Abstract
The rheological properties of acid-induced coagulation of camel and cow milk gels following the addition of calcium chloride (CaCl2) and hydrogen phosphate dehydrate (Na2HPO4*2H2O) were investigated using a dynamic low amplitude oscillatory rheology. For a considered condition, the final values of storage modulus (G′) and loss modulus (G″) of camel milk gels were significantly lower than those of cow milk gels. The increase of the added CaCl2 levels improved significantly the gelation properties of camel and cow milk gels, since a reduction in the gelation time and an increase in the gel firmness were observed. Following the addition of Na2HPO4*2H2O at 10 and 20 mM, no significant effect on the gelation rate and the firmness of camel milk gels was observed, while, a significant decrease in the gelation rate and firmness were observed for cow milk gels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Milk acidification is traditionally induced by using bacterial cultures and/or glucono-δ-lactone (GDL) which converts lactose into lactic acid (Sadeghi et al. 2014). The hydrolysis of GDL leads to the formation of gluconic acid inducing a decrease of pH (Koh et al. 2002). With this decrease: (1) the solubilization of the colloidal calcium phosphate gradually begins and becomes fully soluble at a pH of ~5.20 (Le Graet and Brule 1993); and (2) the surface charges of the casein micelles decreased, leading to reduce the steric and electrostatic stabilization of milk and the formation of gel at pH ~4.80 (Lucey et al. 1997).
Camel milk is mostly consumed as fluid contrary to other types of milk (Yagil et al. 1984). This could be explained by the difficulty of the camel milk transformation regardless of the gelation type i.e., acid, enzymatic or mixed (Attia et al. 2000; El Zubeir and Jabreel 2008). This trend has been attributed by the authors to the casein micelles structure. Indeed, the low content of κ-casein and the large size of casein micelles of camel milk compared with cow with cow milk contribute to prolong the coagulation time (Al Haj and Al Kanhal 2010; Kappeler et al. 2003). Additionally, the presence of several natural antimicrobial agents in camel milk such as lysozyme, lactoferrin, lactoperoxidase and immunoglobulin limit the effect of lactic acid bacteria (Ramet 2001).
Calcium and phosphate are considered as important elements of the mineral fraction of cow and camel milk with total concentrations varying in the 25–35 mM for both types of milk (Bornaz et al. 2009; Gaucheron 2005). These mineral components are in equilibrium state between the micellar and the serum phase and contribute significantly to the formation of coagulum (Ramasubramanian et al. 2008). In addition, the addition of calcium significantly increased the curd yield (Makhal et al. 2015).
In the literature, the rheology properties of acid-induced cow milk protein gels following the addition of calcium have been studied (Gastaldi et al. 1994; Guillaume et al. 2002; Ramasubramanian et al. 2008). One of the main conclusions of these studies was that added calcium decreased the gelation time and improved the firmness of the gels. Regarding the effect of phosphate on the acid-induced coagulation of cow milk, few studies have been published (Mizuno and Lucey 2007; Ozcan et al. 2008). The authors pointed out that the enrichment of milk with phosphate delayed the onset gelation and decreased the gel firmness.
Although several studies were published regarding the effect of calcium and phosphate on the acid gelation properties of cow milk gels, to the best of our knowledge, very limited scientific report were available in the literature concerning the effect of these minerals on camel milk gels. Thus, in the present work, rheological properties of the GDL-induced coagulation of camel and cow milk gels following the addition of CaCl2 and Na2HPO4*2H2O at two concentrations (10 and 20 mM) will be studied.
Materials and methods
Milk samples
Fresh camel milk (2 L distributed into plastic bottles of 30 mL capacity) was obtained from an experimental station located in region Foum Lahcen, province Tata (Moroccan desert). Camels of Crezni genotype, aged of 6 years with two child births were used. Camels were kept on pasture feeding and supplemented with barley and clover.
Fresh cow milk (2 L distributed into plastic bottles of 30 mL capacity) of Holstein–Friesian genotype was collected from a regional farm of Lille (France). Cow were maintained on pasture feeding and supplemented with a corn-based concentrate.
Once arrival to the laboratory, milk samples were kept at −18 °C up to analysis. All the analyses were conducted in duplicate (2 samples for each condition).
