Abstract
This study was performed in order to evaluate efficiency of near-infrared reflectance spectroscopy (NIRS) for the determination of fatty acid composition ratio of sunflower seeds and to compare performance of calibration methods. Calibration equations were developed using modified partial least squares (MPLS) and partial least squares (PLS) regression methods. Ninety-three sunflower seed varieties were from test field of East Mediterranean Agricultural Research Institute. In order to determine the reference fatty acid values needed to construct calibration in NIRS analysis, sunflower seed samples were analyzed by gas chromatography method. Coefficients of determination (R2) in calibration were developed using MPLS and PLS as follows: for palmitic acid 0.706–0.664, for stearic acid 0.615–0.654, for oleic acid 0.996–0.994, for linoleic acid 0.995–0.994, for arachidic acid 0.768–0.643, for linolenic acid 0.818–0.763, for behenic acid 0.891–0.776, for eicosapentaenoic 0.933–0.892, for unsaturated fatty acid 0.837–0.890 and for saturated fatty acid 0.837–0.890 respectively. The results showed that NIRS was a reliable technique that can be used as a tool for rapid pre-screening of fatty acid composition of sunflower seeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sunflower (Helianthus annuus L.) is considered to be one of the most important oil plants having 22–55% oil content (Flagella et al. 2002; Gonzalez-Martin et al. 2013) with an agricultural history dating back to 3000 B.C and cultivation in a large area in the world. Sunflower oil contains approximately 15% saturated, 85% unsaturated fatty acid and consisting of 14–43% oleic and 44–75% linoleic acids in its unsaturated fatty acid content. Standard type sunflower oil is one of the most important vegetable oil in terms of oil composition and among the most important oils in human nutrition. In recent years, high quality sunflower oil has been produced with a range of composition via the development of mid-oleic type (43.1–71.8%) and high-oleic type (75–90.7%) sunflower varieties that has higher oleic acid content than standard sunflower type (Flagella et al. 2002).
For the proper utilization of sunflower oils in food and other industries, oil, moisture and protein contents, fatty acid compositions and quality characteristics of sunflowers should be quickly and reliably evaluated by analytical tools at the harvest, marketing and processing (Biskupek-Korell and Moschner 2006).
The development of fast and effective methods becomes a necessity when the applicational needs are taken into consideration. In recent years, there is a growing interest in fast, reliable and environmentally friendly technologies both in food production and food research. Consequently alternative technologies such as NIR spectroscopy (Cen and He 2007) are being developed. This technology is based on the measurement of absorption of electromagnetic radiation in 400–2500 nm wavelength range (Davies and Granth 1987).
NIR spectroscopy, based on the resolution of the analytical and quality factors from food samples with correlation of electromagnetic absorption at aforementioned wavelength, is used routinely in sensory, physical and chemical analysis of food and agricultural products (Williams 2001; Izneid et al. 2014; Madalozzo et al. 2015; Srikham and Noomhorm 2015). For this purpose, studies were conducted to determine crude fat content of the oil plants and their fatty acid compositions by NIR spectroscopy (Fassio and Cozzolino 2004; Velasco et al. 2004; Biskupek-Korell and Moschner 2006; Koprna et al. 2006).
NIRS technology has been used successfully in the analysis of fatty acid composition of sunflower seeds to assess the content of palmitic acid, stearic acid, oleic acid and linoleic acid (Velasco et al. 2004), the content of oleic and linoleic acids (Biskupek-Korell and Moschner 2006), discriminant analysis of sunflower seeds for fatty acids (Grunvald et al. 2012), the content of moisture, fat, oleic acid (Gonzalez-Martin et al. 2013) and the content of palmitic, palmitoleic, stearic, oleic and linoleic acids (Perez-Vich et al. 1998).
It is necessary to determine fatty acid composition in sunflower seeds which have an important value in national production, importation as well as in food processing and breeding programs, by fast and reliable methods. Since analyses based on NIR spectroscopy do not require labour intensive sample pre-treatment and processing, samples are analysed with simple grinding or as a whole. This study was performed in order to evaluate efficiency of NIR Spectroscopy in determination of fatty acid composition ratio consisting unsaturated (UFA) and saturated fatty acid (SFA) fractions in sunflower seeds.
