Abstract
The quality and preservation of fresh-cut fruits can be improved by applying mild, non-thermal pre-treatments, such as osmotic dehydration (OD). The aim was to model the effect of OD on mass transfer and quality preservation. Apricot pieces were immersed in multi-component solutions comprising glycerol, erythritol, steviol glucosides and mineral salts, at various time–temperature conditions (25–45 °C for 3–240 min, wfruit/wsolution = 1/5). Water loss (WL), solid gain (SG), water activity (aw) as well as main quality indices (colour, texture) were measured during OD. OD apricot exhibited higher quality retention (acceptable level of colour change, increased firmness) suggesting that OD can be an effective pre-processing step in the production of innovative products. A second degree polynomial model was developed, describing the effect of processing conditions and glycerol concentration on WL, SG, aw, colour and texture of osmotically dehydrated apricots, and ANOVA was applied to identify the factors that significantly affect the aforementioned parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osmotic dehydration (OD) involves immersing of a solid food in a hypertonic solution to induce loss of water from the food and solute transfer from the solution. Typically, it is applied to plant origin materials, such as fruits and vegetables that consist of tissues or cell organizations with different characteristics and complexity. Water and solute mass transfer strongly depend on the properties of the osmotic solution (including type and concentration of osmo-active solute and osmotic gradient), working pressure–temperature and the structure of the solid food being processed. To optimize the process and food product quality, it is important to understand parameters affecting mass transfer during osmotic dehydration (Ahmed et al. 2016; Delgado et al. 2017; Yadav and Singh 2014).
New aspects of scientific research include the use of multi component osmotic solutions, consisting of alternative solutes, such as alternative carbohydrates, emulsions, and the combined use of osmotic dehydration and edible coating (Katsoufi et al. 2017; Rodriguez et al. 2016; Salazar-López et al. 2015). Replacement of conventional osmotic agents (the most common osmotic agent being sucrose) with polyhydric alcohols has the potential to improve texture and overall quality (e.g. of the dried fruit) and especially to maintain its moistness (Assis et al. 2017; Mendonça et al. 2017). The use of glycerol as an osmotic agent for a variety of food matrices (e.g. cantaloupe, chestnut, litchi, mandarin, papaya) has been reported. Glycerol has led to browning reaction rate decrease and overall quality improvement (Thalerngnawachart and Duangmal 2016).
In order to describe and identify the main factors influencing processes, such as the OD dehydration, response surface methodology (RSM) has been often proposed in recent literature. RSM is a statistical tool, used in many cases for the optimization of a process when the independent variables have a combined effect on the desired response (Chiu et al. 2017; Jain et al. 2011; Yadav et al. 2012; Roopa et al. 2014).
Apricot (Prunus armeniaca L.) is one of the most widely grown stone fruits of economic and nutritional interest that is consumed fresh, dried or processed (frozen and canned, in cubes or other forms). The marketing of minimally processed apricots ready for consumption is limited by a short postharvest shelf-life and rapid deterioration of their components due to tissue damage as a result of washing/cutting/dicing and ensuing enzyme activity and microbial growth (Ella Missang et al. 2011). Apricot is prone to browning as a result of the enzymatic oxidation of polyphenols in the presence of oxygen by polyphenoloxidase and loss of texture due to water loss and osmotic changes that cause a decrease in turgor and crispiness (Toivonen and Brummell 2008). Consequently, a minimally processed and more fresh-like apricot raw material with extended shelf life could have high commercial interest (Raj et al. 2015).
The objective of this work was to study the effect of osmotic dehydration using a non-conventional multi-component OD solution on mass transfer phenomena and important quality attributes’ change of apricot. During OD processing, glycerol concentration, time and temperature effect was investigated and a polynomial model was proposed to describe the main effects of the procedure on mass transfer (WL, SG), water activity (aw) and quality (colour, texture) as a function of the factor process variables (osmotic solution concentration, osmotic dehydration time and temperature).
Materials and methods
Raw materials
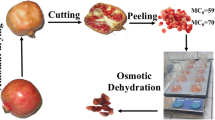
Apricots were selected according to size (uniform size for all apricots) and maturity level (semi-ripened apricots to ensure firm texture), washed, dried, cut in spheres (of average diameter 10 mm), blanched at 80 °C for 30 s and rapidly chilled.