Physicochemical analyses of camel and cow milk
The pH, protein, fat and dry matter contents of camel and cow milk were determined as described by Karoui and Dufour (2003). Prior to pH measurements, the pH meter (Hach sensION + pH3 Laboratory) was calibrated with standard pH solutions prepared using buffer capsules of pH 7 and 4.
Distribution of casein micelles size of milk
Skimmed milk was obtained by centrifugation at 3740 g for 15 min (Froilabo-SW14R, France). Number-based particle size distribution of skimmed milk was obtained with SALD-2300 laser diffraction particle size analyzer (Shimadzu Scientific Instruments, France) using wet type measurement. Milk was directly injected into the dispersion cell (containing deionized water under agitation) until a light intensity distribution more than 10% was reached and the particle size distribution was determined. The refractive index of 1.55 was used according to the findings of Griffin and Griffin (1985) who have used refractive index of 1.57. For the dispersant, a blank was measured with deionized water at which the light intensity distribution is less than 10%.
Samples preparation for the gelation
Milk samples (10 samples in total for each milk species) were thawed during 12 h at 4 °C and then kept at room temperature (~18 °C) for 15 min to avoid thermal shock. Milk samples (25 mL for each sample) were placed in a water-bath equilibrated previously at 36 °C for 5 min. Then, calcium chloride anhydrous (CaCl2, Merck, Darmstadt, Germany) and di-Sodium hydrogen phosphate dehydrate (Na2HPO4*2H2O, Merck Darmstadt, Germany) powders were separately added to the milk at two concentrations (10 and 20 mM). Milk coagulation was performed by progressive acidification with GDL (Glucono-delta-lactone «C6H10O6», Merck schuchardt OHG, Hohenbrunn, Germany) added at 3% (w/v) with stirring for 2 min and rheological measurement was immediately performed. Control milk (without added CaCl2 and Na2HPO4*2H2O) was also studied.
Rheological and pH measurements throughout acid induced coagulation
The experiments were performed using a controlled-strain rheometer (Physica MCR 301, Anton Paar Company, Germany) with low amplitude oscillation and a temperature set at 36 °C by applying a Peltier plate. A volume of 20 mL was placed in two concentric cylinders, with an inner diameter of 26.66 mm, length of 40.02 mm, and a gap of 1.13 mm. A layer of liquid paraffin was placed onto the surface of milk sample to prevent evaporation during coagulation, and then measurement was started. The oscillation experiments were performed by applying a constant frequency of 1 Hz and a strain of 0.05% during 180 min that was in the linear viscoelastic range of these types of gels network. Then, a frequency sweep from 1 to 10 Hz was performed by applying a strain of 0.05%, followed by an amplitude sweep from 0.05 to 150% with a frequency of 1 Hz. Taking into account the elastic modulus (G′), the following parameters were assessed: (1) the gelation time defined as the time when G′ = 1 Pa as reported by Lucey et al. (1998a) and Ramasubramanian et al. (2014); (2) the gelation rate defined as dG′/dt corresponding to the slope obtained by linear regression of G′ versus time from onset of gelation over a period of 15 min; and (3) the asymptotic value of G′ after 180 min of gelation (G′asym).
The pH was determined every 5 min during 180 min.
Statistical analysis
In order to detect difference between milk samples, rheological parameters were compared using a one-way ANOVA (p < 0.05). ANOVA was applied using XLSTAT 2013 (Addinsoft SARL USA, New York, NY, USA) software.
Results and discussion
Physicochemical parameters of camel and cow milk
Physicochemical properties of camel and cow milk showed that dry matter, protein and fat contents of camel milk (108.30 ± 10.74, 27.32 ± 0.06 and 34.50 ± 0.50 g L−1, respectively) were lower than those of cow milk (120.50 ± 1.45, 33.75 ± 0.16 and 37.50 ± 0.71 g L−1, respectively), in agreement with others (Al Haj and Al Kanhal 2010; Hailu et al. 2016; Jumah et al. 2001).
The added GDL induced a decrease of the pH values reaching 3.48 and 4.02 after 180 min for camel and cow milk gels, respectively. This decline in the pH values for both types of milk gels is related to the added GDL, in agreement with the findings of Lucey et al. (1998a).