Materials and methods
Materials
In this study, 93 different sunflower varieties, harvested between 2015 and 2016, (35 low-oleic varieties, 52 mid-oleic varieties and 6 high-oleic varieties) in which reclamation and adaptation studies were performed in East Mediterranean Agronomic Institute testing ground were used as experimental materials.
Oil extraction of samples
Prior to oil analysis sunflower seeds were cleaned, dried in oven (40 °C for 6 h) (Fassio and Cozzolino 2004) until moisture contents fall below 10% and 50 g of samples ground in a waring blender (Waring Commercial, USA) up to 1 mm diameter.
For crude oil analysis, 5 g sunflower seed samples were weighed after grinding and analysed by Soxhlet extraction device (Gerhardt Analytical Systems, Germany) using 150 mL petroleum ether as solvent for 3 h (ISO 2009).
Gas chromatography analysis of fatty acids
The extracted crude oils were then used for gas chromatography (GC) analysis after the esterification. 0.5 g oil was transferred into 10 mL-capacity glass tube. Five millilitres n-heptane was added into the tube. 200 µL 2 M potassium hydroxide solution in methanol was added to this mixture. After mixing for 20 s, upper phase was separated and analysed by GC (TFCC 2010). The GC was equipped with a capillary column (Fused silica, 100 m × 0.25 mm × 0.2 µm) and a FID detector (Agilent 7890A, Agilent Technologies, USA). The GC conditions used to determine fatty acid methyl ester (FAME) were as follows (TFCC 2010); injection volume: 1µL; temperature programme: 175 °C for 10 min, 5 °C min−1 to 210 °C, 5 °C min−1 to 230 °C; final temperature 230 °C for 15 min; detector temperature: 260 °C; injector temperature: 250 °C; gas carrier flow: N2, 1 mL min−1; split: 1:20; total run time: 58.5 min.
Spectral analysis and curve calibration
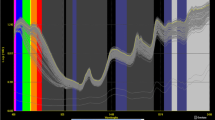
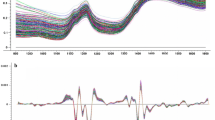
XDS near-infrared Rapid Content Analyser (FOSS NIRSystem, Denmark) apparatus was used to receive spectrums and to determine estimated values of the spectrum of sunflower seed samples which classical analysis were completed. Spectra of ground sunflower seed samples were taken to be every 2 nm in between 400 and 2500 nm wavelength. Calibration equations were constructed using WinISI III v1.61 (Infrasoft International) programme. This programme uses original spectra either directly of after corrections to provide optimal pre-treatment for each parameter evaluated and for the instrument. Applied corrections are 1st and 2nd derivative transformation and standard normal variate and detrend scatter correction (SNV/detrend). Modified partial least squares (MPLS) (Shenk and Westerhaus 1993) and partial least squares (PLS) (Blanco and Villarroya 2002) methods were used to construct calibration equations. The best fitting mathematical model was obtained by using several mathematical models in order to correlate the results of the reference analytical methods to the results obtained by NIR spectroscopy. Calibration statistics include the standard error of calibration (SEC) and the coefficient of determination in calibration (\( {\text{R}}_{\text{cal}}^{2} \)). These statistics were used to develop calibration model. Standard error of prediction (SEP), slope, bias and the coefficient of determination in prediction (R2) were used to determine precision of the validation.
Results and discussion
Calibration statistics using MPLS and PLS regression methods used for the prediction of fatty acid compositions of sunflower seeds are summarized in Table 1. \( {\text{R}}_{\text{cal}}^{2} \) values obtained by MLPS regression method were 0.706, 0.615, 0.996, 0.995, 0.768, 0.818 and 0.891 for palmitic, stearic, oleic, linoleic, arachidic, linolenic, behenic acids, respectively. Whereas \( {\text{R}}_{\text{cal}}^{2} \) value for eicosapentaenoic (EPA) was calculated as 0.933, it was 0.837 for both UFA and SFA.
