Abstract
Effects of virgin coconut oil (VCO) at various levels (0–25%) on the properties of croaker surimi gels were studied. As the levels of VCO increased up to 15%, breaking force continuously decreased. No differences in breaking force, deformation and fracture constant were noticeable when VCO of 15–25% was incorporated. Based on texture profile analysis, hardness and chewiness decreased as the level of added VCO increased up to 10%, while no marked changes were observed with the addition of 10–25% VCO. Addition of VCO had no profound impact on springiness, cohesiveness and resilience. No remarkable change in protein pattern among all surimi gel samples was noticed, regardless of VCO levels. Lower elastic (G′) as well as loss moduli (G″) of surimi paste were observed when VCO was added, compared to the control. Nevertheless, there was no marked difference in the moduli among samples containing VCO at all levels. Whiteness of surimi gel increased, whereas expressible moisture content decreased as VCO levels increased. Microstructure study revealed that VCO droplets were distributed uniformly in gel network. Overall likeness of surimi gel was also increased for gel added with VCO. Therefore, VCO addition directly affected textural properties and improved the whiteness as well as sensory property of surimi gel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surimi is a concentrated myofibrillar proteins obtained by washing fish mince with water. Surimi is the functional ingredient for various surimi-based seafood products. The increasing popularity of surimi-based products is due to its unique textural properties and high nutritive value. Owing to the health concerns associated with animal fat, which possesses high saturated acid and cholesterol contents, most consumers prefer food products containing no animal fat. Thus, different vegetable oils such as peanut, rapeseed, soybean, and corn oils have been incorporated in surimi-based products to modify gel properties (Shi et al. 2014). Also the vegetable oil supplemented surimi products have the increased whiteness caused by light scattering effect of oil droplets in the matrix (Benjakul et al. 2004; Hsu and Chiang 2002). Pietrowski et al. (2011) reported that supplementation of surimi with flaxseed, algae, menhaden, krill oils and blend resulted in the improved nutritional value and colour without affecting the texture. ‘Fish tofu’ is the emulsified surimi gel, which is subsequently fried to obtain the yellow husk/surface. Gel is very white in color and has the smooth and soft texture associated with oil droplets distributed throughout the gel. This product has been popular in Thailand and other countries in Asia.
Virgin coconut oil (VCO) is a rich source of medium-chain fatty acids (MCFAs), notably lauric acid (Marina et al. 2009), which are also found in human breast milk and are easily digestible. Lauric acid has antiviral, antibacterial, anticaries, antiplaque and antiprotozoal functions (German and Dillard 2004). Trilaurin and tripalmitin fractions of coconut oil were reported to inhibit the promotion stage of carcinogenesis (Nolasco et al. 1994). Short chain fatty acids such as capric, caproic and caprylic acids, which are present in considerable amounts in VCO also had antimicrobial and antiviral effects (Bergsson et al. 1998; German and Dillard 2004). In general, VCO finds its application in medicines, baby foods and has been known as weight loss aid. Functional food industry is growing at a faster pace and the consumer demand for health foods has been increasing. Due to its health benefits, the incorporation of VCO instead of other vegetable oils could be a means to prepare a functional surimi gels. However, no information regarding the impact of VCO on the properties of surimi gel exists. The present work was undertaken to incorporate VCO at different levels into surimi gels. Properties of resulting surimi gels were examined.
Materials and methods
Materials and chemicals
Frozen croaker surimi (AA grade) and virgin coconut oil (VCO) were procured from Pacific Fish Processing Co., Ltd. (Songkhla, Thailand) and Posture Trading, Ltd (Pathumthani, Thailand) respectively. Sodium dodecyl sulfate (SDS), Coomassie Blue R-250, N,N,N′,N′-tetramethyl ethylene diamine (TEMED) and all chemicals for electrophoresis were purchased from Bio-Rad Laboratories (Hercules, CA, USA).