Osmotic dehydration (OD)
Apricot pieces were osmotically treated in multi-component aqueous solutions containing non-conventional osmotic agents as follows: glycerol (40–60% w/w), erythritol (12.5% w/w), sodium chloride (3.5% w/w), calcium chloride (1.5% w/w), steviol glucosides (1.25% w/w) and Citrox© (0.20% w/w). The aim was to develop a novel osmo-dehydrated apricot product of reduced aw and improved quality. Glycerol was used as the main osmotic agent to lower aw. Erythritol provided sweetness and enhanced the texture of the final product (Moon et al. 2010). Sodium chloride increased mass transfer rates improving also the sensory characteristics of the final product. Calcium chloride was used in order to minimize tissue damage. Citrox©, a natural product consisting of plant origin ingredients (citrus green extract combined with citric acid and polyphenols), was chosen for its anti-microbial activity (Tsiraki and Savvaidis 2016). Finally, steviol glucosides (with established low calorie, low glycemic index, high-potency sweetener with antioxidant, antimicrobial, antifungal and anticarcinogenic effects) were added into the OD solution to improve the sensory as well as the functional characteristics of the final product (Lemus-Mondaca et al. 2012).
OD treatment was conducted at mild temperatures 25, 35 and 45 °C for a time period up to 240 min and using glycerol concentrations of 40, 50 and 60% w/w to assess the effect of the aforementioned OD processing conditions, on mass transfer and quality changes. The solution to food sample ratio was 5:1 (w/w).
Analysis of physicochemical properties
Water content was determined by drying at 110 °C for 24 h (WTB BINDER 7200E53, Germany). Water activity (aw) was monitored during the process using a dew point water activity meter (Aqua LAB 4TEV, Decagon Devices, Inc., USA).
Mass transfer calculations
Mass transfer parameters were calculated in terms of water loss (WL) and solid gain (SG) according to the following equations (Εqs. 1, 2)
where M0 is the initial mass of fresh material, Mt is the mass after time t of osmotic treatment, mt is the dry mass of apricot after time t of osmotic treatment and m0 is the dry mass of fresh material.
The average effective coefficients of water and solute diffusivity were calculated by fitting the experimental data for OD times to the following analytical solutions based on Fick’s second law for diffusion from a food sample of spherical shape, after suitable assumptions and boundary conditions (Εqs. 3, 4)
where MR and SR are the diffused moisture and solute ratio, respectively, M and S are the moisture and solute content, the subscripts 0, t and ∞ represent the relevant values at time 0, t and at equilibrium, Dew and Des (m2/s) are the effective coefficients of water and solute diffusivity, respectively, r (m) is the average radius of apricot. The effective diffusivities, Dew/es, were determined from the slope (Dew/es * π2/r2) of the plot of ln (MR) or ln (SR) against time, t, respectively.
The effect of temperature on Dew/es was then expressed using an Arrhenius-type relationship (Eq. 5) (Amami et al. 2008).
The effect of glycerol concentration on Dew/es was expressed using the following equation (Eq. 6).
where Dew/es,o1,2 are the effective coefficient constants of water/solid diffusivity (m2/s).
Colour and texture
Instrumentally measured apricot colour (CIELab values, CR-200 Chromameter®, Minolta Co., Japan) was expressed by the value for total color change ΔE according to Eq. (7).
where L0, a0, b0 are the initial values of colour parameters (at time zero).
Texture analysis was conducted by means of a texture analyzer (TA-XT2i of Stable Micro Systems, England). The test was performed on a non-lubricated flat platform using a knife probe with which samples are cut at a fixed rate and depth (1/3 of the initial). The maximum peak force (Fmax, g) was the mechanical parameter considered, and it was reported as hardness. Measurements (8–10 samples) were made in triplicate.
