Abstract
The antifungal effect of chitosan bags added with montmorillonite (MMT) and cinnamon leaf essential oil (CLEO) was evaluated on D'Anjou pear during storage at refrigeration and ambient temperature. Films from low-molecular weight chitosan at 1%, MMT (10%), glycerol (10%), and three concentrations of CLEO (0.25, 0.50, or 0.75%) based on the dry weight of chitosan, were used for the in vivo and in vitro assays on Penicillium crustosum. The in vitro evaluation was carried out through mycelial growth inhibition (MGI) and spore germination (SG) using chitosan films. For in vivo evaluation, the fruit was inoculated with P. crustosum and bagged with the films. Severity index and disease incidence were assessed on pears. Results showed an MGI of 100% and an SG of 3% of P. crustosum when using chitosan films with CLEO at 0.75%. In vivo results with chitosan bags indicated that the same treatment showed the greatest antifungal activity on P. crustosum, reducing the severity of infection by 43% and the disease incidence by 22%. CLEO influenced the content of polyphenol oxidase and total phenols. The chitosan bags with CLEO preserved the pear fruit better resulting in less weight loss, highest firmness, and good color preservation. The results indicated that bags from chitosan added with CLEO at 0.75% and MMT at 10% helped to maintain the quality of fruit during storage and could be used as an alternative for D'Anjou pear preservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
D'Anjou pear (Pyrus communis L.) fruit is consumed due to its slightly-acid sweet taste and high content of vitamin C, potassium, antioxidants, and fiber [1]. However, in postharvest, these fruits are susceptible to contamination by fungal attack mainly of the Penicillium genus, with P. expansum, P. crustosum, and P. solitum standing out because of their pathogenicity. These fungi are considered a severe concern in the fresh fruit industry because they cause significant economic losses [2]. For example, up to 50% of postharvest losses have been attributed to decomposition caused by Penicillium spp. Worldwide [3]. To prevent fungal damage to these fruit during postharvest, synthetic antifungal agents have been applied [4], but the use of synthetic fungicides has triggered antimicrobial resistance, health concerns, and environmental pollution. To replace them, the application of essential oils (EO) for fruit protection could be a natural alternative [5]. Several studies have been carried out using EO in vitro or in vivo experiments against a wide variety of microorganisms for fruit preservation including cinnamon bark essential oil (CEO) against Monilinia fructicola, P. expansum, and Rhizopus spp. In stored peach fruits [6], CEO against P. expansum [7] or CLEO against Aspergillus niger, Botrytis cinerea, and Rhizopus stolonifer [8]. In postharvest, EO has been sprayed directly on the fruit or dipped into the solution; however, the main disadvantage is the odor impregnation of these compounds in the fruit and the skin damage. To overcome this problem, EO has been incorporated into biopolymers and applied in the form of a coating so, high concentration of EO can be added and a controlled release can take place by diffusion during storage. Chitosan is a natural, renewable, and abundant polymer obtained by deacetylation of chitin from shells of crustaceans and shrimp [9]. Chitosan coatings have been applied to preserve guava, papaya, and mango [10,11,12], mixed with MMT to protect bananas [13] and added with cinnamaldehyde to preserve the quality of mandarin fruits [14]. Much of the research has been done with coatings but there is little information about using bags made with films. When using chitosan as a raw material to prepare biodegradable films, EO can be added to decrease water vapor permeability and to increase its antimicrobial capacity. In addition, plasticizers such as glycerol can be incorporated to provide flexibility to the film. Also, sodium montmorillonite (MMT), a biocompatible clay, has an interlaminar structure that allows incorporating the chitosan chains into its structure improving the water vapor barrier properties [15, 16]. Although several studies have been carried out with cinnamon bark essential oil (in which cinnamaldehyde is the major component), few works have studied the cinnamon leaf essential oil in which eugenol is the major component and it can act in a different way.
Essential oils can promote defense mechanisms in fruits including the increase in the activity of polyphenol oxidase (PPO). Also, there is evidence that clove essential oil increased PPO activity in citrus fruit inoculated with P. italicum [17]. Similarly, the thyme and cinnamon essential oils enhanced PPO activity in peaches infected with Monilinia laxa [18], and the oil from Tetradium glabrifolium increased PPO activity in bell pepper fruit inoculated with Phytophthora capsica [19]. In the presence of oxygen, PPO reacts with phenolic compounds forming quinones, which are toxic to the fungi, preventing their growth by synthesizing lignin and thickening the cell wall [20].
Carrying out in vitro and in vivo assessments is important as the response of the fungus could change when the fruit is implicated. In this same sense, to our best knowledge, the antimicrobial activity of CLEO in chitosan bags on P. crustosum has not been reported. The objective of this study was to evaluate the in vitro and in vivo antifungal effect of chitosan bags added with CLEO and MMT as an alternative to control the growth of P. crustosum in stored pears. Also, montmorillonite was added to improve water vapor barrier properties and to prevent water condensation inside the packaging; however, a complete study about this property is beyond the scope of this work.
Materials and methods
Materials
Low-molecular weight chitosan (89 kDa, with a 90% deacetylation degree) (Sigma-Aldrich, CAS No. 9012–76-4), anhydrous glycerol (Sigma-Aldrich, G7757), sodium montmorillonite (MMT) (Sigma-Aldrich, 682,659), cinnamon leaf essential oil (CLEO) (Oils and essences for the food industry, Mexico), tween 80 (Hycel, Mexico), Folin-Ciocalteu (Hysel), and potato dextrose agar (PDA) (Bioxon, Mexico) were used.
Biological Material
Penicillium crustosum was isolated from an infected pear and, after confirming its identity by a molecular identification at the Integrated Phytosanitary Diagnostic Laboratory, the strain was incubated in a PDA medium for 10 days at 28 °C. Pears (Pyrus communis L.) cv. D'Anjou at the physiological maturity stage (pears had reached their maximum development and presented dark green coloration) were acquired in a commercial store at Yautepec, Morelos, México.