Preparation of surimi gels
After thawing for 3–4 h using a running water, surimi with the temperature of 0–2 °C was cut into small pieces and minced in Moulinex Masterchef 350 mixer (Paris, France) for 1 min. Salt (2.5% w/w) dissolved in iced water was added to minced surimi. The mixture was blended for 1 min and the moisture content of paste was adjusted to 80%. Thereafter, VCO at various levels (5, 10, 15, 20 and 25%) was added into surimi paste, followed by mixing for 1 min. The pastes were then stuffed in polyvinylidine casing with a diameter of 2.5 cm and sealed at both ends. The gels were set at 40 °C for 30 min, followed by heating at 90 °C for 20 min in a temperature controlled water baths (Memmert, Schwabach, Germany). All gels were then cooled in iced water. Subsequently gels were stored at 4 °C for 24 h prior to analysis.
Analysis
Textural properties
A penetration test was performed using a Model TA-XT2i texture analyzer (Stable Micro Systems, Surrey, England) equipped with a spherical plunger (diameter of 5 mm, depression speed of 60 mm/min). Cylinder-shaped gel samples of 2.5 cm in length were equilibrated to room temperature (25 °C) for 1 h before testing. Breaking force and deformation were determined (Arfat and Benjakul 2013). The breaking force was defined as the force required in grams to break the gel, and the breaking deformation represented the distance in millimeters travelled by probe from surface of the gel at the point of breakage. Fracture constant (Kf) was calculated as the ratio of breaking force/deformation. Kf provided a measure of relative rigidity or firmness of gels at the failure point (Herranz et al. 2012).
Texture profile analysis (TPA) of the gels was carried out according to Cheret et al. (2005). Cylindrical gels (height of 2.50 cm, diameter of 2.5 cm) were used for the TPA measurement. Gel samples were subjected to two-cycle compression at 50% compression using the texture analyzer with a 70-mm TPA compression plate attachment moving at a speed of 127 mm/min. From the resulting force–time curves, hardness, springiness, cohesiveness, chewiness and resilience were determined.
Expressible moisture content
Expressible moisture content was estimated by the method of Benjakul et al. (2001) with some modifications. A cylindrical gel sample with a thickness of 0.5 cm was weighed (X g) and sandwiched between two Whatman filter papers No. 1 (Whatman International Ltd., Maidstone, England) at the top and three pieces of the same type of filter paper at the bottom. The sample was pressed by a standard weight of 5 kg for 2 min and weighed again (Y g). Expressible moisture content was expressed as percentage of sample weight as follows:
Whiteness
Whiteness of surimi gels was measured using Hunterlab (ColorFlex, Hunter Associates Laboratory, Reston, VA). L* (lightness), a* (redness/greenness) and b* (yellowness/blueness) were firstly determined. Whiteness was then calculated as described by NFI (1991) as follows:
Protein patterns
Protein patterns of the surimi gels were determined by Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli (1970). The protein sample was prepared by adding 3 g of sample to 27 mL of 5% (w/v) SDS solution heated at 85 °C. The mixture was then homogenized for 2 min at a speed of 11,000 rpm using a homogenizer (IKA Labortechnik, Selangor, Malaysia). The homogenate was incubated at 85 °C for 1 h. The samples were centrifuged at 8000×g for 20 min at room temperature (26–28 °C) using a centrifuge (Model MIKRO20, Hettich ZENTRIFUGEN, Tuttlingen, Germany). Protein concentration in the supernatant was determined as per the method of Lowry et al. (1951). Solubilized samples were mixed with the sample buffer (0.5 M Tris–HCl, pH 6.8, containing 4% SDS, 20% glycerol and 10% β-ME) at a ratio of 1:1 (v/v) and boiled for 3 min. Samples (15 µg protein) were loaded onto polyacrylamide gels comprising a 10% running gel and a 4% stacking gel and subjected to electrophoresis at a constant current of 15 mA/gel using a Mini Protein III unit (Bio-Rad Laboratories, Inc., Richmond, CA, USA). After electrophoresis, staining of the gel was carried out with 0.02% (w/v) Coomassie Blue R-250 in 50% (v/v) methanol and 7.5% (v/v) acetic acid and destained with 50% (v/v) methanol and 7.5% (v/v) acetic acid. The protein standards (Bio-Rad Laboratories, Inc., Richmond, CA, USA) containing myosin (200 kDa), β-galactosidase (116 kDa), phosphorylase B (97.4 kDa), serum albumin (66.2 kDa) and ovalbumin (45 kDa) were used to estimate the molecular weight of the proteins.