As inferred from Table 1, PLS method however yielded \( {\text{R}}_{\text{cal}}^{2} \) values of 0.664, 0.654, 0.994, 0.994, 0.643, 0.763 and 0.776 for palmitic, stearic, oleic, linoleic, arachidic, linolenic and behenic acids, respectively. Regression coefficients of calibration using PLS regression were 0.892 for EPA and 0.890 for both UFA and SFA.
Regression coefficients of calibration equations obtained by MPLS method for palmitic, oleic, linoleic, arachidic, linolenic, behenic acids and EPA were found to be greater than those obtained by PLS method. However, \( {\text{R}}_{\text{cal}}^{2} \) values for stearic acid, UFA and SFA were greater in PLS method.
Standard errors of calibration equations obtained by MPLS method were 0.39, 0.43, 1.04, 1.03, 0.018, 0.016, 0.035 and 0.012 for palmitic, stearic, oleic, linoleic, arachidic, linolenic, behenic acids and EPA, respectively. SEC value of UFA and SFA was found as 0.47. Calibration equations obtained by PLS method had standard errors of 0.42, 0.41, 1.21, 1.18, 0.023, 0.018, 0.051 and 0.016 for palmitic, stearic, oleic, linoleic, arachidic, linolenic, behenic acids and EPA, respectively. SEC value for UFA and SFA was 0.38 in this case.
Perez-Vich et al. (1998) found regression coefficient of calibration equations obtained by MLPS method as 0.86, 0.92, 0.86 and 0.85 in their study on the determination of palmitic, stearic, oleic and linoleic acid contents of sunflower intact-seed samples using NIRS. \( {\text{R}}_{\text{cal}}^{2} \) values found by MPLS method for oleic and linoleic acids in this study are higher and regression coefficients for palmitic and stearic acids are lower than those reported by Perez-Vich et al. (1998). SEC values calculated in this study are considerably lower than SEC values calculated by Perez-Vich et al. (1998).
Velasco et al. (2004) used NIRS to determine palmitic, stearic, oleic and linoleic acid contents of sunflowers. Although they reported R2 values as 0.52, 0.80, 0.89 and 0.91 for palmitic, stearic, oleic and linoleic acids, respectively, the regression model employed in their study was not mentioned. Nevertheless R2 values calculated in this study using both MPLS and PLS regression models for palmitic, oleic and linoleic acids were greater than those reported by Velasco et al. (2004). However, R2 value for stearic acid found in this study was lower than that calculated by Velasco et al. (2004) yet acceptable.
Gonzalez-Martin et al. (2013) found R2 value for calibration equation obtained by multiple linear regression (MLR) for oleic acid as 0.999. R2 values obtained by both MPLS and PLS methods in this study were similar to that reported by Gonzalez-Martin et al. (2013).
Table 2 shows validation statistics of calibration equations obtained using MPLS regression method in the determination of fatty acid composition of sunflower seeds. The ranges of predicted percentages of each fatty acid were as follows: 4.26–6.82% for palmitic acid, 2.08–5.01% for stearic acid, 16.97–87.68% for oleic acid, 3.44–68.45% for linoleic acid, 0.155–0.330% for arachidic acid, 0.112–0.271% for linolenic acid, 0.489–0.965% for behenic acid, 0.163–0.375% for EPA, 87.65–92.17% for UFA and 7.82–12.35% for SFA. The mean fatty acid contents were predicted as 5.79, 3.19, 48.11, 40.12, 0.254, 0.179, 0.703, 0.266, 90.03 and 9.97% for palmitic, stearic, oleic, linoleic, arachidic, linolenic, behenic acids, EPA, UFA and SFA, respectively.
Table 2 also shows R2 and SEP values for each fatty acid. As it can be inferred from Table 2, R2 values were 0.722, 0.632, 0.996, 0.996, 0.788, 0.839, 0.904, 0.940, 0.854 and 0.854 for palmitic, stearic, oleic, linoleic, arachidic, linolenic, behenic acids, EPA, UFA and SFA, respectively. SEP values were calculated as 0.373, 0.413, 0.972, 0.959, 0.017, 0.015, 0.033, 0.011, 0.438 and 0.438 for palmitic, stearic, oleic, linoleic, arachidic, linolenic, behenic acids, EPA, UFA and SFA, respectively.