Composition of Cinnamon Leaf Essential Oil
CLEO was diluted in dichloromethane at a 1:20 (v/v) ratio and then analyzed using a 7890A GC System gas chromatograph (Agilent Technologies. Santa Clara, CA. USA) coupled with a mass spectroscopy detector using the following methodology: 2 µL of the sample were injected using helium as a carrier gas at a flow rate of 1 mL/min, with a split ratio 5:1, using a DB-5 MS:1897–60013 column (325 °C, 60 m × 250 µm × 0. 25 µm). For the oven, an initial temperature of 40 °C for 10 min was used, followed by a ramp of 3 °C/min to reach 140 °C for 20 min, another ramp of 3 °C/min to 220 °C for 5 min and one more of 10 °C/min to reach 260 °C for 5 min. The mass spectrum was operated at 1 mL/min with an energy of 69.922 eV, with a mass source temperature of 230 to 250 °C and quadrupole temperature of 150 to 200 °C, in SCAN mode, and a mass range of 33 to 600 m/z.
Preparation of Films and Chitosan Bags
The methodology reported by Wang et al. [21] was followed with some modifications. 1% chitosan solution was prepared in 1% glacial acetic acid solution and mixed with a magnetic stirrer at 25 °C and 750 rpm until complete dissolution. The other components: MMT, glycerol, and CLEO were added to the chitosan solution based on the dry weight of chitosan. For this, a solution of 10% MMT was prepared in distilled water and sonicated (Bransonic 1510R-MTH) for 1 h. The solutions were mixed at 750 rpm at 25 °C for 4 h. Glycerol at 10% was added, stirred for 30 min, then tween-80 at 0.05% (based on the dry weight of chitosan) was added by stirring for 30 min. Finally, the CLEO was added at 0.25, 0.5, or 0.75% (based on the dry weight of chitosan). Each of the solutions was mixed with an ultra Turrax (Politron PT 1200 CL Kinematica, Switzerland) at 2000 rpm for 2 min to decrease the size of the oil droplet. 170 mL of the filmogenic solution was poured into 18 × 28 cm2 acrylic plates and dried at room temperature for 3 days at 25 °C and 50% RH. The films were then removed from the plates. The chitosan bags were prepared from these films. As chitosan films prepared in this study are not heat-sealable, the films were attached using adhesive tape. Before applying the chitosan bags for the in vivo study, the integrity of the sealing was assessed by a simple test closing the bag and exerting a mild pressure by hand to make sure that the trapped air does not scape. Films were used to evaluate the physical properties and to carry out the in vitro test.
Physical Properties of the Films
Mechanical Properties
Tensile strength (TS) and percentage of elongation at break (EB) of the films were evaluated following the ASTM D882-02 method. Prior to analysis, films were cut into 100 × 10 mm rectangles and conditioned in a desiccator at 57% RH achieved with an oversaturated sodium bromide salt solution. Thickness was measured in ten random points before testing and the average was used for further calculations. Then in a TA-XT texturometer (TA Plus, Lloyd Instruments) with an initial distance between grips of 60 mm and crosshead speed of 1 mm/s, eight replicates of each film treatment were measured. The TS and EB were calculated using the Eqs. 1 and 2, respectively.
where TS is tensile strength; maximum force in newtons (N); Thickness of film in m, and width of film in m.
where EB is the percentage of elongation at break; L0 is the film length at the fracture, and L is the initial length of the film.
Water Vapor Permeability
Water vapor permeability was determined following the ASTM E96-66 method (ASTM, 2000). The films were conditioned for 3 days in a desiccator with NaBr solution (57% RH). The conditioned film was placed between two silicon gaskets and allocated inside the bored cap allowing a transfer area of 2.206 × 10–3 m2. The cap was tightened to the 5 × 3.5 cm glass cell filled with water (100% RH) and the cell was then placed on the plate of an analytical balance inside a silica gel chamber with 0% RH at a controlled temperature of 30 °C. The balance was connected to a computer, where the weight was automatically recorded every min for 4 h. The weight was plotted vs time and the slope was used to calculate the water vapor transmission rate (WVTR) and then the water vapor permeability (WVP) was calculated using the Eqs. 3 and 4, respectively [22].
where WVP is in g m−1 s−1 Pa−1; Δm (g) amount of water vapor passing through the film; L (m) is the film thickness; A (m2) is the area of the film; ΔP (Pa) is the differential partial water vapor pressure and t (s) is the time.
In vitro Antifungal Activity
Preparation of Spore Solution
A spore solution was prepared from a P. crustosum strain whose pathogenicity was previously tested using Koch's postulates. 1 mL of sterile distilled water was added to the strain and then gently scraped with a loop to dissolve the spores; this concentrated solution was filtered using a funnel and sterile gauze and poured into an Erlenmeyer flask. The spores were counted using a microscope (Nikon model Alphaphot 2YS-H) with a 40X lens and a Neubauer chamber until a concentration close to 1.2 × 105 was obtained.
Mycelial Growth Inhibition
For mycelial growth inhibition (MGI) [23], six Petri dishes with PDA were used for each treatment. The films were cut into discs of the same diameter as the Petri dish (50 mm) and placed on the PDA agar. 10 µl of a 1.2 × 105 spore solution were poured on the discs under sterile conditions using a UV hood with laminar flow and incubated at 28 °C. The analyzed treatments contained different concentrations of CLEO: 0.25%, 0.50%, and 0.75%. PDA agar and chitosan films without CLEO (FWC) were used as controls. The mycelial growth was measured with a digital vernier every 24 h for 9 days. Mycelium growth inhibition was calculated using the Eq. 5, where A is the growth of the microorganism in the control film and B is the growth of the microorganism in the tested film.