Microstructure
Microstructure of gel samples was analyzed using a scanning electron microscope (Quanta 400, FEI, Brno, Czech Republic). Operation was performed at 20 kV with spot size of 4 under an Environmental SEM mode. Microstructure was obtained using SEI (Secondary Electron Image) with Gaseous Secondary Electron Detector (GSED). A standard working distance of 10 mm and pressure of 600 Torr were used for optimal image quality. The samples were visualized at magnification of 300×.
Sensory property
Surimi gel samples were cut into bite size (1 cm in thickness and 2.5 cm in diameter) and equilibrated at room temperature for 30 min. Each sample was assigned a random 3-digit code and served on white paper dish at room temperature under the fluorescent day-light type illumination. Thirty non-trained panelists (aged between 20 and 32) were the students at the Department of Food Technology, who were acquainted with surimi products. The panelists were asked to evaluate color, texture, odor, flavor and overall likeness of surimi gels using 9-point hedonic scale (1, extremely dislike; 2, very much dislike; 3, moderately dislike; 4, slightly dislike; 5, neither like nor dislike; 6, slightly like; 7, moderately like; 8, very much like; 9, extremely like) as per the method of Meilgaard et al. (1999). Panelists were asked to rinse their mouth after evaluating each sample.
Dynamic rheological properties of surimi paste containing VCO
Dynamic rheological test of surimi pastes containing VCO at various levels was conducted following the method of Shi et al. (2014) with a minor modification. The pastes added with VCO at different levels (5–25%) were subjected to oscillatory testing using a rheometer (HAAKE RheoStress1, ThermoFisher Scientific, Karlsruhe, Germany) equipped with a 40 mm parallel steel plate. A gap of 1 mm was set and silicon oil was used to prevent water evaporation. Stress and frequency sweep tests were conducted to obtain the linear viscoelastic region. Stress of 12.69 Pa and frequency of 1 Hz were used to conduct temperature sweep tests, in which the sample was gradually heated from 20 to 90 °C at a heating rate of 1 °C/min. Storage modulus (G′) loss modulus (G″) and loss tangent (tan δ) values were recorded as a function of temperature.
Statistical analysis
Experiments were carried out in triplicate using three different lots of samples. Data were subjected to analysis of variance (ANOVA). Comparison of means was carried out by the Duncan’s multiple range test. (Steel and Torrie 1980). Statistical analysis was performed using the Statistical Package for Social Science (SPSS 11.0 for windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Properties of surimi gels incorporated with VCO at various levels
Textural properties
Breaking force and deformation of surimi gels incorporated with VCO at different levels are shown in Fig. 1a, b, respectively. Breaking force decreased continuously as VCO was added up to 15% (p < 0.05), in which the decrease by 29% was obtained in gel containing 15%, compared with that of control (without oil). It was noted that no difference in breaking force was found (p > 0.05) when VCO at a level of 5% was added. Similar breaking force was attained in gels containing VCO at 15–25% (p > 0.05). Deformation of all gels was in the range of 12.03–14.31 mm. A decrease in deformation was noticed when VCO level was incorporated at level up to 15% (p < 0.05). No marked change in deformation was found when VCO in the range of 15–25% was added (p > 0.05). The result was in agreement with Benjakul et al. (2004) who found that addition of soybean oil significantly decreased the breaking force but showed no impact on deformation of surimi gel from bigeye snapper (Priacanthus tayenus) and mackerel (Trachurus japonicus). Hsu and Chiang (2002) also reported significant influence of oil concentration on breaking force of golden threadfin bream (Nemipterus virgatus) surimi gel, however the deformation was less affected by oil incorporation. With addition of 4% oil, 50% reduction in the breaking force was obtained, however, only 10% reduction in deformation was found.
