Abstract
The fig (Ficus carica L.) is a fruit native to the Mediterranean region. However, it has spread worldwide. Osmotic dehydration is used to reduce the moisture content in food, preserving its main characteristics. This study aimed to obtain the kinetics of the water loss, solid gain and water activity of green figs that were cut transversely and osmotically dehydrated in different sucrose solutions (40, 50 and 60 °Brix). The osmotic dehydration occurred at 40 °C, with a vacuum pulse of 74 mmHg in the first 5 min of the process in a total time of 240 min. The ratio of sample:solution was kept constant at 1:10 (weight/weight). Periodically, the samples were weighed to calculate the kinetics of water loss, solid gain, and water activity. The increase in the concentration of the sucrose solution from 40 to 60 °Brix promoted a percentage increase of water loss from 8.14 ± 0.80 to 12.80 ± 0.50%, of solid gain (0.89 ± 0.31 to 1.42 ± 0.70%), and the reduction of water activity (0.93 ± 0.02 to 0.91 ± 0.01). The mathematical models of Peleg and Azuara were tested for fitting the kinetics of water loss and solid gain. Both the mathematical models of Peleg and Azuara presented good fitness to the experimental data. However, the latter was more adequate (higher r2 and lower error), with predicted equilibrium conditions closer to the experimental values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fig is a fruit that is consumed both fresh and processed. The drying process is applied to prolong the storability of the figs because they have a short post-harvest life [1]. This is greatly important for nutrition, as it is a source of carbohydrates and vitamins A, B1, B2 and C. Additionally, figs have essential amino acids, aspartic acid and glutamine that are the substances with the highest concentrations similar to other fruits such as oranges [2,3,4].
Osmotic dehydration (OD) is commonly applied as a pretreatment for drying operations. The process involves the immersion of foods in a hypertonic solution for the partial removal of the water due to the pressure difference between the food and the hypertonic solution [5]. The incorporation of solid is also seen, altering the food’s sensory characteristics according to each osmotic agent used [6]. The process can be performed under atmospheric pressure (OD) or with the application of a vacuum pulse in a process called pulsed vacuum osmotic dehydration (PVOD) [7]. During the application of the vacuum pulse, there is an expansion of the pores of plant tissue, promoting the removal of occluded gases. With the resumption of atmospheric pressure, the pores are able to receive solutions with consequently higher mass transfer [8]. This hydrodynamic mechanism is responsible for significant water loss (WL) and solid gain (SG) at the beginning of PVOD due to the pressure gradient generated by the simultaneous action of capillary flow and pressure variations on the subject. The porous structures of plant tissue cause a rapid change in the composition of food with physical consequences in the food matrix [9]. PVOD could minimize browning reactions, nutrient deterioration and the loss of flavor volatile components, as well as reduce the acidity and decrease the structural collapse of the fruit during the drying process [10, 11].
One of the most important aspects of the engineering process is the mathematical modeling, because it reflects the reality of the experimental processes, facilitating its comprehension, and allowing the choice for the most appropriate process for each product taking into account its characteristics [12]. The mathematical models employed to describe the OD process are usually based on Fick’s diffusion law; even if the diffusion equation does not represent several mechanisms of mass transfer inside a food subjected to an osmotic process, it has been very useful to empirically model these processes [5, 13]. Fick’s law is widely used for the analysis and optimization of various theoretical and practical mass transfer processes [14,15,16,17]. The model equations are specific for the certain geometry of the samples as plates, cylinders, and spheres [18]. However, in the model of Azuara et al. [19], the model equation is based on mass balance, with no geometric specification used to estimate mass transfer coefficients and the final equilibrium point during a relatively short period of time.
Peleg [20] proposed a two-parameter sorption equation and tested the prediction accuracy during the water adsorption of milk powder and whole rice and the soaking of whole rice [20]. This equation was used to describe sorption processes in various foods with good adjustment [21, 22]. Therefore, the main objective of this work was to study mass transfer in the pulsed vacuum osmotic dehydration process in green figs (Ficus carica L.) that were cut transversely and osmotically dehydrated in different sucrose solutions, as well as the mathematical models for the prediction of the osmotic dehydration kinetics and physicochemical characterization of vegetables.
2 Material and methods
2.1 Raw material
Green figs (Ficus carica L.) were cultivated in São Sebastião do Paraíso, in the state of Minas Gerais, Brazil. The figs selected were those with uniform maturation characteristics of green peel color.
The compositions of the figs used in the trials were determined according to the Official Methods of Analysis (AOAC, 2007). All data were obtained by triplicate analyses and expressed as the mean ± standard deviation. The carbohydrates were calculated by the difference between 100 and the sum of the percentage of the moisture content, lipids, proteins and minerals.