Spore Germination
The test was carried out in spore germination chambers that consisted of Petri dishes with a glass slide where three PDA disks (15 mm diameter) were placed. On the PDA disks, the film was placed in the form of a 15 mm diameter disk (each disk represented a time of incubation of 8, 10, and 12 h). Then, 10 µl of a 1.2 × 105 spore solution was poured over each film and incubated at 28 °C. A drop of lactophenol blue was added to each disc to stop spore germination at each incubation time. The germinated spores were counted by observing the discs on a microscope (Nikon model Alphaphot 2YS-H) with a ×40 objective. Three replicates were carried out for each treatment.
In vivo Antifungal Activity
Preparation of the Spore Solution
A solution of P. crustosum spores was prepared to inoculate the pears, following the same procedure as for the in vitro antifungal activity, changing only the spore concentration of the final solution of c.a. 3 × 106.
Inoculation of D’Anjou Pears
The pears cv. D'Anjou were rinsed with potable water, immersed in 1% sodium hypochlorite (NaClO) for 2 min, and in distilled water for another 2 min to remove the excess of NaClO, and left to dry. Then, the fruit was randomly selected for the treatment and inoculated with P. crustosum. Using a sterile puncture needle, each pear was perforated in the equatorial zone (1 mm wide and 10 mm deep) and inoculated with 100 µL of the 3 × 106 spore solution. Once inoculated, the pears were placed inside the chitosan bags as follows: (a) pears with a bag made from chitosan film + MMT + glycerol + CLEO (PCLF); (b) bag from chitosan film + MMT + glycerol without CLEO (PF); (c) pears without chitosan bag (PWF). The pears were placed in clean plastic boxes and kept in a chamber at 4 °C and 95% RH for 15 days (Fig. 1). After that, pears were removed from the cold chamber and stored at 25 °C and 95% RH, for 10 days to simulate storage and distribution conditions. Thirty fruits were used for each treatment.
Evaluation of Disease Severity and Incidence
The presence of P. crustosum was evaluated as severity index and disease incidence (%). The severity index was measured considering the damage extension in the treated fruit stored at 4 °C for 15 days and 25 °C for 10 days. For this, a scale formed by 5 grades (1–5) was considered: 1 = 0%, 2 = 1–25%, 3 = 26–50%, 4 = 51–75% and 5 = 76–100% of the fruit surface with symptoms of the disease.
Equation 6 was used to calculate the severity index where Xi = number of damaged fruits for each degree of damage; the numbers (0, 1, 2, 3, 4, 5) are the degree of damage on the scale used. N is the number of fruits per experimental unit.
Equation 7 was used to determine the fruit disease incidence.
Evaluation of PPO Enzymatic Activity and Total Phenolic Compounds
PPO (EC 1.10.3.2)
To determine the activity of polyphenol oxidase (PPO), 1 g of pear pulp, including the peel, was weighed, and homogenized in a mortar and pestle with 5 mL of 0.2 M sodium phosphate buffer (Na2HPO4, pH 6.4) and 50 mg of polyvinylpyrrolidone (PVP). The mixture was centrifuged at 6000 rpm for 30 min at 4 °C, and the supernatant was recovered (enzymatic source). Subsequently, 1 mL of the supernatant was mixed with 1 mL of 0.2 M catechol, and PPO activity was measured at λ = 410 nm for 60 s in a Genesys 10S UV–VIS spectrophotometer (Thermo Scientific, USA). 1 mL 0.2 M Na2HPO4 buffer and 1000 µL catechol were used as the control group. Enzyme activity was reported as international units (IU), defined as the amount of enzyme causing a 0.1 units increase in absorbance per minute [24].
Determination of Protein Content by the Bradford Method
To determine the protein concentration, 700 µL of Bradford reagent were added to 100 µL of supernatant recovered from the enzymatic assay. The mixture was incubated in the dark for 5 min. The absorbance was measured at 590 nm in a spectrophotometer (Thermo Scientific Genesis 10S UV–VIS, USA). A standard curve (1 to 10 mg/mL) was prepared using a stock solution of bovine serum albumin (Bio-Rad, USA) dissolved in water. PPO activity was reported as IU mg−1 protein [25].
Total Phenolic Compounds
To determine the total phenolic compounds, 3 g of pear pulp with peel were macerated in 5 mL of 80% methanol. The mixture was centrifuged at 8000 rpm for 10 min and the supernatant, containing the phenolic compounds, was recovered. Then, the reagents were placed in a test tube in the following order: 3850 µL of distilled water, 750 µL of 20% sodium bicarbonate (NaHCO3), 250 µL of Folin Cicalteau reagent, and 150 µL of the supernatant from the centrifuged sample. The mixture was incubated for 2 h in the dark. A change from yellow to dark green/blue color was taken as indicative of the presence of phenolic compounds. After that time, the samples were measured in a spectrophotometer (Thermo Scientific Genesis 10S UV–VIS, USA) at λ = 760 nm. Distilled water was used as a blank. The samples were analyzed in triplicate. To determine the concentration of total phenolic compounds, a standard curve was obtained from a stock solution of gallic acid at six concentrations from 30 to 180 µg [26].
Evaluation of Quality Variables During Storage of D’Anjou Pears
Weight Loss
The fruit was weighted during the storage period, and the weight loss was calculated using the Eq. 8. Fifteen pears per treatment were used for each evaluation day; the analysis was done by triplicate, with 5 pears for each replicate.
where W1 is the percentage of weight loss. Wi (g), and Wf (g) are the initial and final weights, respectively.
Total Soluble Solids
The percentage of total soluble solids (TSS) was determined using a manual refractometer (Atago®, Japan) with a scale from 0 to 32°Brix. One drop of pear juice from 5 pears per treatment was taken and placed on the refractometer. Results were averaged and expressed as the percentage of TSS.
Firmness
Firmness was measured using a fruit firmness tester (model 53205, Turoni®, Italy). The pear surface was penetrated on two opposite sides at the equatorial zone of 5 fruit per treatment. The results were averaged and reported in Newtons (N).