Determination of factor interactions during osmotic pre-treatment
A second degree polynomial model (Eq. 8) was chosen for the description of the response variables Y (WL, SG, aw, colour/ΔE, texture/hardness) as a function of the factor variables Xi [Cglycerol, T and t] (Terkmane et al. 2016).
where α0 the constant, αi represents the linear, αii the quadratic and αij the interaction effects of the factors. From the experimental results, model coefficients were calculated by non-linear regression using SYSTAT 12® Software, UK. The variables present in linear terms represent the coordinates of the maximum value predicted, those in quadratic terms represent the surface curvature, and the bi-factorial cross products represent the directions of the axes of the geometric figure obtained by sectioning the surface area (Terkmane et al. 2016).
Results and discussion
Mass transfer kinetics
In Fig. 1, water loss and solid gain (Eqs. 1, 2) occurring during osmotic dehydration of apricot pieces at the experimental conditions studied are presented. It can be observed that both water loss and solid gain highly depend on process conditions. Higher glycerol concentration (from 40 to 60% w/w) and temperature (from 25 to 45 °C) enhanced the rates of WL and SG during OD. Ispir and Togrul (2009) also reported that temperature (25–45 °C) and concentration (sucrose: 40–70% w/w) increase of osmotic medium caused increased water loss and solid gain during osmotic dehydration of apricot.
The experimental data presented in Fig. 1 were fitted to analytical solutions of Eqs. (3) and (4). The effective diffusion coefficients of water values (Dew, * 10−9 m2/s) were calculated as: 0.281 (25 °C), 0.551 (35 °C), 1.000 (45 °C) for 40% glycerol concentration, 0.317 (25 °C), 0.797 (35 °C), 1.330 (45 °C) for 50% glycerol concentration, 0.461 (25 °C), 1.152 (35 °C), 2.000 (45 °C) for 60% glycerol concentration. Respective effective diffusion coefficients of solids values (Des, * 10−9 m2/s) were: 0.279 (25 °C), 0.384 (35 °C), 1.000 (45 °C) for 40% glycerol concentration, 0.367 (25 °C), 0.657 (35 °C), 1.679 (45 °C) for 50% glycerol concentration, 0.442 (25 °C), 0.799 (35 °C), 2.100 (45 °C) for 60% glycerol concentration. These values of Dew and Des agree with published work (e.g. Dermesonluoglu et al. 2016; Mercali et al. 2011). Effective diffusivities for both water and solids increased with the increase of temperature and glycerol concentration, which is in agreement with the findings of Toğrul and Ispir (2008), Ispir and Togrul (2009), Mercali et al. (2011), da Silva et al. (2014). The activation energy Ea for diffusion (Eq. 5) during OD ranged from 50.06 (40% w/w) to 58.01 (60% w/w) kJ/mol for water loss and from 50.67 (40% w/w) to 60.63 kJ/mol (60% w/w) for solids gain showing similar temperature effect on water and solids diffusion. The effect of glycerol concentration on both water and solids diffusion was adequately expressed by an exponential increase, as shown by the R2 values obtained (> 0.975), (Eq. 6). The results were similar for the lower temperatures of 25 and 35 °C (Dew/es,o2 ~ 0.110 * 10−9 m2/s); the effect of glycerol concentration was significant for the highest temperature of 45 °C (Dew/es,o2 ~ 0.220 * 10−9 m2/s).
Water activity
Water activity was substantially lowered with OD time, as well as with the increase of OD temperature and glycerol concentration. At the end of osmotic dehydration aw values were 0.8989 (25 °C–240 min), 0.8969 (35 °C–180 min) and 0.8939 (45 °C–120 min) (50% w/w glycerol concentration), when the initial aw value of the fresh-cut apricot was measured at 0.9756 ± 0.0012. The respective aw values were: 0.9131 (25 °C–240 min), 0.9073 (35 °C–180 min) and 0.9045 (45 °C–120 min) (40% w/w glycerol concentration), and 0.8886 (25 °C–240 min), 0.8849 (35 °C–180 min) and 0.8847 (45 °C–120 min) (60% w/w glycerol concentration) (Supplementary Fig. 1). The mass transfer achieved by the removal of water and the solute uptake (glycerol effect) into the apricot tissue during OD reduced aw leading to a stable product without comprising sensory quality suitable for further processing. Temperature and glycerol concentration increase promoted aw decrease (Assis et al. 2017).