Perez-Vich et al. (1998) reported R2 values as 0.82, 0.85, 0.76 and 0.78 for palmitic, stearic, oleic and linoleic acids, respectively in their study on the validation of calibrations obtained by MPLS regression method for sunflower intact-seed samples. In this study R2 values for oleic and linoleic acids were greater than those reported by Perez-Vich et al. (1998) whereas R2 values for palmitic and stearic acids calculated in this study were lower than those reported in Perez-Vich et al. (1998). SEP values calculated in this study were lower than those reported by Perez-Vich and his colleagues.
Biskupek-Korell and Moschner (2006) also used MPLS regression methodology to construct calibration equations in their validation study. R2 values of calibration equations were 0.96 and 0.98 for oleic and linoleic acids, respectively. R2 values found in our study were higher and SEP values were lower than those reported by Biskupek-Korell and Moschner (2006).
Validation statistics for the calibration equations obtained using PLS regression methodology in order to predict fatty acid composition of sunflower seeds are shown in Table 3. The ranges of each fatty acid were found to be as follows 4.20–6.86, 2.06–4.90, 17.52–87.54, 3.66–67.90, 0.166–0.333, 0.109–0.262, 0.517–0.935, 0.152–0.385, 87.13–92.53 and 7.47–12.87% for palmitic, stearic, oleic, linoleic, arachidic, linolenic, behenic acids, EPA, UFA and SFA, respectively. The mean percentages of these fatty acids in the above stated order were 5.80, 3.20, 48.46, 39.50, 0.255, 0.179, 0.703, 0.265, 90.05 and 9.96%. Table 3 also shows R2 and SEP values of calibration equations obtained by PLS regression. R2 values were found as 0.688, 0.684, 0.995, 0.994, 0.674, 0.784, 0.796, 0.903, 0.903 and 0.903 whereas SEP values were calculated as 0.387, 0.387, 1.142, 1.132, 0.022, 0.017, 0.048, 0.015, 0.358 and 0.358 for palmitic, stearic, oleic, linoleic, arachidic, linolenic, behenic acids, EPA, UFA and SFA, respectively.
Velasco et al. (1999) found R2 values as 0.88 for both oleic and linoleic acids in their validation study in which they compared NIRS and GC. We found that NIRS and GC comparison, R2 values determined by using PLS and MPLS regression methods were greater than those reported by Velasco et al. (1999). In the study of Velasco et al. (2004) the regression methodology used has not been indicated, R2 values were found to be 0.83, 0.92 and 0.93 for stearic, oleic and linoleic acid, respectively. In our study comparing GC and NIRS, R2 values found for oleic and linoleic acids by both regression methodologies were greater than those reported by Velasco et al. (2004). However, R2 values calculated for stearic acid were lower than R2 value reported in the study of Velasco et al. (2004). SEP values calculated in their study were very low for all fatty acids. This might be due to the higher number of samples analysed.
Gonzalez-Martin et al. (2013) reported R2 values as 0.987 for oleic acid in their study in which they compared the reference values and values obtained by NIR calibration using MLR regression method. R2 values calculated in this study are similar to R2 values reported by Gonzalez-Martin et al. (2013).
The comparison of NIRS predicted fatty acid composition ratio and fatty acid composition ratio obtained by GC analysis (reference values) is shown in Figs. 1 and 2. Since the calibration coefficients (R2 = RSQ) were higher, tangents of the calibration lines were equal or closer to 1. When the standard error of prediction (SEP) is close to zero and R2 is close to 1, it means the calibration model is the most suitable one. This shows that predicted values are closely correlated with real values.
Conclusion
This study demonstrated that NIRS can be reliably used to determine fatty acid composition of sunflower seeds. Higher R2 values were found by the MPLS regression method than PLS regression method. In addition, it showed that NIRS analysis can be fast and effective method in both vegetable oil industry and sunflower seed trade and marketing.