Color
The L*, a*, and b*color parameters were measured with a BC-10 colorimeter (Kónica Minolta, Sensing, Américas, Inc.). The chroma (C*) and hue (h°) values were determined from these coordinates. The letter L* indicates luminosity, and the values range from 0 (dark) to 100 (light). The value of a* represents green (− a*) to red (+ a*). The value of b* represents yellow (+ b*) to blue (− b*). The value of chroma (C*) indicates the saturation, and the hue angle (h°) is the hue of the color. The color was assessed in 15 fruits by measuring the surface of the pears in two zones of the equatorial area.
Statistical Analysis
A completely randomized analysis of variance was used for in vitro mycelial growth and a factorial arrangement design for the remaining treatments. For the comparison of means, Tukey's test was used with p ≤ 0.05. Statistical software used was InfoStat 2019.
Results and Discussion
Mechanical Properties and Water Vapor Permeability
It is important to measure the mechanical and barrier properties to know is they are suitable to maintain the structural integrity of fruits during storage and preservation.
The results indicated a tensile strength of 36.90 ± 1.40 MPa, an elongation at break of 3.09 ± 0.25%, and water vapor permeability of 1.22 × 10–10 ± 2.36 × 10–11 g m−1 s−1 Pa−1. Only 0.75% CLEO was used in the film formulation because it is the amount necessary to confer antimicrobial activity without damaging fruit metabolism. These properties are the results of the interactions taking place among all added components in the film. MMT hinders water vapor permeability by promoting a tortuous diffusion path of water molecules through the film [27], as it is dispersed in the polymeric matrix [28] in exfoliated or intercalated form promoting the interaction between the metallic ions of MMT and the functional groups of chitosan [29]. CLEO affects permeability because it influences the hydrophilic/hydrophobic balance of films [30]. Essential oils decrease the fracture toughness because the oil breaks the strong polymer–polymer interactions by forming weaker polymer-oil interactions [31]. Glycerol is a plasticizer that works by weakening the intermolecular forces between polymer chains increasing their elongation percentage and the permeability in the films [32].
Composition of Cinnamon Leaf Essential Oil
The results from GC/MS indicated the presence of 125 compounds in CLEO. Table 1 shows the concentration of the first 23 compounds and the composition analysis shows that the CLEO used in this study had more similarity with clove essential oil since, according to literature, the content of eugenol in this oil ranges from 69.68 to 80.09% [33,34,35]. Compared to 54.12% found as the main component (Table 1) of CLEO. In addition, other compounds comparable to clove oil were also detected, but in lower amounts, among them, β-caryophyllene, chavicol, α-cubebene, and eugenyl acetate were identified. The composition of CLEO (cinnamon leaf) differs from that of cinnamon bark essential oil. Even when they come from the same tree, cinnamon bark has a higher content of cinnamaldehyde with 44.25% [36] and lower content of eugenol with 3.19% [37].
In Vitro Mycelial Growth and Spore Germination
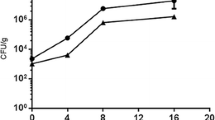
The MGI values were statistically different (p ≤ 0.05) among treatments after 9 days of incubation. The growth of P. crustosum was totally inhibited in the treatments containing CLEO at 0.50 and 0.75%, followed by the treatment with CLEO at 0.25%. The lowest inhibition value was observed in the control sample grown on PDA and FWC (Fig. 2). For spore germination, the effect of the incubation time and CLEO concentration was statistically significant (p ≤ 0.05) (Table 2). After incubation for 6 h, no spore germination was observed in any treatments, including the control group; however, at 14 h, spores germinated reaching values of 100%. As observed in Table 2, germination decreased when the CLEO concentration increased. The film with no essential oil added also showed a strong inhibitory effect. Perdones et al. [8] evaluated the antifungal effect of chitosan-based films added with CLEO on A. niger, B. cinerea, and R. stolonifer. In that study, the growth of the three fungi was inhibited as a function of concentration and the highest fungal inhibition was reached at the highest dose. A similar behavior was observed in this work. Also, Wang et al. [21] tested chitosan films with CEO at 2.5 and 10% against A. oryzae and P. digitatum demonstrating antimicrobial activity at these two concentrations. In our study, the CLEO concentrations tested were lower and showed higher inhibition effect than the other treatments reported in the literature. This difference in toxicity can be attributed to compounds derived from phenols (eugenol) from CLEO having a higher antimicrobial effect than the compounds with aldehyde groups (cinnamaldehyde) from CEO [38]. Essential oils have antimicrobial activity due to their lipophilicity and the presence of phenolic compounds with functional groups OH that allow them to interact through hydrogen bonds with membrane proteins, altering the morphology of the fungus, loss of rigidity and integrity of the cell wall [39]. Eugenol has a cytotoxic activity at concentrations as low as 0.03% [40] and it has antifungal activity against several postharvest fungi including P. expansum, P. glabrum, P. italicum [41, 42] Cladosporium spp., Rhizopus oryzae [43], Aspergillus flavus [44], and Botrytis cinerea [45].
Effect of different formulations based on chitosan, MMT, and CLEO at different concentrations, on the mycelial growth of P. crustosum after incubation for 9 days. One-way ANOVA with Tukey's mean comparison (p ≤ 0.05). Different letters indicate significant differences among treatments. PDA potato dextrose agar; FWC film without CLEO; CLEO chitosan-based film + MMT + CLEO at concentrations of 0.25, 0.50, and 0.75%
In this work, during the in vitro test, P. crustosum was in direct contact with the chitosan films without CLEO that were placed on PDA agar; therefore, the inhibition of mycelial growth and spore germination observed in these samples could be due to the polycationic structure of chitosan interacting with the electronegative charges of cell membranes, modifying the permeability of the cell wall, and releasing the salt ions and proteins, as it has been stablished by other researchers [46].