In general, protein content of surimi gels decreased as VCO was present, especially at high level. VCO droplets also showed the interfering effect on protein–protein interaction in the gel matrix, thus lowering the strength as evidenced by the decreased breaking force. Breaking force and breaking distance of surimi gels from Alaska pollock, common carp, grass carp, and silver carp were well correlated with the protein concentrations of surimi (Luo et al. 2001). When oil was added, oil–protein interaction could occur in gel network. Oil might act as filler in the voids of gel matrix in meat products, thus limiting the movement of the matrix (Dickinson and Chen 1999; Wu et al. 2009; Yost and Kinsella 1992). Generally, the reduced surimi protein content in the network structure could not be compensated by the increased oil content (Shi et al. 2014). Therefore, the decreases in breaking force and deformation were found with increasing VCO level, except in the range of 15–25%, where VCO had no profound effect on reduction of those two parameters. The fracture constant (Kf) is the ratio of breaking force and deformation and it provides a measure of relative rigidity or firmness of gels at the failure point (Herranz et al. 2012). The fracture constant slightly decreased with increasing VCO and ranged from 37.55 to 49.22 g/mm (Fig. 1c). The result indicated the less rigidity in texture of gel added with VCO. Although the decrease in fracture constant was noticed between the control sample and those added with VCO at levels above 10%, no difference in fracture constant was found in gel added with VCO in the range of 15–25%. Therefore, level of VCO added could significantly influence the textural properties of surimi gel.
Texture profile analysis (TPA) is a rheological method to imitate the mastication process (Figura and Teixeira 2007). The test involves two complete cycles of compression and decompression of a food material (Lu and Abbott 2004). Table 1 shows the TPA parameters of surimi gel incorporated with VCO at various levels. The hardness, representing the force required to compress the sample to attain a given deformation, displayed a gradual decrease with increasing VCO levels, and ranged from 52 to 60 N. The decreases in hardness were noticed only after addition of VCO at the levels of 10% or above. The result was concomitant with the breaking force. Chang et al. (2015) reported that the surimi gels containing higher soybean oil concentration showed lower hardness and chewiness values. Hardness and chewiness were probably related to the fish protein content. The increasing soybean oil concentration directly resulted in the lower content of fish protein. Pietrowski et al. (2011) also found that corn oil addition decreased the hardness and chewiness of Alaska pollock surimi gel.
There were no differences in springiness, the elastic recovery that occurs when the compressive force is removed, and resilience, which is a measure of how the sample recovers from deformation, among all gels tested, regardless of VCO level used (p > 0.05). Chang et al. (2015) also noticed insignificant difference in the springiness values of gels with different soybean oil concentrations. The cohesiveness, the capability of breaking down the internal structure, ranged from 0.80 to 0.83 and was rarely changed with increasing VCO levels. However, slight decrease in cohesiveness was observed when VCO of 15% was added (p < 0.05). Chewiness, representing the energy required for chewing a solid food until it is ready for swallowing, ranged from 39 to 44 N. Chewiness displayed the gradual decrease with increasing VCO levels up to 10%. Nevertheless, no difference was observed among gels containing VCO ranging from 10 to 25% (p > 0.05).