Colour
Carotenoids are responsible for the apricot colour and its change plays a key role in the degradation of total quality and sensory acceptance of fresh cut tissue. The main colour pigment of apricot is all-trans-β-carotene accounting for > 50% of the total carotenoid content (Fratianni et al. 2016). Being highly unsaturated, carotenoids are expected to undergo degradation and isomerization depending on factors such as food matrix, pH, heat, oxygen, and presence of other antioxidants (Penicaud et al. 2011). Thermal treatments induce losses, and lead to structural modifications such as cis-isomerization (Fratianni et al. 2016).
L, a and b values of fresh cut and osmotically dehydrated apricot presented statistically significant differences (p > 0.05). The initial L, a and b colour parameters for fresh-cut apricot were 57.09 ± 1.33, 6.44 ± 1.38 and 40.61 ± 2.89, respectively. At the end of the osmotic dehydration, the corresponding values for L, a and b values were: 25 °C–240 min: 53.54 ± 3.04, 13.52 ± 1.28, 47.02 ± 2.55, 35 °C–180 min: 53.05 ± 4.57, 13.21 ± 1.95, 48.46 ± 0.71, 45 °C–120 min: 52.83 ± 2.39, 13.96 ± 1.49, 48.04 ± 3.99. It was concluded that osmotic dehydration modified the colour of apricot leading to lower L values (decreased lightness or whiteness) and higher a (increase of red) and b (increase of yellow) values sensorially perceived as light brown colour. On the other hand, a and b increase, and no L change of osmo-dehydrated apricots, as a function of processing time (sucrose, maltose or sorbitol syrups at 65% w/w, with ascorbic acid and sodium chloride addition) was reported by Forni et al. (1997). Total colour change of osmotically dehydrated apricot cuts expressed by ΔE (Eq. 5), increased immediately after OD application (from initial times) and during progress (> 3 up to 17 at the end of OD processing) as presented in Fig. 2a. Colour change observed during osmotic dehydration could be due to liquid phase composition and shrinkage induced by OD (Heredia et al. 2010). Combined processes of osmotic dehydration and air-drying or freezing have been reported to obtain intermediate moisture apricot ingredients with natural colour, without the addition of commonly used sulphur dioxide (Riva et al. 2005).
Texture
A major problem encountered in the processing of apricots (referred as instantaneous pressure softening), is their high sensitivity to textural damage. Hence, it is a challenge for food technology to develop techniques to improve the integrity of this fruit tissue and to limit the texture losses during processing.
In Fig. 2b, hardness (Fmax) values of osmotically dehydrated apricot cuts are illustrated. Hardness increased with time and temperature of osmotic dehydration (p > 0.05) and glycerol concentration in the osmotic solution (p > 0.05). The values up to 600 g were characterized as “acceptable and desirable” according to sensory analysis conducted (data not shown). All OD treated apricot samples presented increased hardness compared to fresh ones showing that one of the main goals of the application of the osmotic dehydration had been achieved. The application of OD induces a greater firmness by replacing the osmotic solution in pores due to air loss, thus, obtaining, a more compact and less deformed tissue (Moreno et al. 2012). In the presence of calcium, the free carboxyl groups on different pectin chains can be cross-linked, resulting in a network of pectin chains and gel formation, which improves the plant tissue firmness (Liu et al. 2017). Moreover, it has been reported that when a treatment for aw reduction is applied changes of texture in tissue are more dependent on physical and chemical changes due to the transformation of protopectin to soluble pectin and to a sugar diffusion in intercellular spaces, which causes a loss of turgor and ion movement form the cell wall to the media (Moreno et al. 2012).