Severity and Disease Incidence
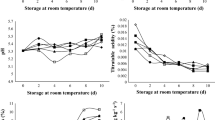
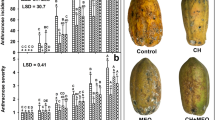
On severity, the effect of the treatment-time interaction was statistically significant (p ≤ 0.05) after 15 days at 4 °C and then after 10 days at 25 °C. The result of the severity test indicated that until day 12 at 4 °C, P. crustosum did not grow, probably due to the low storage temperature. At this temperature, after 15 days of storage, the pears in all treatments showed infection signals with significantly less fungal growth in the PCLF treatment (Fig. 3). At 25 °C, P. crustosum growth was evident since day 1 of storage. Overall, infection increased as the storage time increased. During the 10 days of storage at 25 °C, the PCLF treatment showed the lowest infection compared to the other treatments.
Effect of films on the severity index of P. crustosum in pears stored at 4 and 25 °C. Different letters indicate significant differences. Two-way ANOVA with mean comparison using Fisher LSD test (p ≤ 0.05). PWF no bagged pear, PF pears bagged in chitosan and montmorillonite film, PCLF pears bagged in chitosan, montmorillonite, and CLEO film
On disease incidence, the effect of the treatment-time interaction was statistically significant (p ≤ 0.05) after storage at 4 and 25 °C. No infected fruit were observed during the first 9 days of storage at 4 °C in any of the treatments, including the control. On day 15, the fruit showed infection symptoms and the lowest incidence was recorded in pears for the PCLF treatment. On the other side, since day 1 of storage at 25 °C, the incidence of P. crustosum was higher compared to 4 °C and increased, in general, with the storage time (Fig. 4). During storage from day 3 to 10 at 25 °C, the disease incidence of pears was up to 70% for the bagged fruit with or without essential oil. At the end of the storage at both temperatures, pears showed typical symptoms of fungal infection, such as brown growing halos and softening in the inoculated areas. As observed in Fig. 5, pears from the PWF and PF treatments showed larger affected areas. Also, these treatments showed softening of the endocarp, acetic acid smell, color changes from green to yellow, and mycelium growth in some of them. Pears from the PCLF treatment preserved their firmness and kept the green color with no acetic acid odor. According to the literature, the in vivo antimicrobial activity of EO depends on several factors including the EO composition, concentration, type of microorganism, fruit stage, and how EO was added to the fruit. Perdones et al. [8] reported antifungal activity against B. cinerea when testing chitosan films with CLEO at 0.25, 0.5, and 1% on strawberries; in addition, a shelf-life extension was observed at 10 °C. The CEO at 300 μL L−1 showed antifungal effect against P. expansum when tested in vitro; however, when applied to pears, no inhibition of the fungus was observed [7]. In this work, CLEO in chitosan bags was effective in vitro as well as in vivo tests. Some essential oils can contribute to defense mechanisms including the essential oil thyme in avocado [47] the essential oil of thyme and cinnamon in peaches [18] and tea tree oil in strawberries [48]. In this study, the in vivo antifungal effect is attributed to the presence of CLEO which activates the natural defense mechanisms of pears, along with the antifungal activity of the essential oil.
Effect of films on the percentage of disease incidence of P. crustosum in pears stored at 4 and 25 °C. Different letters indicate significant differences. Two-way ANOVA with means comparison of Fisher (p ≤ 0.05). PWF no bagged pears, PF pears bagged in chitosan and montmorillonite film, PCLF pears bagged in chitosan, montmorillonite, and CLEO film
Enzymatic Activity
The PPO activity of fruit after storage at 4 °C showed significant differences (p ≤ 0.05) among treatments. Fruit from PCLF treatment had a PPO value of 14.23 IU/mg protein, while PWF had 6.68 IU/mg protein, and PF showed a value of 5.95 IU/mg protein. After 10-days storage at 25 °C, the PPO activity increased significantly (p ≤ 0.05) in all treatments. The PPO in fruits treated with the essential oil had an activity value of 32.43 IU/mg, showing the highest activity among all treatments, while PWF and PF had activity values of 20.66 and 16.14 IU/mg, respectively (Fig. 6). Chen et al. (2016) [49] reported that applying carboxymethyl cellulose coatings with clove essential oil on mandarin oranges increased the PPO activity, making the fruit more resistant to infection. The thyme essential oil increased the activity of this enzyme in pear fruit, indicating that thyme oil may have induced antimicrobial resistance. PPO is a defense enzyme that oxidizes phenolic compounds to quinones that are toxic compounds with antimicrobial activity; in addition, PPO promotes cell lignification in infected fruits, preventing the spread of the pathogen [50].
Effect of films on polyphenol oxidase activity in pears. Different letters indicate significant differences. Two-way ANOVA with means comparison using Tukey's test (p ≤ 0.05). PWF no bagged pear, PF pears bagged in chitosan and montmorillonite film, PCLF pears bagged in chitosan, montmorillonite, and CLEO film
Total Phenolic Compounds
At the end of storage at 4 °C, the total phenolic compounds decreased significantly (p ≤ 0.05) in all treatments, with PCLF-treated fruit showing the lowest amount (0.26 mg/g). After 10-days storage at 25 °C, the total phenolic compounds in PF (0.62 mg/g) increased significantly (p ≤ 0.05) compared to the other treatments. PWF (0.49 mg/g) and PCLF (0.53 mg/g) treatments did not show significantly different (p ≤ 0.05) values (Fig. 7). The phenols could have been used as substrate by PPO and therefore they were not quantified. Total phenolic compounds are important, due to their antioxidant and antifungal capacity, can protect the fruit by inhibiting the production of extracellular enzymes of pathogens, capable of breaking the cell wall of the fruit [51].