References
Biskupek-Korell B, Moschner CR (2006) Near-infrared spectroscopy (NIRS) for quality assurance in breeding, cultivation and marketing of high-oleic sunflowers. Helia 29(45):73–80
Blanco M, Villarroya I (2002) NIR spectroscopy: a rapid response analytical tool. Trends Anal Chem 21:240–250
Cen H, He Y (2007) Theory and application of near infrared reflectance spectroscopy in determination of food quality. Trends Food Sci Technol 18(2):72–83
Davies AMC, Granth A (1987) Near-infrared analyses of foods. Int J Food Sci Technol 22:191–207
Fassio A, Cozzolino D (2004) Non-destructive prediction of chemical composition in sunflower seeds by near infrared spectroscopy. Ind Crops Prod 20:321–329
Flagella Z, Rotunno T, Tarantino E, Di Caterina R, De Caro A (2002) Changes in seed yield and oil fatty acid composition of high oleic sunflower (Helianthus annuus L.) hybrids in relation to the sowing date and the water regime. Eur J Agron 17:221–230
Gonzalez-Martin I, Villaescusa-Garcia V, Lopez-González F, Oiz-Jiménez C, Lobos-Ortega IA, Gordillo AB, Hernández-Hierro JM (2013) Control of quality and silo storage of sunflower seeds using near infrared technology. Grasas Aceites 64(1):30–35
Grunvald AK, Carvalho CGP, Leite RS, Mandarino JMG, Gonçalves JL, Andrade CAB, Scapim CA (2012) Discriminant analysis of sunflower seeds for fatty acid composition using NIR spectroscopy. J Am Oil Chem Soc 89:995–1001
ISO (2009) Oilseeds—determination of oil content (Reference method). International Organization for Standardization ISO 659: 2009, Geneva
Izneid BA, Fadhel MI, Al-kharazi T, Ali M, Miloud S (2014) Design and develop a nondestructive infrared spectroscopy instrument for assessment of mango (Mangifera indica) quality. J Food Sci Technol 51(11):3244–3252
Koprna R, Nerušıl P, Kolovrat O, Kučera V, Kohoutek A (2006) Estimation of fatty acid content in intact seeds of oilseed rape (Brassica napus L.) lines using near-infrared spectroscopy. Czech J Genet Plant Breed 42(4):132–136
Madalozzo ES, Sauer E, Nagata N (2015) Determination of fat, protein and moisture in ricotta cheese by near infrared spectroscopy and multivariate calibration. J Food Sci Technol 52(3):1649–1655
Perez-Vich B, Velasco L, Fernandez-Martinez JM (1998) Determination of seed oil content and fatty acid composition in sunflower through the analysis of intact seeds, husked seeds, meal and oil by near infrared reflectance spectroscopy. J Am Oil Chem Soc 75:547–555
Shenk JS, Westerhaus MO (1993) Analysis of agriculture and food products by near infrared reflectance spectroscopy. Infrasoft International Port, Matilda, PA, USA
Srikham W, Noomhorm A (2015) Milling quality assessment of Khao Dok Mali 105 milled rice by near-infrared reflectance spectroscopy technique. J Food Sci Technol 52(11):7500–7506
TFCC No: 2010/36, Turkish Food Codex Communique on analysis methods of olive oil and olive-pomace oil notification, Turkish Food Codex Legislation, Official Newspaper 07.08.2010/27665
Velasco L, Perez-Vich B, Fernandez-Martinez JM (1999) Non-destructive screening for oleic and linoleic acid in single sunflower achenes by near infrared reflectance spectroscopy. Crop Sci 39:219–222
Velasco L, Perez-Vich B, Fernandez-Martinez JM (2004) Use of near-infrared reflectance spectroscopy for selecting for high stearic acid concentration in single husked achenes of sunflower. Crop Sci 44:93–97
Williams PC (2001) Implementation of near-infrared technology. In: Williams PC, Norris KH (eds) Near-infrared technology in the agriculture and food industries, 2nd edn. AACC Inc, St Paul, Minnesota, pp 145–170
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Akkaya, M.R. Prediction of fatty acid composition of sunflower seeds by near-infrared reflectance spectroscopy. J Food Sci Technol 55, 2318–2325 (2018). https://doi.org/10.1007/s13197-018-3150-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3150-x