Determination of factor interactions during osmotic pre-treatment
The coefficients obtained from the second degree polynomial model (Eq. 8), indicating the effect of processing time, OD temperature and glycerol concentration on WL, SG, aw, texture/hardness and colour/ΔE-value of osmotically dehydrated apricots are presented in Table 1. In general, a positive value of the equation coefficient represents an effect which promotes the response, and a negative value indicates an inverse relationship between the factor and response. The quality of these models and their predictive power are closely dependent on the coefficients of variance (Terkmane et al. 2016). Glycerol concentration and temperature have a negative effect on WL showing a synergistic action (positive interaction coefficient). OD time duration has a positive effect on WL, accompanied by a synergistic action with temperature and antagonistic with glycerol concentration. Temperature was the prevailing factor affecting WL. Temperature and time duration have a positive linear effect on SG acting synergistically. Glycerol concentration shows negative effect on SG having synergistic action with temperature and antagonistic action with time duration. Temperature is also the prevailing factor affecting SG. Glycerol concentration and temperature have positive effect on aw acting antagonistically. Time duration has a negative effect acting antagonistically with glycerol concentration as well as temperature. The highest effect on hardness values is that of glycerol concentration (positive effect) acting antagonistically with temperature and synergistically with time duration. Temperature and time duration have lower negative and positive effects on hardness, respectively, with synergistic effect. Temperature has the highest positive effect on colour followed by a negative glycerol concentration effect with synergistic action. It is worthy noticing that in almost all factors studied, the largest effect had the linear terms of glycerol concentration (X1) and temperature (X2), followed by the quadratic term of temperature (X 22 ), confirming the principal effect of this parameter on the results of the osmotic process.
Once polynomial equations’ coefficients have been calculated and the model has been constructed, analysis of variance (ANOVA) can then be applied to assess how well the model represents the data. In order to be able to use ANOVA statistical tool, the normality of the data was checked using a plot of the residuals versus the predicted values. It was shown that the residuals were scattered randomly around zero with no outliers or unexpected errors (Li et al. 2014), thereby indicating that all results were within the range required to validate the model (graph not shown). Assessing ANOVA results, the associated p value is used as an indicator of statistical significance. According to the low values of the probability factor (p < 0.05) obtained, the models are considered statically significant. Therefore, the fitted models significantly (p < 0.05) represented the data for all response factors studied (Mune Mune and Minka 2017). Another important criterion for the goodness of fit is the coefficient of correlation (R2 (adj.)); the high values estimated for all parameters suggested that the models are appropriate. Additionally, plots of observed (experimental) versus predicted values showed an acceptable level of agreement (R2 > 0.8, Supplementary Fig. 2). From the above, it can be concluded that the quadratic models adequately represented the data and could be used to describe the design space.
As far as the contribution of each factor (linear, quadratic, interaction) is concerned, ANOVA was used to identify which factors are significant (Table 1), if a confidence level of 95% is considered.
The RSM was employed to study the effect of the main OD process parameters on mass transfer phenomena. For this purpose, the three dimensional response surface graphs were drawn to illustrate the combined effects of the independent variables and contour plots were generated for each of the fitted models as a function of two variables, while keeping the third variable constant (at the central value). Figure 3 shows the effect of treatment time and temperature (while keeping glycerol concentration constant) on WL, SG, water activity, texture and colour. At the beginning of the OD process, due to the high osmotic concentration gradient between the OD solution and the fresh sample, the rate of water removal and solid gain was high. As basic principles of osmotic dehydration describe, most indices (WL, SG, aw, colour) reach a plateau after approximately 120 min. Solute impregnation (SG increase) into the surface layers of the product reduces the osmotic driving force between the product and osmotic solution, impeding further mass exchange and lowering substantially WL rates. Temperature seems to have an almost linear effect on WL, SG and hardness, results that agree with the expected trend for mass exchange phenomena. Increasing temperature with processing time rises up both WL and SG rapidly. It is worthy noticing that higher process temperatures seem to promote mass transfer phenomena, reducing the time required to reach the equilibrium concentrations. On the other hand, at short processing times, increasing temperature favours WL more than SG, a phenomenon that could be attributed to a different degree of diffusion between water and solutes as related to their molar masses (Eren and Kaymak-Ertekin 2007).
The same trends regarding the effect on WL and SG are observed in Fig. 4, which shows the positive effect of glycerol concentration and treatment time (while keeping temperature constant). On the other hand, glycerol concentration increase does not seem to have a significant impact on aw decrease, whereas samples’ hardness increases almost linearly with both factors increase. As expected, colour loss is promoted by glycerol concentration and treatment time increase.