Effect of films on the concentration of total phenols in pears. Different letters indicate significant differences. Two-way ANOVA with means comparison using Tukey's test (p ≤ 0.05). PWF no bagged pears, PF pears bagged in chitosan and montmorillonite film, PCLF pears bagged in chitosan, montmorillonite, and CLEO film
Quality of Pears During Storage
Table 3 shows the physicochemical properties of treated pears. The most noticeable changes were observed in pears stored at 25 °C as there were significant differences in weight loss and firmness (p ≤ 0.05). The PCLF treatment had the lowest weight loss (4.2%) compared to the control (6.3%) and samples without CLEO (6%). The firmness of the PCLF treatment (22 N) was also statistically different (p ≤ 0.05) compared to the other two treatments where firmness values were lower with 14.8 N and 18.1 N for PWF and PF, respectively. The coloration of pears changed at the end of storage at 25 °C as the h° values, indicating yellow tonality in the PWF (97.63) and PF (99.35) treatments, were significantly different (p ≤ 0.05) from PCLF treatment (105.45) which showed a green tonality (Table 4). The a* value in the PCLF treatment was located at the green color area, while the other treatments were located far from this color. The b* value in all treatments increased slightly indicating a shift to yellow color. The color change is due to the loss of chlorophyll and the appearance of carotenoids [52].
The behavior of chitosan films with CLEO could be explained because it behaves as a semi-permeable barrier to water vapor and oxygen [53] that, in addition to the presence of exfoliated sheets of MMT, resulted in a tortuous path that difficulted the diffusion of water and oxygen molecules through the film as it has been reported by [54]. Furthermore, the phenolic compounds of the CLEO conferred antioxidant properties to the film, reducing the ambient oxygen before it can oxidize the pear. This could explain the decrease in respiration rate caused by the CLEO films preventing early deterioration of the fruit. A similar behavior has been reported in the conservation of several fruits coated with chitosan including grapes [55], mango [56], pineapple [57], strawberries [58], kiwi [59], melon [60], and pears [61, 62].
Conclusion
Eugenol was the major component in CLEO. The film with 0.75% CLEO inhibited the in vitro growth of P. crustosum and showed antifungal activity in vivo by reducing the fungal growth on the inoculated D’Anjou pears during storage in chitosan bags. The presence of CLEO also increased the activity of PPO and, consequently, the total phenols. The fruit quality was maintained in the PCLF treatment. Chitosan bags added with cinnamon leaf essential oil, montmorillonite, and glycerol could be applied as an active packaging to extend the shelf-life of D’Anjou pears since bagged pears demonstrated protection against P. crustosum and no side effects during the ripening process of the fruit were observed.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Reiland H, Slavin J (2015) Systematic review of pears and health. Nutr Today 50:301–305. https://doi.org/10.1097/NT.0000000000000112
Scholtz I, Korsten L (2016) Profile of Penicillium species in the pear supply chain. Plant Pathol 65:1126–1132. https://doi.org/10.1111/ppa.12494
Louw JP, Korsten L (2014) Pathogenic Penicillium spp. on apple and pear. Plant Dis 98:590–598. https://doi.org/10.1094/PDIS-07-13-0710-RE
Sugar D, Basile SR (2008) Timing and sequence of postharvest fungicide and biocontrol agent applications for control of pear decay. Postharvest Biol Technol 49:107–112. https://doi.org/10.1016/j.postharvbio.2007.12.008
Sivakumar D, Bautista-Baños S (2014) A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot 64:27–37. https://doi.org/10.1016/j.cropro.2014.05.012
Montero-Prado P, Rodriguez-Lafuente A, Nerin C (2011) Active label-based packaging to extend the shelf-life of “ Calanda” peach fruit: changes in fruit quality and enzymatic activity. Postharv Biol Technol 60:211–219. https://doi.org/10.1016/j.postharvbio.2011.01.008
Valenzuela NL, Marcelo F, Viveros L et al (2016) Essential oil of Cynnamomum zeylanicum : control alternative for Penicillium expansum on pear in postharvest. Rev Mex Ciencias Agrícolas 7:1017–1028
Perdones Á, Vargas M, Atarés L, Chiralt A (2014) Physical, antioxidant and antimicrobial properties of chitosan-cinnamon leaf oil films as affected by oleic acid. Food Hydrocoll 36:256–264. https://doi.org/10.1016/j.foodhyd.2013.10.003
Priyadarshi R, Rhim JW (2020) Chitosan-based biodegradable functional films for food packaging applications. Innov Food Sci Emerg Technol 62:102346. https://doi.org/10.1016/j.ifset.2020.102346
Amina RMZ, Rashid A (2012) Efficacy of edible chitosan formulation in quality maintenance and shelf life of guava (Psidium guajava L.) fruit during cold storage. Sarhad J Agric 38:266–274
Vilaplana R, Chicaiza G, Vaca C, Valencia-Chamorro S (2020) Combination of hot water treatment and chitosan coating to control anthracnose in papaya (Carica papaya L.) during the postharvest period. Crop Prot. https://doi.org/10.1016/j.cropro.2019.105007
Khalil HA, Abdelkader MFM, Lo’ay AA et al (2022) The combined effect of hot water treatment and chitosan coating on mango (Mangifera indica L. cv. Kent) fruits to control postharvest deterioration and increase fruit quality. Coatings 12:83. https://doi.org/10.3390/coatings12010083
Wantat A, Seraypheap K, Rojsitthisak P (2022) Effect of chitosan coatings supplemented with chitosan-montmorillonite nanocomposites on postharvest quality of ‘Hom Thong’ banana fruit. Food Chem 374:131731. https://doi.org/10.1016/j.foodchem.2021.131731
