidity, and color are consistent with several works in which similar coatings are used to coat mango Ataulfo (Salvador-Figueroa et al. 2011), grapes (Chauhan et al. 2014) and papaya Maradol (Salvador-Figueroa et al. 2017).
Presence of fungi on fruit
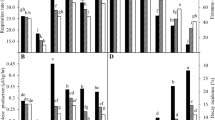
During the first 2 days of storage, only fruit coated with the A80Ch20-15 treatment presented visible evidence of fungal growth (Fig. 3). From day 4, the fruit of all treatments had some incidence of fungus. Contrary to our expectations, at this phase of the study, the A80Ch20-15 and Mancozeb® treatments (the treatments with the highest inhibitions in the in vitro tests) showed no inhibitory effects on the development of fungi, even the fungal incidence of these fruits was greater during the first 9 days than was seen in the fruit that did not receive any treatment (absolute control). Although the A80Ch20-15 treatment in solution exhibited activity against the R. stolonifer fungus in the in vitro test (Table 2), when the fruits were coated with this solution, the fungicidal effects were weaker than those of the A60Ch40-15 treatment, which shows that there is no direct relationship between the concentration of A. vera and chitosan, but there is a synergistic effect of the combination gel-chitosan-EOs. The coated fruit showed lower incidences of disease compared to what was reported by Bosquez-Molina et al. (2010), who used Mexican thyme and lime EOs in papaya fruit, as well as those reported by Salvador-Figueroa et al. (2017), who reported a 75% incidence of disease when using chitosan coatings with the EO of cinnamon 1%-lime 1% in the fruit of papaya Maradol. Additionally, only the fruit coated with the treatments A90Ch10 and A60Ch40-15 exhibited lower incidence compared to those reported by Lakshmi et al. (2011), which demonstrates the effectiveness of these coatings in the control of postharvest fungi.
Incidence (a) and severity index (b) of fungi on papaya fruit coated with Aloe vera-chitosan films mixed with essential oils during 12 days of storage at 30 ± 2 °C and 80% RH. For severity index: 0 = no damage, 1 = initially damaged, 2 = slightly damaged, 3 = moderately damaged and 4 = severely damaged
From day two, the fruit coated with the treatment A80Ch20-15 presented a severity of grade 1 (initial damage); from days 4 to 6, the fruits of all treatments showed severities between grades 1 and 2 (initial damage and slightly damaged, respectively). However, as the storage duration increased, the severity indices increased to grades 3 and 4 (Fig. 3a). Thus, the fruit coated with the A90Ch10 treatment showed the lowest severity of infection (16–30%, moderately damaged) at day 12 among the treatments; all others were severely damaged. The fruit of the A60Ch40-15 treatment also had a lower incidence of disease, but these fruits were severely damaged at day 12. The inhibitory effect of Aloe vera gel was improved by mixing it with chitosan and EOs, as mentioned by Ramos-García et al. (2012) and Bakkali et al. (2008).
Conclusion
The appropriate physicochemical characteristics for films that are to be applied in fruit and possibly in other vegetables were obtained with the A60Ch40-15 treatment, and this treatment presented promising antifungal activity in vitro. Similarly, the solutions A90Ch10 and A80Ch20-15 (which did not form films) were shown to have higher antifungal activities. The use of such coatings in papaya Maradol did not affect the quality parameters of the fruit during ripening. In contrast, their use reduced the rate of CO2 production and retained papaya firmness for at least 10 days under ambient tropical conditions (average of 30 °C). In addition, the coatings A90Ch10 and A60Ch40-15 were able to reduce the incidence of fungi on the surface of fruit with values lower than 50% during 12 days of storage and reduced the severity of the infection during 11 days of storage.