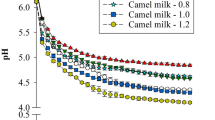
Compared with control camel and cow milk gels, the addition of CaCl2 and Na2HPO4*2H2O to milk induced change in the pH, particularly for added Na2HPO4*2H2O (Fig. 1a, b). For a considered time, an increase and a decrease of pH values were observed for milk gels enriched with phosphate and calcium. After 180 min of added GDL, milk samples added with CaCl2 presented pH values of 3.46 and 3.84 for camel and cow milk gels, respectively, while those added with Na2HPO4*2H2O presented higher pH values of 3.75 and 4.45 for respectively camel and cow milk gels.
Size distribution of casein micelles
The size distribution of casein micelles of camel and cow milk are presented in Fig. 2. The casein micelles size of camel milk was of 468 ± 1.00 nm, while it was only of 137 ± 1.50 nm for cow milk. This result was in agreement with the findings of Bornaz et al. (2009) reporting that casein micelles diameter of camel milk varied in the 280–550 nm range, larger than that of cow milk having a diameter in the 90–210 nm range. This difference between the both types of milk might be explained by the variation in the physico-chemical composition (e.g., κ-casein content, etc.) where a smaller micelle size was known to be associated with a higher amount of κ-casein ~3.47 and 13% for camel and cow milk, respectively as pointed out by others (Davies and Law 1980; Kappeler et al. 2003; Walsh et al. 1998).
Rheological properties of calcium/phosphate-induced camel and cow milk gels
The evolution of G′ following the addition of CaCl2 and Na2HPO4*2H2O to cow and camel milk gels as a function of time are shown in Fig. 3a, b. Calcium/phosphate-added milk induced the gelation of both camel and cow milk, irrespective of the CaCl2 and Na2HPO4*2H2O concentration. For both types of gels: (1) the higher the calcium concentration, the higher the gel firmness; and (2) the higher the phosphate concentration, the lower the gel firmness (Fig. 3a, b). The G′asym of control camel milk gels showed average value of 1.61 Pa and increased significantly (p < 0.05) to 2.23 and 3.81 Pa with the addition of CaCl2 at 10 and 20 mM, respectively (Fig. 3a; Table 1). The low elasticity and the weakness of camel gels could be ascribed to the lack of network structure through the hydrophobic interactions which involved low energy and little resistant to mechanical treatment (Mahaut et al. 2000).
The gelation time and gelation rate of the calcium/phosphate-induced camel milk gels compared with control milk gels were determined (Table 1). As observed, shorter gelation time and higher gelation rate were observed for camel milk gels with added calcium (20 mM) since a significant decrease of gelation time (p < 0.05) and an increase in the gelation rate compared with control camel milk gels were observed with added CaCl2 at 20 mM. These modifications in the gelation time and gelation rate with added CaCl2 could be attributed to the increase of ionic calcium concentration inducing: (1) a pH decrease resulting from the negative charge decrease at the surface of caseins; and (2) the formation of more bridges between casein proteins and calcium (Koutina et al. 2016; Ramasubramanian et al. 2012).
As observed in Fig. 3a and Table 1, no significant differences (p < 0.05) were noticed between the gelation rates and G′asym values of control camel milk gels and those added with Na2HPO4*2H2O (10 and 20 mM). However, the addition of Na2HPO4*2H2O at 20 mM induced a significant delay (p < 0.05) of the gelation time compared with control camel milk gels and those added with 10 mM phosphate. This trend could be probably attributed to the formation of a phosphocalcic complex between calcium and added phosphate as pointed out by Tsioulpas et al. (2010).