Gao Y, Kan C, Chen M et al (2018) Effects of chitosan-based coatings enriched with cinnamaldehyde on mandarin fruit cv. Ponkan during room-temperature storage. Coatings. https://doi.org/10.3390/COATINGS8100372
Hong SI, Lee JH, Bae HJ et al (2010) Effect of Shear rate on structural, mechanical, and barrier properties of chitosan/montmorillonite nanocomposite film. J Appl Polym Sci 119:2742–2749. https://doi.org/10.1002/app.31767
Abdollahi M, Rezaei M, Farzi G (2012) A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J Food Eng 111:343–350. https://doi.org/10.1016/j.jfoodeng.2012.02.012
Chen C, Cai N, Chen J, Wan C (2019) Clove essential oil as an alternative approach to control postharvest blue mold caused by Penicillium italicum in citrus fruit. Biomolecules. https://doi.org/10.3390/biom9050197
Cindi MD, Soundy P, Romanazzi G, Sivakumar D (2016) Different defense responses and brown rot control in two Prunus persica cultivars to essential oil vapours after storage. Postharv Biol Technol 119:9–17. https://doi.org/10.1016/j.postharvbio.2016.04.007
Wang B, Li P, Yang J et al (2022) Inhibition efficacy of Tetradium glabrifolium fruit essential oil against Phytophthora capsici and potential mechanism. Ind Crops Prod. https://doi.org/10.1016/j.indcrop.2021.114310
Duan X, OuYang Q, Tao N (2018) Effect of applying cinnamaldehyde incorporated in wax on green mould decay in citrus fruits. J Sci Food Agric 98:527–533. https://doi.org/10.1002/jsfa.8490
Wang L, Liu F, Jiang Y et al (2011) Synergistic antimicrobial activities of natural essential oils with chitosan films. J Agric Food Chem 59:12411–12419. https://doi.org/10.1021/jf203165k
Sifuentes-Nieves I, Flores-Silva PC, Gallardo-Vega C et al (2020) Films made from plasma-modified corn starch: chemical, mechanical and barrier properties. Carbohydr Polym 237:116103. https://doi.org/10.1016/j.carbpol.2020.116103
Correa-Pacheco ZN, Bautista-Baños S, Valle-Marquina MÁ, Hernández-López M (2017) The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides growth in vitro and on cv Hass avocado and fruit quality. J Phytopathol 165:297–305. https://doi.org/10.1111/jph.12562
Arnnok P, Ruangviriyachai C, Mahachai R et al (2010) Optimization and determination of polyphenol oxidase and peroxidase activities in hot pepper (Capsicum annuum L.) pericarb. Int Food Res J 17:385–392
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Xiao C, Zhu L, Luo W et al (2010) Combined action of pure oxygen pretreatment and chitosan coating incorporated with rosemary extracts on the quality of fresh-cut pears. Food Chem 121:1003–1009. https://doi.org/10.1016/j.foodchem.2010.01.038
Cao S, Liu M, Zou L et al (2022) Addition of montmorillonite to improve the barrier and wetting properties of chitosan-based coatings and the application on the preservation of Shatang mandarin. Food Packag Shelf Life 33:100889. https://doi.org/10.1016/j.fpsl.2022.100889
Lai Y, Wang W, Zhao J et al (2022) Chitosan Na-montmorillonite films incorporated with citric acid for prolonging cherry tomatoes shelf life. Food Packag Shelf Life 33:100879. https://doi.org/10.1016/j.fpsl.2022.100879
Souza VGL, Pires JRA, Rodrigues PF et al (2018) Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus officinalis essential oil: development and physical characterization. Food Packag Shelf Life 16:148–156. https://doi.org/10.1016/j.fpsl.2018.03.009
Wang W, Zhang Y, Yang Z, He Q (2021) Effects of incorporation with clove (Eugenia caryophyllata) essential oil (CEO) on overall performance of chitosan as active coating. Int J Biol Macromol 166:578–586. https://doi.org/10.1016/j.ijbiomac.2020.10.215
Shojaee-Aliabadi S, Hosseini H, Mohammadifar MA et al (2013) Characterization of antioxidant-antimicrobial κ-carrageenan films containing Satureja hortensis essential oil. Int J Biol Macromol 52:116–124. https://doi.org/10.1016/j.ijbiomac.2012.08.026
Priyadarshi R, Sauraj KB, Negi YS (2018) Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr Polym 195:329–338. https://doi.org/10.1016/j.carbpol.2018.04.089
Xu JG, Liu T, Hu QP, Cao XM (2016) Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 21:1–13. https://doi.org/10.3390/molecules21091194
Kaur K, Kaushal S, Rani R (2019) Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential oil, its major compound and its derivatives. J Essent Oil-Bearing Plants 22:1195–1217. https://doi.org/10.1080/0972060X.2019.1688689
Radovic M, Adamovic T, Pavlovic J et al (2019) Supercritical CO2 impregnation of gelatin-chitosan films with clove essential oil and characterization thereof. Chem Ind Chem Eng Q 25:119–130. https://doi.org/10.2298/CICEQ180323025R
Nikkhah M, Hashemi M, Habibi Najafi MB, Farhoosh R (2017) Synergistic effects of some essential oils against fungal spoilage on pear fruit. Int J Food Microbiol 257:285–294. https://doi.org/10.1016/j.ijfoodmicro.2017.06.021
Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH (2010) Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem 122:161–166. https://doi.org/10.1016/j.foodchem.2010.02.033
Yahyazadeh M, Omidbaigi R, Zare R, Taheri H (2008) Effect of some essential oils on mycelial growth of Penicillium digitatum Sacc. World J Microbiol Biotechnol 24:1445–1450. https://doi.org/10.1007/s11274-007-9636-8
Da Cruz CL, Fernández Pinto V, Patriarca A (2013) Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int J Food Microbiol 166:1–14. https://doi.org/10.1016/j.ijfoodmicro.2013.05.026
Prashar A, Locke IC, Evans CS (2006) Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif 39:241–248. https://doi.org/10.1111/j.1365-2184.2006.00384.x
Neri F, Mari M, Brigati S (2006) Control of Penicillium expansum by plant volatile compounds. Plant Pathol 55:100–105. https://doi.org/10.1111/j.1365-3059.2005.01312.x