Analyses of water activity (aw) were carried out with the use of a hygrometer (Aqualab CX-2 T Decagon Devices Inc., Pullman, USA) at 25 °C.
The soluble solids content was measured after fruit maceration in distilled water, filtration and direct reading in a digital refractometer (Hanna Instruments model 96,801, USA).
The color of the samples was measured with an electronic colorimeter (Minolta Camera Co-CR 400, Japan) according to the following standards: L* (lightness), a* (red intensity) and b* (yellow intensity). The parameters were measured using as references the CIE 1964 Standard Observed (10° visual field) and the CIE standard illuminant D65.
2.2 Sample preparation
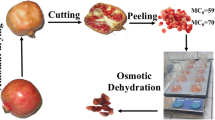
The selected fruits were blanched (97 °C/8 s) and transversely cut with the aid of a stainless steel knife as shown in Fig. 1. The cut aimed to improve mass transfer between the fruit and the osmotic solution.
Blanching inactivates oxidative enzymes, as well as improves color and texture. Furthermore, the process improves water loss and solid gain [9, 23, 24]. Then, the figs were washed in tap water and immersed in a solution of sodium hypochlorite (200 mg L−1 for 15 min) for sanitization [9].
2.3 Pulsed vacuum osmotic dehydration (PVOD)
The equipment used in the osmotic dehydration experiments consisted of a jacketed stainless steel (internal volume of 50 L) chamber designed to work at atmospheric pressure as well as under vacuum and water that was used as the thermal fluid. The temperature and inner pressure were automatically controlled. For the PVOD treatments, the samples were initially weighed and then immersed in a hypertonic solution of sucrose. The osmotic solution was prepared with commercial sucrose at 40, 50 and 60 °Brix. The ratio of the sample to the solution was kept constant at 1:10 (weight/weight) to avoid the dilution of the solution [25]. The experiments were developed for 240 min at 40 °C with the application of the vacuum pulses in the first 5 min under a reduction pressure of 74 mmHg relative to the local atmospheric pressure.
After the PVOD, the samples were removed, rinsed with water and placed on absorbent paper to remove the solution from the surface [26].
The samples were periodically weighed with a semi-analytical scale (Shimadzu AUY220, Kyoto, Japan), and the PVOD was maintained until the samples presented no appreciable variation in weight. The samples were analyzed for water loss (WL) and solid gain (SG) using Eqs. (1) and (2), respectively.
where WL is water loss (kg kg−1), W is sample mass (kg), Xw is the moisture content in the wet basis (kg water kg sample−1). The sub-indice 0 refers to fresh fruit.
where SG is a solid gain (kg kg−1), W is sample mass (kg), S is the solid content (kg of solid per kg of fruit). The sub-indice 0 refers to fresh fruit. The kinetics of reduction of water activity (aw) of the osmotically dehydrated samples were also found. The samples were taken out of the osmotic solution at predetermined times and subjected to aw readings using a hygrometer at 25 °C.
2.4 Mathematical models
Fick’s model might have been used to describe the diffusional osmotic dehydration process. However, diffusion is predominant in a single dimension for characteristic geometries such as spheres, slabs, and cylinders [27]. The geometry of the half fig is almost a semi-sphere but the approximation is not adequate. As a result, the models used in previous work (Azuara and Peleg) were not based on specific geometries [28].
Azuara et al. [19] proposed a model revealing the limitations of Fick’s diffusion model and found good accuracy in the prediction of the mass transport during osmotic dehydration, which can be linearized according to Eq. (3).
where WL is the fraction of water loss, S1 is the model constant related to the rate of water loss, and WL∞ is the fraction of water loss at equilibrium.
The values of S1 and WL can be found from the linearization of Eq. (4).
where WL is the fraction of water loss, S1 is the model constant related to the rate of water loss, WL∞ is the fraction of water loss at equilibrium and t is the time.
Just as Eq. (4) is used for water loss, Eq. (5) is used for solid gain.
where SG is the fraction of soluble solids gained, S2 is a model constant related to the rate of solid gain, SG∞ is the fraction of soluble solids gained at equilibrium and t is the time.
The Peleg model was employed to fit the experimental data. It is an empirical model with two parameters initially established to describe curves that approach equilibrium [13].
where Xw is the moisture content in the wet basis (kg water kg sample−1), k1 and k2 are Peleg model parameters and t is process time. The sub-indice 0 refers to fresh fruit. To determine water loss and solids gain from the model proposed by Peleg, Eqs. (7) and (8) are used [29]:
where Xw is the moisture content in the wet basis (kg water kg sample−1), k1 and k2 are model parameters and t is process time. The sub-indice 0 refers to fresh fruit.
where Xs is the solid content (wet basis), k1 and k2 are model parameters and t is process time. The sub-indice 0 refers to fresh fruit.