A similar trend was observed for cow milk gels following the addition of CaCl2; however, the effect of calcium was more pronounced in cow milk gels than that of camel milk gels (Fig. 3b; Table 1). From the obtained results, it could be concluded that G′asym values exhibited higher significant values (p < 0.05) with the increase of added CaCl2 concentration. Indeed, according to Table 1, the highest G′asym value was observed following the addition of 20 mM CaCl2 (i.e., 231 Pa), while it was only of 20.28 Pa for control cow milk gels, in agreement with others who observed after 360 min of skim cow milk gels acidification G′asym values of 319 (Lucey et al. 1998a) and 244 Pa (Oh et al. 2007). In addition, the difference in G′asym values between control and those enriched with calcium could be attributed to the increase of the micellar calcium phosphate amount leading to higher establishment of calcium bridges between milk proteins and the formation of more elastic gel (Guillaume et al. 2002). From Fig. 3b, it was observed that the G′ values of gels added with CaCl2 increased rapidly during the first 18 min, corresponding to pH value of ~5.20. This trend could be related to the dissociation and rearrangement of casein that increase with the solubilization rate of colloidal calcium phosphate (Gastaldi et al. 1996). After the first 18 min, the G′ values showed small change with the decrease of pH from 5.20 to 4.70 that could be attributed to the reincorporation of casein into the micelle structure (Gastaldi et al. 1996). In the 24–62 min range, the G′ values increased, where the pH value was of ~4.70. Beyond 62 min, the G′ values showed a slight decrease during ~10 min, and after remains constant until the end of gelation (180 min). Regarding the gelation time and gelation rate of cow milk gels, a similar trend was observed for camel milk. Indeed, the increase of the added CaCl2 levels to 20 mM reduced significantly the gelation time (p < 0.05) and increased the gelation rate compared with control milk (Table 1). These modifications could be explained by the increase of the neutralization rate of the negatively charged casein, resulting from the increase in ionic calcium amount (Dalgleish 1983).
The impact of Na2HPO4*2H2O added to cow milk gels was found more pronounced than that of camel milk gels, since a significant delay in the gelation onset, a reduction of the gelation rate, and the formation of a weak gels were obtained with the increase of the added Na2HPO4*2H2O levels. Indeed, from Fig. 3b and Table 1, it could be observed that the gelation rate and G′asym values decreased significantly (p < 0.05) with the increase of Na2HPO4*2H2O concentration in comparison with control milk gels (Table 1). The decline of curd-firming rate and G′asym values could be explained by the decrease in the colloidal calcium phosphate cross-linking leading to the dispersion of casein as described by Mizuno and Lucey (2007) and Ozcan et al. (2008) reporting that the addition of low concentrations of tetrasodium pyrophosphate to milk induced slow protein gelation. As shown in Table 1, the gelation time increased significantly (p < 0.05) with the increase of the level of added phosphate. This trend could be attributed to the formation of a phosphocalcic complex, leading to decrease the calcium ions and thus increase the negative charges of casein micelles (Tsioulpas et al. 2010).
The evolution of G′ as a function of frequency and strain amplitude sweep tests are reported in Figs. 3 and 4. According to Fig. 3c, d, an increase in the G′ values of calcium-induced milk gels was observed with the increase of frequency from 1 to 10 Hz, irrespective of milk species. Considering each milk gels, separately, the difference between G′ and G″ (data not shown) was less than 1 log, particularly for camel milk gels, indicating the formation of weak gels (Lapasin and Pricl 1995). Indeed, when frequency increased from 1 to 10 Hz, G′ values of control camel and cow milk gels varied from 2 to 19 Pa and from 22 to 46 Pa, respectively (Fig. 3c, d). The obtained results were in agreement with the findings of Martin et al. (2009) who reported that G′ and G″ of GDL-induced skim milk gels increased in the 0.016–1.60 Hz range.
Compared with control milk gels, the incorporation of calcium at 10 and 20 mM induced an increase in the G′ and G″ values as a function of applied frequency (i.e., 1–10 Hz range) (Fig. 3c, d). These results were in agreement with those of Ramasubramanian et al. (2014) who observed an increase of G′ for calcium-induced cow milk gels with increase of frequency from 0.1 to 10 Hz, reflecting that the formed bonds in the gels matrix have less time to relax when the time scale of the applied stress is shorter (Mishra et al. 2005). Similar results for control milk gels were obtained with added phosphate (Fig. 3c, d). For example, with added Na2HPO4*2H2O at 20 mM, an increase in the G′ values from 1.55 to 21 Pa and from 16.31 to 41.27 Pa was observed with increasing frequency from 1 to 10 Hz for camel and cow milk gels, respectively.