Figure 5 shows the effect of glycerol concentration and temperature (at a fixed time of 120 min) on WL, SG, water activity, texture and colour. As expected, WL, SG and colour change increase and aw decrease as glycerol concentration and temperature increase. The effect of temperature is more evident at high glycerol concentrations, which could be explained by the improved water transfer characteristics on the product surface due to lower viscosity of the osmotic medium. At all processing times (here shown at 120 min of OD process), WL increases gradually with glycerol concentration, whereas SG was more affected at the higher temperatures. Hardness of apricot samples seems to slightly decrease as glycerol concentration increases, while temperature increase hinders tissue softening. RSM methodology is not only for optimizing process conditions; it has been proved that the response surface and contour plots could be practical tools for optimization of water loss, solid gain, water activity reduction, tissue softening and colour change during osmotic dehydration of apricot. Moreover, RSM represents a useful tool for determination of the most favourable conditions to obtain desirable product attributes (Roopa et al. 2014). Depending on the final product characteristics and post-processing purposes (for example maximum WL and minimum SG and aw for shelf life extension (Eren and Kaymak-Ertekin 2007), SG optimization for solid enrichment purposes in the case of candied products) the optimal OD conditions (time, temperature, glycerol concentration) could be obtained.
Conclusion
From this study, it was found that osmotic dehydration can be considered an efficient method with respect to apricot cuts’ quality retention, since it contributes to water removal from the tissue without affecting its colour and texture, which are the most important quality attributes of the fruit. An interesting finding was the potential of using alternative multi-component OD solutions to extend shelf life and develop new, commercially available apricot products, possibly consumed as a snack or as fresh like ingredients for apricot containing food recipes or products. In order to assess mass transfer phenomena, Fick’s second law of diffusion and its analytical solution allowed for the reliable estimation of diffusion coefficients. A second order polynomial model was proven to adequately describe the effect of the prevailing OD processing factors (temperature, time, glycerol concentration). As demonstrated in this study, mass transfer phenomena during osmotic dehydration are strongly dependent on the process variables. This leads to modified quality characteristics of the final product, so that carefully designed products, with specific attributes, can be achieved by selecting certain levels of process variables.
Abbreviations
- α0 :
-
Constant of the second degree polynomial model
- αi :
-
Coefficients representing the linear effects of the factors of the second degree polynomial model
- αii :
-
Coefficients representing the interaction effects of the factors of the second degree polynomial model
- a :
-
Red (positive)–green (negative)
- a 0 :
-
a at initial osmotic treatment time zero (0)
- b :
-
Yellow (positive)–blue (positive)
- b 0 :
-
b at initial osmotic treatment time
- aw :
-
Water activity
- C glycerol :
-
Glycerol concentration (% w/w)
- D es :
-
Effective coefficient of solid diffusivity (m2/s)
- D es,ο1,2 :
-
Effective coefficient constant of solid diffusivity (m2/s)
- D ew :
-
Effective coefficient of water diffusivity (m2/s)
- D ew,ο1,2 :
-
Effective coefficient constant of water diffusivity (m2/s)
- E a :
-
Activation energy (kJ/mol)
- F max :
-
Maximum peak force (g)
- L :
-
Lightness or whiteness (100 for white to 0 for black)
- L 0 :
-
L at initial osmotic treatment time zero (0)
- M 0 :
-
Initial mass of fresh material (g)
- m 0 :
-
Dry mass of fresh material (g)
- M t :
-
Mass after time t of osmotic treatment (g)
- m t :
-
Dry mass after time t of osmotic treatment (g)