The Peleg rate constant k1 and k2 relates to the water loss and solid gain rates, respectively, at the initial time, t = t0 [21], according to Eqs. (9a) and (9b):
where Xw is the moisture content (wet basis), Xs is the solid content (wet basis), k1 and k2 are rate constant of Peleg model.
The constant k1 is obtained by linear regression of the experimental data.
where Xw is the moisture content (wet basis), kw1 is rate constant of Peleg model for water loss. The sub-indices 0 and ∞ refer to fresh fruit and condition equilibrium, respectively.
where Xs is the solid content (wet basis), ks2 is rate constant of Peleg model for solid gain. The sub-indices 0 and ∞ refer to fresh fruit and condition equilibrium, respectively.
These equations were used to describe mass transfer during osmotic dehydration in different foods [21, 29].
The correlation coefficient (R2) and error standard were used for selection of the best mathematical model.
where Xexp,i is the experimental dates (water loss or solid gain), Xpre,i is the predict values (water loss or solid gain) and n is the number of observations.
The evaluations were conducted via the nonlinear estimation procedure using Statistica 5.0® (Statsoft, Tulsa, USA) software.
3 Results and discussion
3.1 Characterization of figs in nature
The results of the chemical composition of the fresh fruits are given in Table 1.
As shown in Table 1, fresh green figs are a product with high moisture content. Additionally, carbohydrates are more prominent than the other solid constituents of the fruit. According to TACO (2011), fresh figs have 88.22% moisture, 10.20 g carbohydrates, 1.00 g protein, 0.20 g lipids, and 0.40 g minerals per 100 g of fresh product.
The physicochemical parameters are shown in Table 2.
The values of water activity (aw), total soluble solids and color parameters indicate that the analyzed figs were in the initial stage of maturation. The total soluble solids were lower than those found in the literature, which reported values from 20.60 to 55.01 °Brix [11, 30]. This difference can be explained by the variety of fig used in addition to the fruit maturation stage. The value of aw is similar to that found in data reported in the literature [31].
Among the color parameters evaluated, the figs had a light near white, due to the high value of L*, with green and yellow tones characteristic of fresh figs, justified by the values of a* and b*.
3.2 Kinetics of PVOD
The results of water loss (WL) and solid gain (SG) kinetics during the process of PVOD, as well as the fitting of the Azuara model, are shown in Figs. 2 and 3, respectively.
The analysis of Figs. 2 and 3 shows that higher WL and SG were found in experiments with more concentrated solutions. This is explained mainly by the large osmotic pressure gradient across the interface of the product and the solution caused by an increase in the concentration of the dehydrating solution. Previous studies found similar results during the PVOD of guavas, figs and pineapple [5, 32, 33]. Furthermore, in the first 120 min, mass transfer rates were higher, as observed in previous works [5, 6, 33]. For studies with solutions of 40 and 50 °Brix, after this time, constant values of WL and SG were observed, indicating that the process tends to equilibrium after this period.
The mass transfer between the product and the dehydrating solution is strictly affected by the nature of the product (species, variety, maturation stage, porosity, shape, and size). Previous works of osmotic dehydration used samples with smaller dimensions than those in the present work. As the process is mainly diffusive, the higher the sample dimensions, the lower the mass transfer [30, 32, 33]. Moreover, the use of vacuum pulses is more prominent in porous samples. Vianna et al. [27] tested PVOD with cladodes and verified that the vacuum was not influent.
The PVOD kinetic curves were fit by the models of Peleg [20] and Azuara et al. [19]. The parameters obtained in fitting these models for WL and SG are presented in Tables 3 and 4.
There is a relationship between the S1 parameter established by the Azuara model and mass transfer rates. The largest values of S1 indicate higher mass transfer rates for WL. Therefore, a relationship between water loss and S1 was established, and the analyzed parameter increased with the increasing concentration of the dehydration solution. However, for a solid gain, the same relationship was not seen. The parameters of the model with mass transfer rates were also studied by Assis et al. [34].
The Peleg model parameters were studied by Corzo et al. [21], who inferred that the higher the values of the constant K, the smaller the transfer rates during the osmotic dehydration process, which was also seen in this study.
The increase in the concentration of the solutions also favored an increase in the equilibrium conditions. These data are consistent with the assumption that the more saturated the solution, the larger the solid gain rates and the more often water loss will occur [21].