Over the entire frequency range (from 1 to 10 Hz), the slopes obtained using a linear regression of log G′ versus log frequency exhibited similar values i.e., ~1.17, R2 = 0.96 for all camel milk samples indicating that the evolution of G′ depend on the frequency, and reflecting the formation of a weaker gel structure (Fig. 3c). Those of cow milk gels showed lower values, where slopes of ~0.17 (R2 = 0.99) and ~0.35 (R2 = 0.92) were observed with 10 and 20 mM calcium-induced milk gels, and control milk gels, respectively (Fig. 3d). Similar results were obtained with added phosphate (Fig. 3c, d).
The low slopes obtained from cow milk gels reflects that the evolution of G′ values was independent of frequency, indicating the formation of gels with higher firmness that could be related to the increase of the number of formed bonds in the gel matrix. No data was available concerning the slopes obtained for camel milk gels. However, those obtained from cow milk gels were in line with the literature (Lucey et al. 1998a; Oh et al. 2007). Indeed, Sandra et al. (2012) observed fair slope values as a function of frequency following the addition of CaCl2 suggesting a higher extent of cross-linking as a result of calcium bridges formed in the milk gels. Figure 4a, b illustrates that the highest breaking stress values of camel and cow milk gels were obtained with added CaCl2 (10 and 20 mM). Indeed, the breaking stress of camel milk gels exhibited a maximum value of 1.89 and 1.94 Pa with 10 and 20 mM added CaCl2, while it was only of 1.80 for control milk gels (Fig. 4a). The breaking strain showed similar values of 102% for the three cases.
A similar trend was observed for cow milk gels (Fig. 4b), since the breaking stress for control milk gels and those added with 10 and 20 mM CaCl2 was respectively of 49.31, 97.92 and 131.75 Pa, with a breaking strain of 102% for control milk gels and 48% for those with added CaCl2. The increase of the breaking stress with the addition of CaCl2 could be explained by the increase of the insoluble calcium level of casein micelles, leading to decrease the large pores and increase the strength interactions and bonds between caseins particles (Lucey et al. 1997).
Maximum breaking stress values of 1.47 and 1.45 Pa and a breaking strain of 102% were observed, respectively for 10 and 20 mM phosphate-induced camel milk gels (Fig. 4a). For cow milk gels (Fig. 4b), the maximum breaking stress following the addition of 10 and 20 mM Na2HPO4*2H2O showed similar values of ~46 Pa, with a breaking strain of 150 and 102%, respectively. The decrease in the breaking stress compared to added calcium could be ascribed to the reduction in the number of colloidal calcium phosphate cross-links, and the dispersion of casein particles as reported by Ozcan et al. (2008).
Comparison of the final storage modulus of camel and cow milk gels as a function of added mineral levels
Changes in G′asym values of Na2HPO4*2H2O and CaCl2-induced camel and cow milk gels were in the following order: cow milk gels > camel milk gels (Fig. 5). Indeed, for a considered concentration of Na2HPO4*2H2O and CaCl2, the G′asym of cow milk samples exhibited significant higher values (p < 0.05) than those obtained with camel milk gels. Furthermore, the G′asym of calcium-induced milk gels was significantly higher (p < 0.05) than that of phosphate-induced milk gels. The difference in the physico-chemical composition of both types of milk such as dry matter, protein and fat might be considered as the principal reason of this variation. In fact, the low contents of dry matter, protein and fat in camel milk compared with cow milk induced some modifications in the gelation properties. In this context, Jumah et al. (2001) reported that the lower levels of dry matter and protein of camel milk, the weaker acid-induced gel. This could be explained by the fact that: (1) the lower fat content of camel milk induced the formation of a weak gel since fat globules could interact with the protein matrix improving the firmness of recombined milk gels (El Zubeir and Jabreel 2008; Lucey et al. 1998b); and/or (2) the larger size of camel casein micelles affect the rheological properties of milk gels (Glantz et al. 2010), in agreement with the results obtained in Fig. 2 indicating that casein micelles size of camel milk (468 nm) is larger than that of cow milk (137 nm).
Conclusion
The results of the present study showed some difference between the rheological properties of acid-induced camel and cow milk gels. The increase of the added CaCl2 concentration from 10 to 20 mM reduced significantly the gelation time and increased the firmness for both camel and cow milk gels compared with control milk gels.