- M ∞ :
-
Mass at equilibrium (g)
- MR :
-
Diffused moisture ratio
- OD:
-
Osmotic dehydration
- R :
-
Universal gas constant (8.314 kJ/mol K)
- RSM:
-
Response surface methodology
- r :
-
Average radius of apricot (m)
- S 0 :
-
Initial solid content of fresh material (g)
- S t :
-
Solid content after time t of osmotic treatment (g)
- S ∞ :
-
Solid content at equilibrium (g)
- SG:
-
Solid gain (g of total solids/g initial dry matter)
- SR :
-
Diffused solid ratio
- T:
-
Osmotic treatment temperature (°C)
- T :
-
Temperature (K) of the Arrhenius equation
- t :
-
Osmotic treatment time (min)
- Xi :
-
Factor variables of the second degree polynomial model
- Y:
-
Response variables of the second degree polynomial model
- WL:
-
Water loss (g of water/g initial dry matter)
- ΔE:
-
Total color change or difference
- ∞ :
-
At equilibrium
- 0:
-
Initial (at time zero)
- t :
-
At time t
References
Ahmed I, Qazi IM, Jamal S (2016) Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov Food Sci Emerg 34:29–43
Amami E, Khezami L, Vorobiev E, Kechaou N (2008) Effect of pulsed electric field and osmotic dehydration pre-treatment on the convective drying of carrot tissue. Dry Technol 26:231–238
Assis FR, Morais RMSC, Morais AMMB (2017) Osmotic dehydration with sorbitol combined with hot air convective drying of apple cubes. J Food Sci Technol 54(10):3152–3160
Chiu M-T, Tham HJ, Lee J-S (2017) Optimization of osmotic dehydration of Terung Asam (Solanum lasiocarpum Dunal). J Food Sci Technol 54(10):3327–3337
da Silva WP, de Farias Aires JE, de Castro DS, da Silva e Silva CMDP (2014) Numerical description of guava osmotic dehydration including shrinkage and variable effective mass diffusivity. LWT Food Sci Technol 59(2–1):859–866
Delgado T, Pereira JA, Ramalhosa E, Casal S (2017) Osmotic dehydration effects on major and minor components of chestnut (Castanea sativa Mill.) slices. J Food Sci Technol 54(9):2694–2703
Dermesonluoglu E, Zachariou I, Andreou V, Taoukis PS (2016) Effect of electric fields on mass transfer and quality of osmotically dehydrated kiwifruit. Food Bioprod Process 100(B):535–544
Ella Missang C, Maingonnat J-F, Renard CMGC, Audergon J-M (2011) Texture variation in apricot fruit: intra-fruit heterogeneity, impact of thinning and relation with the texture after cooking. Food Res Int 44:46–53
Eren İ, Kaymak-Ertekin F (2007) Optimization of osmotic dehydration of potato using response surface methodology. J Food Eng 79:344–352
Forni E, Sormani A, Scalise S, Torreggiani D (1997) The influence of sugar composition on the colour stability of osmodehydrofrozen intermediate moisture apricots. Food Res Int 30:87–94
Fratianni A, Niro S, Messia MC, Cinquanta L, Panfili G, Albanese D, Di Matteo M (2016) Kinetics of carotenoids degradation and furosine formation in dried apricots (Prunus armeniaca L.). Food Res Int 99(2):862–867
Heredia A, Peinado I, Rosa E, Andrés A (2010) Effect of osmotic pre-treatment and microwave heating on lycopene degradation and isomerization in cherry tomato. Food Chem 123:92–98
İspir A, Togrul TI (2009) The influence of application of pre-treatment on the osmotic dehydration of apricots. J Food Process Preserv 33:58–74
Jain SK, Verma RC, Murdia LK, Jain GK, Sharma GP (2011) Optimization of process parameters for osmotic dehydration of papaya cubes. J Food Sci Technol 48(2):211–217
Katsoufi S, Lazou AE, Giannakourou MC, Krokida MK (2017) Mass transfer kinetics and quality attributes of osmo-dehydrated candied pumpkins using nutritious sweeteners. J Food Sci Technol 54(10):3338–3348
Lemus-Mondaca R, Vega-Gálvez A, Zura-Bravo L, Ah-Hen K (2012) Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: a comprehensive review on the biochemical, nutritional and functional aspects. Food Chem 132(3):1121–1132
Li H, Wei B, Wub C, Zhang B, Xu X, Jin Z, Tian Y (2014) Modelling and optimisation of enzymatic extrusion pretreatment of broken rice for rice wine manufacture. Food Chem 150:94–98