Expressible moisture content
As shown in Fig. 1d, expressible moisture content of surimi gel was decreased with increasing VCO level up to 15% (p < 0.05). No further changes were noticeable when VCO was added at the range of 15–25% (p > 0.05). The decrease in expressible moisture was observed when VCO was incorporated, more likely associated with the lower water in gel. This was caused by dilution effect of VCO in the gel system. Furthermore, oil droplets might act as water migration barrier from gel network, in which the water could be trapped. However, Shi et al. (2014) reported that increasing vegetable oil concentration increased expressible water content of surimi gel.
Whiteness
Figure 1e displays the whiteness of surimi gels incorporated with VCO at various levels. Whiteness of surimi gels increased as VCO levels progressively increased and ranged from 79.23 to 86.30. The highest whiteness was observed when VCO at a level of 25% was added (p < 0.05). Benjakul et al. (2004) found the increased whiteness for mixed surimi from bigeye snapper (SSA and SA grade) and mackerel with the addition of soybean oil. The increased whiteness of surimi with added vegetable oil is attributed to the light scattering effect of the emulsion that is created when oil is comminuted with surimi and water (Park 2000). Shi et al. (2014) also found the increased whiteness of silver carp surimi gel with increasing oil content. Corn oil added Alaska pollock surimi gels had higher whiteness, compared to those without oil (Pietrowski et al. 2011). Therefore, VCO addition directly improved the whiteness of emulsified surimi gels.
Protein pattern
Protein patterns of surimi gels without and with the addition of VCO at different levels are depicted in Fig. 2. Surimi paste contained myosin heavy chain (MHC) as the dominant protein, followed by actin and tropomyosin. Disappearance of MHC band was found in the control as well as all VCO containing samples, compared to that observed in surimi paste. The decrease in band intensity of MHC indicated the formation of ε-(γ-glutamyl) lysine intra- and inter-molecular crosslinks of proteins induced by endogenous transglutaminase (TGase) (Kaewudom et al. 2013). However, no marked changes in actin and tropomyosin bands were observed. Endogenous TGase plays a role in setting of surimi by forming non-disulfide covalent bonds (Benjakul and Visessanguan 2003). Crosslinks were not dissociated by the mixture of SDS and β-mercaptoethanol used for electrophoresis (DeJong and Koppelman 2006). Therefore, VCO incorporation in the range of 5–25% had no remarkable effect on the protein crosslinking of the surimi gel, particularly by covalent bonds mediated by endogenous TGase.
Microstructure
The microstructure of croaker surimi gel without and with VCO added at different levels (5–25%) is shown in Fig. 3. A typical fibrous structure was observed in the control sample, however VCO droplets were embedded in the gel matrix of VCO containing samples. It was noted that the number of oil droplets was increased with increasing amounts of VCO added. Benjakul et al. (2004) reported the spherical soybean oil droplets dispersed in the matrix of the mixed snapper and mackerel surimi gels. At higher VCO levels, the larger oil globules were also observed. Lower proportion of myofibrillar proteins in the paste containing higher amount of VCO more likely resulted in their reduced emulsifying capacity. This was evidenced by the coalescence, in which the larger oil droplets were formed. The shape of VCO droplets varied from spherical to oval. Some irregular shaped droplets were also embedded in the matrix. The light scattering by the VCO droplets in the gel matrix was responsible for whiter color of VCO added gels, compared to the control gel (Fig. 1e). Moreover, oil droplets showed the higher interfering effect on protein–protein interaction in the gel matrix by increasing the intermolecular distances between protein chains, thus lowering the strength as evidenced by the decreased breaking force (Fig. 1a) and hardness (Table 1). Such a phenomenon was more pronounced as VCO levels increased. However, Zhou et al (2017) reported that camellia tea oil could occupy the void spaces of surimi protein matrix and formed a firmer gel.