The addition of Na2HPO4*2H2O at 10 and 20 mM exhibited a slight effect of the gelation properties of camel milk gels compared with control milk gels. This effect was more pronounced for cow milk gels, since a significant delay in the gelation onset and decreases of the gel firmness were observed. However, the detailed mechanism by which added CaCl2 and phosphate affects the rheological properties of acid-induced camel milk gels needs to be further investigated.
The different measurements were performed on frozen-thawed milk samples and it would be interesting to test these results on fresh milk samples although a recent study conducted on caprine milk pointed out that milk freezing up to 2 months at −27 °C did not present significant effect on the coagulation properties (Kljajevic et al. 2016).
References
Al Haj OA, Al Kanhal HA (2010) Compositional, technological and nutritional aspects of dromedary camel milk. Int Dairy J 20:811–821
Attia H, Kherouatou N, Nasri M, Khorchani T (2000) Characterization of the dromedary milk casein micelle and study of its changes during acidification. Lait 80:503–515
Bornaz S, Sahli A, Attalah A, Attia H (2009) Physicochemical characteristics and renneting properties of camels’ milk: a comparison with goats’, ewes’ and cows’ milks. Int J Dairy Technol 62:505–513
Dalgleish DG (1983) Coagulation of renneted bovine casein micelles: dependence on temperature, calcium ion concentration and ionic strength. J Dairy Res 50:331–340
Davies DT, Law AJR (1980) Content and composition of protein in creamy milk in south-west Scotland. J Dairy Res 47:83–90
El Zubeir IEM, Jabreel SO (2008) Fresh cheese from camel milk coagulated with Camifloc. Int J Dairy Technol 61:90–95
Gastaldi E, Pellegrini O, Lagaude A, Tarodo de la Fuente B (1994) Functions of added calcium in acid milk coagulation. J Food Sci 59:310–320
Gastaldi E, Lagaude A, Tarodo de la Fuente BT (1996) Micellar transition state in casein between pH 5.5 and 5.0. J Food Sci 61:59–64
Gaucheron F (2005) The minerals of milk. Reprod Nutr Dev 45:473–483
Glantz M, Devold TG, Vegarud GE, Lindmark-Månsson H, Stålhammar H, Paulsson M (2010) Importance of casein micelle size and milk composition for milk gelation. J Dairy Sci 93:1444–1451
Griffin MCA, Griffin WC (1985) A simple turbidimetric method for the determination of the refractive index of large colloidal particles applied to casein micelles. J Colloid Interface Sci 104:409–415
Guillaume C, Marchessaeau A, Laugaude A, Cuq JL (2002) Effect of salt addition on the micellar composition of milk subjected to pH reversible CO2 acidification. J Dairy Sci 85:2098–2105
Hailu Y, Hansen EB, Seifu E, Eshetu M, Ipse R (2016) Factors influencing the gelation and rennetability of camel milk using camel chymosin. Int Dairy J 60:62–69
Jumah RY, Shaker RR, Abu-Jdayil B (2001) Effect of milk source on the rheological properties of yogurt during the gelation process. Int J Dairy Technol 54:89–93
Kappeler S, Farah Z, Puhan Z (2003) 5′-Flanking regions of camel milk genes are highly similar to homologue regions of other species and can be divided into two distinct groups. J Dairy Sci 86:498–508
Karoui K, Dufour É (2003) Dynamic testing rheology and fluorescence spectroscopy investigations of surface to centre differences in ripened soft cheeses. Int Dairy J 13:973–985
Kljajevic NV, Jovanovic ST, Miloradovic ZN, Macej OD, Vucic TR, Zdravkovic IR (2016) Influence of the frozen storage period on the coagulation properties of caprine milk. Int Dairy J 58:36–38
Koh MWW, Merino LM, Dickinson E (2002) Rheology of acid-induced sodium caseinate gels containing added gelatin. Food Hydrocoll 16:619–623
Koutina G, Christensen M, Bakman M, Andersen U, Skibsted LH (2016) Calcium induced skim-milk gelation during heating as affected by pH. Dairy Sci Technol 96:79–93