The correlation coefficients (R2) found indicate a satisfactory fit with the model applied, in agreement with Corzo et al. [21], who compared the fitting of the Peleg and Azuara models.
Therefore, the fit obtained by the Azuara model was better because it had the highest R2, the lowest values for standard errors (SE), and it better predicted the values of equilibrium conditions. Such behaviors were observed for WL and SG.
Figure 3 demonstrates the water activity kinetics during the process of PVOD.
It was found (Fig. 4) that the reduction in water activity was directly related to the concentration of the osmotic solution. An increase in the concentration of the solution, as well as promoting greater water loss, also led to a greater incorporation of the solute by the product. As a consequence of both phenomena, the water activity was reduced [34]. Figure 4 also demonstrates that the greatest reduction in water activity occurs in the first 120 min, with stable equilibrium reached.
Water activity is an intrinsic factor in food. It is related to the available water in the food. Representing the free moisture. This free water enables microbial growth and accelerates biochemical reactions affecting the quality of the products. Thus, the reduction of aw becomes indispensable for the better conservation of foods [35].
4 Conclusions
High water loss and solid gain values were observed in the first part of the PVOD process. Furthermore, treatment with higher concentrations of osmotic solutions showed higher values of WL and SG and higher reductions in water activity.
Primarily due to the high moisture content and consumer preference for fresh figs, osmotic dehydration using vacuum pulses proved to be effective when applied to transversely cut figs. It may be a product conservation alternative that is still more effective when applied as a pretreatment to conventional drying. Moreover, it was possible to observe the effect of the concentration of the osmotic solution in relation to water loss, solid gain and the reduction of water activity.
The two models studied presented good fitness to the experimental data. However, the values predicted by the model for the Azuara equilibrium conditions were more consistent with the experimental values than the values found with the adjustments to the Peleg model.
In Brazil, green fig is generally industrialized in the preparation of sweet syrup, with high solid content (usually sucrose). Thus, the PVOD process of green fig provides the initial incorporation of solutes, in this case, sucrose, similarly to what occurs in the industrial process. Furthermore, the samples have greater stability after the application of PVOD due to water loss, increasing the quality of the samples and consequently of the future co-products.
References
Şahin U, Öztürk HK (2016) Effects of pulsed vacuum osmotic dehydration (PVOD) on drying kinetics of figs (Ficus carica L). Innovative Food Sci Emerg Technol 36:104–111
Solomon A, Golbowicz S, Yablowicz Z, Grossman S, Bergaman M, Gottlieb H (2006) Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J Agric Food Chem 50:7717–7723
Verbric R, Colaric M, Stampar F (2008) Phenolic acids and flavonoids of fig fruit (Ficus carica L .) in the northern Mediterranean region. Food Chem 106(1):153–157
Roussos PA (2011) Phytochemicals and antioxidant capacity of orange (Citrus sinensis (L.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in Greece. Sci Hortic 129(2):253–258
Silva MAC, Silva ZE, Mariani VC (2012) Mass transfer during the osmotic dehydration of west Indian cherry. Int J Food Sci Technol 45(2):246–252
Chauan OP, Singh A, Raju PS, Bawa AS (2011) Effects of osmotic agents on color, textural, structural, thermal, and sensory properties of apple slices. Int J Food Prop 14:1037–1148
Corrêa JLG, Pereira LM, Vieira GS, Hubinger MD (2010) Mass transfer kinetics of pulsed vacuum osmotic dehydration of guavas. Int J Food Eng 6(4):498–504
Fito P (1994) Modelling of vacuum osmotic dehydration of food. Int J Food Eng 22(1–4):313–328
Corrêa JLG, Ernesto DB, José GLF, Andrade RS (2014) Optimisation of vacuum pulsed osmotic dehydration of blanched pumpkin. Int J Food Sci Technol 49:2008–2014
Garcia CC, Mauro MA, Kimura M (2007) Kinetics of osmotic dehydration and air-drying of pumpkins (Cucurbita moschata). Int J Food Eng 82:284–291
Naikwadi PM, Chavan UD, Pawar VD, Amarowicz R (2010) Studies on dehydration of figs using different sugar syrup treatments. Int J Food Sci Technol 47(4):442–445