Likeness score
Likeness score of surimi gel supplemented with VCO at various concentrations is presented in Table 2. VCO incorporation increased the color likeneness of surimi gels but there was no difference in score between samples added with VCO at 10–25% (p > 0.05). With increasing VCO levels, the higher whiteness was attained (Fig. 1e). Gel with whiter color is more desirable and VCO droplets played a role in improved whiteness. For texture likeness, no difference in score was found among all samples (p > 0.05). In other words, the panellists could not perceive the difference in the texture between the control and VCO added samples. The likeness score for odor significantly increased with the addition of VCO (p < 0.05), compared to the control. For flavor, higher score was gained for gels added with VCO at all concentrations used, compared to the control. The overall likeness also increased with increasing VCO concentrations. Nonetheless, there was no difference between the control and 5% VCO added sample (p > 0.05). Also similar scores were found among samples incorporated with VCO ranging from 10 to 25% (p > 0.05). Therefore, the addition of VCO was able to improve sensory property of surimi gel, particularly when VCO at higher amount was added.
Dynamic rheological properties of surimi paste incorporated with VCO at various levels
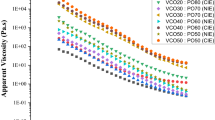
Rheological behavior of surimi paste containing VCO at various levels in comparison with the control during thermal treatment from 20 to 90 °C is illustrated in Fig. 4. For the control (surimi paste without VCO), G′ steadily increased and reached the peak value at around 40 °C. The initial increase of G′ at low temperature could be related to actomyosin molecular interactions and formation of protein network structure via hydrogen bonds between protein molecules, which formed a weaker three-dimensional gel network (Lefevre et al. 1998; Zhang et al. 2013). The lower values of G′ for the samples incorporated with VCO at temperature above 25 °C, compared to the control, could be due to the plasticizing effect of liquefied fat globules distributed in three dimensional protein network. This led to the reduction of the attractive forces between protein chains, thus making it more flexible (Shima and Tanimoto 2016).
Subsequently, G′ decreased at 40 °C and then dropped to the minimum abruptly at around 50 °C for all the samples. At the higher temperature, a large number of hydrogen bonds were destroyed, thus destabilizing the protein aggregate previously formed (Liu et al. 2007). Additionally, the breakdown of gel structure might take place by degradation of myosin mediated by endogenous proteolytic enzymes in this temperature range termed ‘Modori’ (Wasson 1992). Increased protein mobility and breakdown of the gel network structure also occurred due to the dissociation of actin–myosin complex and the denaturation of myosin tail (Shi et al. 2014). Upon further heating, G′ increased until reaching the temperature of 65 °C and then remained almost constant up to 90 °C. The phenomenon might be caused by the formation of a stable gel structure associated with enhanced protein aggregation (Liu et al. 2007). The G′ values correlated well with the springiness values of the samples. The progress and patterns of the G′ as a function of heating temperature were similar, regardless of VCO levels added. All the VCO added samples displayed higher values of moduli (G′ and G″) in the temperature range of 20–24 °C, compared to the control. Since the melting point of VCO was in the range of 22–27 °C (Srivastava et al. 2017), VCO was solidified at temperature lower than 25 °C. VCO in solid state might function as a filler. Consequently, it could increase viscoelasticity of paste. When heat was applied, the phase change from solid to liquid state took place in the VCO and resulted in an abrupt decline in G′ of VCO added samples in the temperature range of 23–24 °C.
In general, the G″ curves were similar to those of G′, but G″ values were much lower than G′ values over the entire temperature range tested (Fig. 4). VCO containing pastes had the sharp decrease in G″ at around 24 °C, which could be due to phase transition of VCO from solid to liquid state. On the other hand, G″ value for the control increased gradually until 35 °C and then rapidly decreased and reached the minimum value at 50 °C. Upon further heating, G″ remained almost constant over the remaining temperature up to 90 °C.