References
Aloui H, Khwaldia K (2016) Natural antimicrobial edible coatings for microbial safety and food quality enhancement. Compr Rev Food Sci Food Saf 15:1080–1103. https://doi.org/10.1111/1541-4337.12226
Alvarado-González JS, Chanona-Pérez JJ, Welti-Chanes JS, Calderón-Domínguez G, Arzate-Vázquez I, Pacheco-Alcalá SU, Garibay-Febles V, Gutiérrez-López G (2012) Optical, microstructural, functional and nanomechanical properties of Aloe vera gel/gellan gum edible films. Rev Mex Ing Quím 11(2):193–210
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475. https://doi.org/10.1016/j.fct.2007.09.106
Barnett HL, Hunter BB (1972) Illustrated genera of imperfect fungi. Burgess Publishing Co, Minneapolis, p 218
Benítez S, Achaerandio I, Pujolá M, Sepulcre F (2015) Aloe vera as an alternative to traditional edible coatings used in freshcut fruits: a case of study with kiwifruit slices. LWT Food Sci Technol 61:184–193. https://doi.org/10.1016/j.lwt.2014.11.036
Binsi PK, Ravishankar CN, Srinivasa TK (2013) Development and characterization of an edible composite film based on chitosan and virgin coconut oil with improved moisture sorption properties. J Food Sci 78(4):1–9. https://doi.org/10.1111/1750-3841.12084
Bosquez-Molina E, Ronquillo E, Bautista-Baños S, Verde-Calvo JR, Morales-López J (2010) Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biol Technol 57:132–137. https://doi.org/10.1016/j.postharvbio.2010.03.008
Chauhan S, Gupta KC, Agrawal M (2014) Application of biodegradable Aloe vera gel to control post-harvest decay and longer the shelf life of grapes. Int J Curr Microbiol Appl Sci 3(3):632–642
Cheng-Pei C, Be-Jen W, Yih-Ming W (2010) Physiochemical and antimicrobial properties of edible aloe/gelatin composite films. Int J Food Sci Technol 45:1050–1055. https://doi.org/10.1111/j.1365-2621.2010.02235.x
Chillo S, Flores S, Mastromatteo M, Conte A, Gerschenson L, Del Nobile M (2008) Influence of glycerol and chitosan on tapioca starch-based edible film properties. J Food Eng 88:159–168. https://doi.org/10.1016/j.jfoodeng.2008.02.002
Du WX, Olsen RJ, Avena-Bustillos TH, Mchugh CE, Levin R, Friedman MM (2009) Antibacterial effects of allspice, garlic, and oregano essential oils in tomato films determined by overlay and vapor-phase methods. J Food Sci 74:390–397. https://doi.org/10.1111/j.1750-3841.2009.01289.x
FAO, Food and Agriculture Organization of the United Nations (2016) FAOSTAT statistics database. Rome
Galus S, Lenart A (2013) Development and characterization of composite edible films based on sodium alginate and pectin. J Food Eng 115:459–465. https://doi.org/10.1016/j.jfoodeng.2012.03.006
Khoshgozaran-Abras S, Hossein M, Hamidy Z, Bagheripoor-Fallah N (2012) Mechanical physicochemical and color properties of chitosan based-films as a function of Aloe vera gel incorporation. Carbohydr Polym 87:2058–2062. https://doi.org/10.1016/j.carbpol.2011.10.020
Lakshmi SL, Abirami R, Pushkala R, Srividya N (2011) Enhancement of storage life and quality maintenance of papaya fruits using Aloe vera based antimicrobial coating. Indian J Biotechnol 10:83–89
López-Mata M, Ruiz-Cruz S, Silva-Beltrán N, Ornelas-Paz J, Zamudio-Flores P, Burruel-Ibarra S (2013) Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 18:13735–13753. https://doi.org/10.3390/molecules181113735
Manoj H, Sreenivas K, Shankarappa T, Krishna H (2016) Studies on chitosan and Aloe vera gel coatings on biochemical parameters and microbial population of bell pepper (Capsicum annuum L.) under ambient condition. Int J Curr Microbiol Appl Sci 5(1):399–405. https://doi.org/10.20546/ijcmas.2016.501.039
Martínez-Romero D, Zapata PJ, Guillén F, Paladines D, Castillo S, Valero D, Serrano M (2017) The addition of rosehip oil to Aloe gels improves their properties as postharvest coatings for maintaining quality in plum. Food Chem 217:585–592. https://doi.org/10.1016/j.foodchem.2016.09.035