Szadzińska J, Kowalski SJ, Stasiak M (2016) Microwave and ultrasound enhancement of convective drying of strawberries: experimental and modeling efficiency. Int J Heat Mass Transf 103:1065–1074
Corzo O, Bracho N, Rodríguez J (2012) Comparison of Peleg and Azuara et al. models in the modeling mass transfer during pile salting of goat sheets. Int J Food Sci Technol 46(2):448–452
Zhao W, Cheng Y, Jiang H, Wang H, Li W (2017) Modeling and experiments for transient diffusion coefficients in the desorption of methane through coal powders. Int J Heat Mass Transf 110:845–854
Wang C, Xu R, Song Y, Jiang P (2017) Study on water droplet flash evaporation in vacuum spray cooling. Int J Heat Mass Transf 112:279–288
Ilie F (2018) Diffusion and mass transfer mechanisms during frictional selective transfer. Int J Heat Mass Transf 116:1260–1265
Chen L, Xia S, Sun F (2018) Entropy generation minimization for isothermal crystallization processes with a generalized mass diffusion law. Int J Heat Mass Transf 116:1–8
El-Aquar AA, Murr FEX (2003) Estudo e modelagem da cinética de desidratação osmótica do mamão formosa (Carica papaya L.). Cienc Tecnol Aliment 23(1):69–75
Azuara E, Cortés R, Garcia HS, Beristain CI (1992) Kinetic model for osmotic dehydration and its relationship with Fick’s second law. Int J Food Sci Technol 27(4):409–418
Peleg M (1993) Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. J Food Process Eng 16(1):21–37
Corzo O, Bracho N (2006) Application of Peleg model to study mass transfer during osmotic dehydration of sardine sheets. J Food Eng 75:533–541
Atarés L, Chiralt A, Gonzáles-Martínez C (2008) Effect of solute on osmotic dehydration and rehydration of vacuum impregnated apple cylinders (cv. Granny Smith). J Food Eng 89:49–56
Gonçalves EM, Pinheiro J, Abreu M, Brandão TRS, Silva CLM (2007) Modelling the kinetics of peroxidase inactivation, colour and texture changes of pumpkin (Cucurbita maxima L.) during blanching. J Food Eng 81:693–701
Silva KS, Caetano LC, Garcia CC, Romero JT, Santos AB, Mauro MA (2011) Osmotic dehydration process for low temperature blanched pumpkin. J Food Eng 105:56–64
Viana AD, Corrêa JLG, Justus A (2014) Optimisation of the pulsed vacuum osmotic dehydration of cladodes of fodder palm. Int J Food Sci Technol 49:726–732
Corrêa JLG, Justus A, Oliveira LF, Alves JG (2015) Osmotic dehydration of tomato assisted by ultrasound, evaluation of the liquid media on mass transfer and product quality. Int J Food Eng 11(4):2008–2014
Crank J (1995) The mathematics of diffusion. In: 2nd. Oxford University Press, New York
Assis FR, Morais RMSC, Morais AMMB (2016) Mass transfer in osmotic dehydration of food products: comparison between mathematical models. Food Eng Rev 8(2):116–133
Khin MM, Zhou W, Perera CO (2006) A study of the mass transfer in osmotic dehydration of coated potato cubes. J Food Eng 77:84–95
Silva JM, Cantu MG, Rodrigues V, Mazutti MA (2013) Influence of osmotic pre-treatment on convective drying kinetics of figs. Int J Food Eng 9(2):187–196
Piga A, Pinna I, Ozer KB, Agabbio M, Aksoy U (2004) Hot air dehydration of figs (Ficus carica L.): drying kinetics and quality loss. Int J Food Sci Technol 39:793–799
Arreola SI, Rosas ME (2007) Aplicación de vacío en la deshidratación osmótica de higos (Ficus carica). Inf Tecnol 18(2):43–48.32
Vasconcelos JIL, Andrade SAC, Maciel MIS, Guerra NB, Vasconcelos MAS (2012) Osmotic dehydration of the Indian fig (Opuntia ficus indica) with binary and ternary solutions. Int J Food Sci Technol 47(11):2359–2365
Kotovicz V, Ellendersen LSN, Clarinso MM, Masson ML (2014) Influence of process conditions on the kinetics of the osmotic dehydration of yacon (Polymnia sonchifolia) in fructose solution. J Food Process Preserv 38:1385–1397
Fu N, Chen XD (2011) Towards a maximal cell survival in convective thermal drying processes. Food Res Int 44(5):1127–1149
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG). Thanks also to the Department of Agricultural Development of São Sebastião do Paraíso and the Tozzi Food Industry for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Mello Jr, R., Corrêa, J.L.G., Lopes, F.J. et al. Kinetics of the pulsed vacuum osmotic dehydration of green fig (Ficus carica L.). Heat Mass Transfer 55, 1685–1691 (2019). https://doi.org/10.1007/s00231-018-02559-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-018-02559-w