With increasing temperature, the value of tan δ was decreased, indicating the formation of stronger gel network. Smaller value of tan δ reflects stronger gel structure and more elastic behavior (Malik and Saini 2017). However, a sharp decrease in tan δ was noticed in the temperature range of 40–50 °C, implying that this temperature range was crucial for a stronger gel network formation. Overall, the final G′, G″ and tan δ of VCO added gels were lower than those of control. This was consistent with the lower breaking force of surimi gel added with VCO.
Conclusion
Addition of VCO significantly influenced the texture and whiteness of surimi gel. Breaking force and deformation decreased as VCO level increased up to 15%. However whiteness of the gel increased with increasing VCO levels. Viscoelastic study revealed that VCO addition affected elastic (G′) as well as loss moduli (G″) of surimi paste. Nevertheless, addition of VCO had no impact on protein pattern of all gels. Uniform distribution and anchoring of VCO droplets in the protein matrix were noticed. VCO incorporation increased the likeness scores for color, odor and overall likeness but had no impact on texture and flavor likeness. Thus, VCO at an appropriate level could be used to replace other vegetable oils, in which health benefits could be claimed, especially as a source of medium chain fatty acids.
References
Arfat YA, Benjakul S (2013) Gel strengthening effect of zinc salts in surimi from yellow stripe trevally. Food Biosci 3:1–9
Benjakul S, Visessanguan W (2003) Transglutaminase mediated setting in bigeye snapper surimi. Food Res Int 36:253–266
Benjakul S, Visessanguan W, Srivilai C (2001) Porcine plasma proteins as gel enhancer in bigeye snapper (Priacanthus tayenus) surimi. J Food Biochem 25:285–305
Benjakul S, Visessanguan W, Kwalumtharn Y (2004) The effect of whitening agents on the gel-forming ability and whiteness of surimi. Int J Food Sci Technol 39:773–781
Bergsson G, Arnfinnsson J, Karlsson SM, Steingrímsson O, Thormar H (1998) In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob Agents Chemother 42:2290–2294
Chang T, Wang C, Wang X, Shi L, Yang H, Cui M (2015) Effects of soybean oil, moisture and setting on the textural and color properties of surimi gels. J Food Qual 38:53–59
Cheret R, Chapleau N, Delbarre-Ladrat C, Verrez-Bagnis V, Lamballerie MD (2005) Effects of high pressure on texture and microstructure of sea bass (Dicentrarchus labrax L.) fillets. J Food Sci 70:477–483
DeJong G, Koppelman S (2006) Transglutaminase catalysed reactions: impact on food applications. J Food Sci 67:2798–2806
Dickinson E, Chen J (1999) Heat-set whey protein emulsion gels: role of active and interactive filler particles. J Dispers Sci Technol 20:197–213
Figura LO, Teixeira AA (2007) Food physics physical properties-measurement and applications. Springer Publishers, Berlin
German JB, Dillard CJ (2004) Saturated fats: What dietary intake? Am J Clin Nutr 80(550):559
Herranz B, Borderias AJ, Solo-de-Zaldívar B, Solas MT, Tovar CA (2012) Thermostability analyses of glucomannan gels. Concentration influence. Food Hydrocoll 29:85–92
Hsu CK, Chiang BH (2002) Effects of water, oil, starch, calcium carbonate and titanium dioxide on the colour and texture of threadfin and hairtail surimi gels. Int J Food Sci Technol 37:387–393
Kaewudom P, Benjakul S, Kijroongrojana K (2013) Properties of surimi gel as influenced by fish gelatin and microbial transglutaminase. Food Biosci 1:39–47
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lefevre F, Fauconneau B, Ouali A, Culioli J (1998) Thermal gelation of brown trout myofibrils: effect of muscle type, heating rate and protein concentration. J Food Sci 63:299–304