Lapasin R, Pricl S (1995) Rheology of industrial polysaccharides: theory and applications. In: Lapasin R, Pricl S (eds) 1st edn. Blackie Academic and Professional, Glasgow, pp 250–494
Le Graet Y, Brule G (1993) Les équilibres minéraux du lait: influence du pH et de la force ionique. Lait 73:51–60
Lucey JA, Teo CT, Munro PA, Singh H (1997) Rheological properties at small (dynamic) and large (yield) deformations of acid gels made from heated milk. J Dairy Res 64:591–600
Lucey JA, Tamehana M, Singh H, Munro PA (1998a) A comparison of the formation, rheological properties and microstructure of acid skim milk gels made with a bacterial culture or glucono-δ-lactone. Food Res Int 31:147–155
Lucey JA, Munro PA, Singh H (1998b) Rheological properties and microstructure of acid milk gels as affected by fat content and heat treatment. J Dairy Sci 63:660–664
Mahaut M, Jeantet R, Brule G (2000) Initiation à la technologie fromagère. Technique and documentation. Lavoisier, Paris
Makhal S, Kanawjia SK, Giri A (2015) Role of calcium chloride and heat treatment singly and in combination on improvement of the yield of direct acidified Cottage cheese. J Food Sci Technol 52:535–541
Martin F, Cayot N, Marin A, Journaux L, Cayot P, Gervais P, Cachon R (2009) Effect of oxidoreduction potential and of gas bubbling on rheological properties and microstructure of acid skim milk gels acidified with glucono-δ-lactone. J Dairy Sci 92:5898–5906
Mishra R, Govindasamy-Lucy S, Lucey JA (2005) Rheological properties of rennet-induced gels during the coagulation and cutting process: impact of processing conditions. J Texture Stud 36:190–212
Mizuno R, Lucey JA (2007) Properties of milk protein gels formed by phosphates. J Dairy Sci 90:4524–4531
Oh EH, Anema SG, Wong M, Pinder DN, Hemar Y (2007) Effect of potato starch addition on the acid gelation of milk. Int Dairy J 17:808–815
Ozcan T, Lucey JA, Horne DS (2008) Effect of tetrasodium pyrophosphate on the physicochemical properties of yogurt gels. J Dairy Sci 91:4492–4500
Ramasubramanian L, Restuccia C, Deeth HC (2008) Effect of calcium on the physical properties of stirred probiotic yogurt. J Dairy Sci 91:4164–4175
Ramasubramanian L, D’Arcy BR, Deeth HC (2012) Heat-induced coagulation of whole milk by high levels of calcium chloride. Int J Dairy Technol 65:183–190
Ramasubramanian L, D’Arcy BR, Deeth HC, Eustina OhH (2014) The rheological properties of calcium-induced milk gels. J Food Eng 130:45–51
Ramet JP (2001) The technology of making cheese from camel milk (Camelus dromedary). In: Animal production and health paper, no. 113. Food and Agriculture Organization, Rome
Sadeghi M, Madadlou A, Khosrowshahi A, Mohammadifar M (2014) Acid-induced gelation behavior of casein/whey protein solutions assessed by oscillatory rheology. J Food Sci Technol 51:2113–2119
Sandra S, Ho M, Alexander M, Corredig M (2012) Effect of soluble calcium on the renneting properties of casein micelles as measured by rheology and diffusing wave spectroscopy. J Dairy Sci 95:75–82
Tsioulpas A, Koliandris A, Grandison AS, Lewis MJ (2010) Effects of stabiliser addition and in-container sterilisation on selected properties of milk related to casein micelle stability. Food Chem 122:1027–1034
Walsh CD, Guinee TP, Reville WD, Harrington D, Murphy JJ, O’kennedy BT, Fitzgerald RJ (1998) Influence of κ-casein genetic variant on rennet gel microstructure, cheddar cheesemaking properties and casein micelle size. Int Dairy J 8:707–714
Yagil R, Saran A, Etzion Z (1984) Camels milk: for drinking only? Comp Biochem Physiol A Physiol 78:263–266
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamal, M., Foukani, M. & Karoui, R. Rheological and physical properties of camel and cow milk gels enriched with phosphate and calcium during acid-induced gelation. J Food Sci Technol 54, 439–446 (2017). https://doi.org/10.1007/s13197-016-2480-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2480-9