Campaniello D, Corbo MR, Sinigaglia M (2010) Antifungal activity of eugenol against Penicillium, Aspergillus, and Fusarium species. J Food Prot 73:1124–1128. https://doi.org/10.4315/0362-028X-73.6.1124
Abbaszadeh S, Sharifzadeh A, Shokri H et al (2014) Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J Mycol Med. https://doi.org/10.1016/j.mycmed.2014.01.063
Trajano VN, de Lima E, O, de Souza FS, (2012) Antifungal activity of the essential oil of Cinnamomum zeylanicum blume and eugenol on Aspergillus flavus. J Essent Oil-Bearing Plants 15:785–793. https://doi.org/10.1080/0972060X.2012.10644121
Melgarejo-Flores BG, Ortega-Ramírez LA, Silva-Espinoza BA et al (2013) Antifungal protection and antioxidant enhancement of table grapes treated with emulsions, vapors, and coatings of cinnamon leaf oil. Postharvest Biol Technol 86:321–328. https://doi.org/10.1016/j.postharvbio.2013.07.027
Zhang H, Li R, Liu W (2011) Effects of chitin and its derivative chitosan on postharvest decay of fruits: a review. Int J Mol Sci 12:917–934. https://doi.org/10.3390/ijms12020917
Sellamuthu PS, Sivakumar D, Soundy P, Korsten L (2013) Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol Technol 81:66–72. https://doi.org/10.1016/j.postharvbio.2013.02.007
Shao X, Wang H, Xu F, Cheng S (2013) Effects and possible mechanisms of tea tree oil vapor treatment on the main disease in postharvest strawberry fruit. Postharvest Biol Technol 77:94–101. https://doi.org/10.1016/j.postharvbio.2012.11.010
Chen CY, Zheng JP, Wan CP et al (2016) Effect of carboxymethyl cellulose coating enriched with clove oil on postharvest quality of “Xinyu” Mandarin oranges. Fruits 71:319–327. https://doi.org/10.1051/fruits/2016019
Jin L, Cai Y, Sun C et al (2019) Exogenous L-glutamate treatment could induce resistance against Penicillium expansum in pear fruit by activating defense-related proteins and amino acids metabolism. Postharvest Biol Technol 150:148–157. https://doi.org/10.1016/j.postharvbio.2018.11.009
Mohamed MSM, Saleh AM, Abdel-Farid IB, El-Naggar SA (2017) Growth, hydrolases and ultrastructure of Fusarium oxysporum as affected by phenolic rich extracts from several xerophytic plants. Pestic Biochem Physiol 141:57–64. https://doi.org/10.1016/j.pestbp.2016.11.007
Kowalczyk D, Kordowska-Wiater M, Zięba E, Baraniak B (2017) Effect of carboxymethylcellulose/candelilla wax coating containing potassium sorbate on microbiological and physicochemical attributes of pears. Sci Hortic 218:326–333. https://doi.org/10.1016/j.scienta.2017.02.040
Duan C, Meng X, Meng J et al (2019) Chitosan as a preservative for fruits and vegetables: a review on chemistry and antimicrobial properties. J Bioresour Bioprod 4:11–21
Qu B, Luo Y (2021) A review on the preparation and characterization of chitosan-clay nanocomposite films and coatings for food packaging applications. Carbohydr Polym Technol Appl 2:100102. https://doi.org/10.1016/j.carpta.2021.100102
Zhao X, Tian R, Zhou J, Liu Y (2022) Multifunctional chitosan/grape seed extract/silver nanoparticle composite for food packaging application. Int J Biol Macromol 207:152–160. https://doi.org/10.1016/j.ijbiomac.2022.02.180
Kumar N, Pratibha N et al (2021) Effect of chitosan–pullulan composite edible coating functionalized with pomegranate peel extract on the shelf life of mango (Mangifera indica). Coatings 11:25–40. https://doi.org/10.3390/coatings11070764
Maharsih IK, Pusfitasari MD, Putri CAS, Hidayat MT (2021) Performance evaluation of cassava peels starch-based edible coating incorporated with chitosan on the shelf-life of fresh-cut pineapples (Ananas comosus). IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/733/1/012017
Quintana SE, Llalla O, García-Risco MR, Fornari T (2021) Comparison between essential oils and supercritical extracts into chitosan-based edible coatings on strawberry quality during cold storage. J Supercrit Fluids 171:105198. https://doi.org/10.1016/j.supflu.2021.105198
Kumarihami HMPC, Cha GH, Kim JG et al (2020) Effect of preharvest Ca-Chitosan application on postharvest quality of ‘Garmrok’ kiwifruit during cold storage. Hortic Sci Technol 38:239–248. https://doi.org/10.7235/HORT.20200023
Carvalho RL, Cabral MF, Germano TA et al (2016) Chitosan coating with trans-cinnamaldehyde improves structural integrity and antioxidant metabolism of fresh-cut melon. Postharv Biol Technol 113:29–39. https://doi.org/10.1016/j.postharvbio.2015.11.004
Deng Z, Jung J, Simonsen J, Zhao Y (2018) Cellulose nanocrystals Pickering emulsion incorporated chitosan coatings for improving storability of postharvest Bartlett pears (Pyrus communis) during long-term cold storage. Food Hydrocoll 84:229–237. https://doi.org/10.1016/j.foodhyd.2018.06.012
Tran VT, Kingwascharapong P, Tanaka F, Tanaka F (2021) Effect of edible coatings developed from chitosan incorporated with tea seed oil on Japanese pear. Sci Hortic (Amsterdam) 288:110314. https://doi.org/10.1016/j.scienta.2021.110314
Acknowledgements
The authors would like to thank CONACYT, EDI and COFAA for its support.
Funding
This study was financially supported by project SIP 20210914 from the Instituto Politécnico Nacional.
Author information
Authors and Affiliations
Contributions
BEAP: Conceptualization, investigation, methodology, writing—original draft. SBB: Conceptualization, writing—review & editing, resources. GV: Formal analysis, writing—review and editing. MHL: Methodology. RIVA: Methodology. CARB: Investigation, conceptualization, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alvarez-Perez, B.E., Bautista-Baños, S., Velazquez, G. et al. Application of Chitosan Bags Added with Cinnamon Leaf Essential Oil as Active Packaging to Inhibit the Growth of Penicillium crustosum in D'Anjou Pears. J Polym Environ 31, 1160–1172 (2023). https://doi.org/10.1007/s10924-022-02659-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02659-z