Liu R, Zhao SM, Xiong SB, Xie BJ, Liu HM (2007) Studies on fish and pork paste gelation by dynamic rheology and circular dichroism. J Food Sci 72:399–403
Lowry QH, Rosebrough NJ, Farr LA, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:256–275
Lu R, Abbott JA (2004) Force/deformation techniques for measuring texture. In: Kilcast D (ed) Texture in food: Solid foods. Woodhead Publishing Limited, Cambridge, pp 109–145
Luo YK, Kuwahara R, Kaneniwa M, Murata Y, Yokoyama M (2001) Comparison of gel properties of surimi from Alaska pollock and three freshwater fish species: effects of thermal processing and protein concentration. J Food Sci 66:548–554
Malik MA, Saini CS (2017) Polyphenol removal from sunflower seed and kernel: effect on functional and rheological properties of protein isolates. Food Hydrocoll 63:705–715
Marina A, Man YC, Nazimah S, Amin I (2009) Chemical properties of virgin coconut oil. J Am Oil Chem Soc 86:301–307
Meilgaard M, Civille GV, Carr BT (1999) Sensory evaluation techniques, 3rd edn. CRC Press, Florida
NFI (1991) A manual of standard methods for measuring, specifying the properties of surimi. National Fisheries Institute, Washington, DC
Nolasco NN, Balboa JG, Serrame E, Lim-Sylianco CY (1994) Effect of coconut oil, trilaurin and tripalmitin on the promotion stage of carcinogenesis. Philipp J Sci 123:161–169
Park JW (2000) Ingredient technology and formulation development. In: Park JW (ed) Surimi and surimi seafood. Marcel Dekker, New York, pp 343–392
Pietrowski BN, Tahergorabi R, Matak KE, Tou JC, Jaczynski J (2011) Chemical properties of surimi seafood nutrified with ω-3 rich oils. Food Chem 129:912–919
Shi L, Wang X, Chang T, Wang C, Yang H, Cui M (2014) Effects of vegetable oils on gel properties of surimi gels. LWT Food Sci Technol 57:586–593
Shima H, Tanimoto M (2016) Effect of milk fat content on the viscoelasticity of mozzarella-type cheese curds. Eur Food Res Technol 242:157–162
Srivastava Y, Semwal AD, Sajeevkumar VA, Sharma GK (2017) Melting, crystallization and storage stability of virgin coconut oil and its blends by differential scanning calorimetry (DSC) and Fourier transform infrared spectroscopy (FTIR). J Food Sci Technol 54:45–54
Steel RGD, Torrie JH (1980) Principles and procedures of statistics: a biometrical approach, 2nd edn. McGraw-Hill, New York
Wasson DH (1992) Fish muscle proteases and heat-induced myofibrillar degradation: a review. J Aquat Food Prod Technol 1:23–41
Wu MG, Xiong YL, Chen JY, Tang X, Zhou GH (2009) Rheological and microstructural properties of porcine myofibrillar protein–lipid emulsion composite gels. J Food Sci 74:207–217
Yost RA, Kinsella JE (1992) Microstructure of whey protein isolate gels containing emulsified butterfat droplets. J Food Sci 57:892–897
Zhang F, Fang L, Wang C, Shi L, Chang T, Yang H, Cui M (2013) Effects of starches on the textural, rheological, and color properties of surimi–beef gels with microbial transglutaminase. Meat Sci 93:533–537
Zhou X, Jiang S, Zhao D, Zhang J, Gu S, Pan Z, Ding Y (2017) Changes in physicochemical properties and protein structure of surimi enhanced with camellia tea oil. LWT Food Sci Technol 84:562–571
Acknowledgements
This work was supported by the Thailand’s Education Hub for Southern Region of ASEAN Countries (TEH-AC, 2015) scholarship. The TRF Distinguished Research Professor Grant was also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gani, A., Benjakul, S. & Nuthong, P. Effect of virgin coconut oil on properties of surimi gel. J Food Sci Technol 55, 496–505 (2018). https://doi.org/10.1007/s13197-017-2958-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2958-0