Liu H, Chen F, Lai S, Tao J, Yang H, Jiao Z (2017) Effects of calcium treatment and low temperature storage on cell wall polysaccharide nanostructures and quality of postharvest apricot (Prunus armeniaca). Food Chem 225:87–97
Mendonça K, Correa J, Junqueira J, Angelis-Pereira M, Cirillo M (2017) Mass transfer kinetics of the osmotic dehydration of yacon slices with polyols. J Food Process Pres 41:e12983
Mercali GD, Marczak LDF, Tessaro IC, Norena CZ (2011) Evaluation of water, sucrose and NaCl effective diffusivities during osmotic dehydration of banana (Musa sapientum, shum). LWT Food Sci Technol 44(1):82–91
Moon HJ, Jeya M, Kim IW, Lee JK (2010) Biotechnological production of erythritol and its applications. J Appl Microbiol Biotechnol 86:1017–1025
Moreno J, Simpson R, Pizarro N, Parada K, Pinilla N, Reyes JE, Almonacid S (2012) Effect of ohmic heating and vacuum impregnation on the quality and microbial stability of osmotically dehydrated strawberries (cv. Camarosa). J Food Eng 110:310–316
Mune Mune MA, Minka SR (2017) Production and characterization of cowpea protein hydrolysate with optimum nitrogen solubility by enzymatic hydrolysis using pepsin. J Sci Food Agric 97(8):2561–2568
Penicaud C, Achir N, Dhuique-Mayer C, Dornier M, Bohuon P (2011) Degradation of β-carotene during fruit and vegetable processing or storage: reaction mechanisms and kinetic aspects: a review. Fruits 66:417–440
Raj D, Sharma PC, Sharera SK (2015) Studies on osmo-air dehydration of different Indian apricot (Prunus armeniaca L.) cultivars. J Food Sci Technol 52(6):3794–3802
Riva M, Campolongo S, Leva AA, Maestrelli A, Torreggiani D (2005) Structure-property relationships in osmo-air-dehydrated apricot cubes. Food Res Int 38:533–542
Rodriguez A, García MA, Campañone LA (2016) Experimental study of the application of edible coatings in pumpkin sticks submitted to osmotic dehydration. Dry Technol 34(6):635–644
Roopa N, Chaukan OP, Raju PS, Das Gupta DK, Singh RKR, Bawa AS (2014) Process optimization for osmo-dehydrated carambola (Averrhoa carambola L.) slices and its storage studies. J Food Sci Technol 51(10):2472–2480
Salazar-López EI, Jiménez M, Salazar R, Azuara E (2015) Incorporation of microcapsules in pineapple intercellular tissue using osmotic dehydration and microencapsulation method. Food Bioprocess Technol 8(8):1699–1706
Terkmane N, Krea M, Moulai-Mostefa N (2016) Optimisation of inulin extraction from globe artichoke (Cynara cardunculus L. subsp. scolymus (L.) Hegi) by electromagnetic induction heating process. Int J Food Sci Technol 51(9):1997–2008
Thalerngnawachart S, Duangmal K (2016) Influence of humectants on the drying kinetics, water mobility, and moisture sorption isotherm of osmosed air-dried papaya. Dry Technol 34(5):574–583
Toğrul IT, İspir A (2008) Equilibrium distribution coefficients during osmotic dehydration of apricot. Food Bioprod Process 86(4):254–267
Toivonen P, Brummell DA (2008) Biochemical baes of appearance and texture changes, in fresh-cut fruit and vegetables. Postharvest Biol Technol 48(1):1–14
Tsiraki MI, Savvaidis IN (2016) The effects of citrus extract (Citrox©) on the naturally occurring microflora and inoculated pathogens, Bacillus cereus and Salmonella enterica, in a model food system and the traditional Greek yogurt based salad Tzatziki. Food Microbiol 53:150–155
Yadav AK, Singh SV (2014) Osmotic dehydration of fruits and vegetables: a review. J Food Sci Technol 51(9):1654–1673
Yadav BS, Yadav RB, Jatain M (2012) Optimization of osmotic dehydration conditions of peach slices in sucrose solution using response surface methodology. J Food Sci Technol 49(5):547–555
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dermesonlouoglou, E.K., Giannakourou, M.C. Modelling dehydration of apricot in a non-conventional multi-component osmotic solution: effect on mass transfer kinetics and quality characteristics. J Food Sci Technol 55, 4079–4089 (2018). https://doi.org/10.1007/s13197-018-3334-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3334-4