Ochiki S, Morwani G, Ngwela R, Ngwela W (2015) Effect of Aloe vera gel coating on postharvest quality and shelf life of mango (Mangifera indica L.) fruits var. “Ngowe”. J Hortic For 7(1):1–7. https://doi.org/10.5897/jhf2014.0370
Official methods of analysis. Association of Official Analytical Chemists, AOAC (2010) In: Horwitz W, Latimer G (eds), 18th edn. Third revision. Maryland, USA
Poverenov E, Zaitsev Y, Arnon H, Granit R, Alkalai-Tuvia S, Perzelan Y, Weinberg T, Fallik E (2014) Effects of a composite chitosan-gelatin edible coating on postharvest quality and storability of red bell peppers. Postharvest Biol Technol 96:106–109. https://doi.org/10.1016/j.postharvbio.2014.05.015
Ramos-García M, Bosquez-Molina E, Hernández-Romano J, Zavala-Padilla G, Terrés-Rojas E, Alicia-Tejacal I, Barrera-Necha L, Hernández-López M, Bautista-Baños S (2012) Use of chitosan-based edible coatings in combination with other natural compounds, to control Rhizopus stolonifer and Escherichia coli DH5α. Crop Prot 38:1–6. https://doi.org/10.1016/j.cropro.2012.02.016
Salvador-Figueroa M, Aragón-González W, Hernández-Ortiz E, Vázquez-Ovando A, Adriano-Anaya M (2011) Effect of chitosan coating on some characteristics of mango (Mangifera indica L.)“Ataulfo” subjected to hydrothermal process. Afr J Agric Res 6(27):5800–5807. https://doi.org/10.5897/AJAR10.784
Salvador-Figueroa M, Castillo-López D, Adriano-Anaya L, Gálvez-López D, Rosas-Quijano R, Vázquez-Ovando A (2017) Chitosan composite films: physicochemical characterization and their use as coating in papaya Maradol stored at room temperature. Emir J Food Agric 29(10):779–791. https://doi.org/10.9755/ejfa.2017.v29.i10.1303
Thakhiew W, Devahastin S, Soponronnarit S (2010) Effects of drying methods and plasticizer concentration on some physical and mechanical properties of edible chitosan films. J Food Eng 99:216–224. https://doi.org/10.1016/j.jfoodeng.2010.02.025
Vásconez M, Florez S, Campo C, Alvarado J, Gerschenson L (2009) Antimicrobial activity and physical properties of chitosan-tapioca starch based edible films and coating. Food Res Int 42:762–769. https://doi.org/10.1016/j.foodres.2009.02.026
Vieira JM, Flores-López ML, Jasso de Rodríguez D, Sousa MC, Vicente A, Martins J (2016) Effect of chitosan-Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol Technol 116:88–97. https://doi.org/10.1016/j.postharvbio.2016.01.011
Wang L, Liu F, Jiang Y, Chai Z, Li P, Cheng Y, Jing H, Leng X (2011) Synergistic antimicrobial activities of natural essential oils with chitosan films. J Agric Food Chem 59:12411–12419. https://doi.org/10.1021/jf203165k
Yulianingsih R, Maharani D, Hawa L, Sholikhah L (2013) Physical quality observation of edible coating made from Aloe vera on Cantaloupe (Cucumis melo L.) minimally processed. Pak J Nutr 12:800–805. https://doi.org/10.3923/pjn.2013.800.805
Ziani K, Oses J, Coma V, Maté J (2008) Effect of the presence of glycerol and Tween 20 on the chemical and physical properties of film based on chitosan with different degree of deacetylation. LWT Food Sci Technol 41:2159–2664. https://doi.org/10.1016/j.lwt.2007.11.023
Acknowledgements
This work was partly supported by SEP-Mexico through the program PROFOCIE-2014-07MSU0001H-11, purpose “Formación del Cuerpo Académico Biotecnología Alimentaria”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
13197_2018_3397_MOESM1_ESM.pdf
Dendrogram of Euclidean distance. Constructed with data obtained from the physicochemical parameters of the films (a) and with data on the inhibition of mycelial growth of the fungi Colletotrichum gloeosporioides and Rhizopus stolonifer (b) (PDF 10 kb)
Rights and permissions
About this article
Cite this article
Monzón-Ortega, K., Salvador-Figueroa, M., Gálvez-López, D. et al. Characterization of Aloe vera-chitosan composite films and their use for reducing the disease caused by fungi in papaya Maradol. J Food Sci Technol 55, 4747–4757 (2018). https://doi.org/10.1007/s13197-018-3397-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3397-2