Abstract
Several coffee brews, including classical and commercial beverages, were analyzed for their diterpene esters content (cafestol and kahweol linoleate, oleate, palmitate and stearate) by high performance liquid chromatography with diode array detector (HPLC–DAD) combined with spectral deconvolution. Due to the coelution of cafestol and kahweol esters at 225 nm, HPLC–DAD did not give accurate quantification of cafestol esters. Accordingly, spectral deconvolution was used to deconvolve the co-migrating profiles. Total cafestol and kahweol esters content of classical coffee brews ranged from 5–232 to 2–1016 mg/L, respectively. Commercial blends contained 1–54 mg/L of total cafestol esters and 2–403 mg/L of total kahweol esters. Boiled coffee had the highest diterpene esters content, while filtered and instant brews showed the lowest concentrations. However, individual diterpene esters content was not affected by brewing procedure as in terms of kahweol esters, kahweol palmitate was the main compound in all samples, followed by kahweol linoleate, oleate and stearate. Higher amounts of cafestol palmitate and stearate were also observed compared to cafestol linoleate and cafestol oleate. The ratio of diterpene esters esterified with unsaturated fatty acids to total diterpene esters was considered as measure of their unsaturation in analyzed samples which varied from 47 to 52%. Providing new information regarding the diterpene esters content and their distribution in coffee brews will allow a better use of coffee as a functional beverage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The coffee plant belongs to the family of Rubiaceae which has more than 70 species. Global coffee production comes from Coffea arabica (Arabica) and Coffea canephora var. robusta (Robusta) with nearly 75% originating from the former and 25% from the latter (Mussatto et al. 2011). In terms of chemical composition, coffee oil consists predominantly of triglycerides (75–80 wt%), although it is source of other valuable products such as sterols (2–5.4 wt%) and diterpene esters (7–20 wt%) (Novaes et al. 2015). The latter include cafestol, kahweol and 16-O-methylcafestol (16-OMC) that can be thermally degraded during the roasting process to form decomposed derivatives such as dehydrocafestol, dehydrokahweol, cafestal and kahweal (Speer and Kölling-Speer 2006). The occurrence of cafestol as the main cholesterol-raising factor has been reported in all commercial coffee species, while kahweol is mainly present in Arabica coffee and it is only found as minor component in Robusta beans (Kitzberger et al. 2013). Robusta beans contain 16-OMC which is absent in Arabica and is described as a chemical marker of Robusta coffee (Kurzrock and Speer 2007). The pentacyclic diterpenes exist in coffee oil as monoesters on C17 by saturated or unsaturated fatty acids (Novaes et al. 2015) as 14 and 12 different fatty acid esters has been detected for cafestol and kahweol, respectively. Also, 12 fatty acid esters were identified for 16-OMC. Nevertheless, from quantitative point of view, C16, C18, C18:1, C18:2, C20, and C22 esters of fatty acids predominate as the total content of these six cafestol esters in sum ranged from 9.4 to 21.2 g/kg dry weight in different Arabica coffees (Speer and Kölling-Speer 2006). The chemical structure of the main eight diterpene esters of Arabica coffee are presented in Fig. 1. Recently, more attention has been dedicated to desirable effect of diterpenes and diterpene esters against cancer and diabetes. Other beneficial properties like induction of the degradation of toxic substances as well as protection against aflatoxin B1 had been also reported for diterpenes (Cavin et al. 2002).
Free diterpenes (either originally present or after cleavage of the esters) can be analyzed using liquid or gas chromatography (Erny et al. 2015) as well as spectrophotometric method (Dias et al. 2013). However, to provide information on individual esters, diterpene esters should be analyzed through HPLC only after gel permeation chromatography or solid phase extraction (Kurzrock and Speer 2007). To the best of our knowledge, baseline separation of diterpene esters where both cafestol and kahweol esters are present has never been achieved. In this case a MS detector should be used to adequately monitor the target species. However those instruments are costly and are not always available. Spectral deconvolution (SD) is a mathematical tool for analysis of dataset. Through analysis of samples with HPLC–DAD, a two dimensional matrixes are obtained with chromatographic profile (retention time dimension) in one dimension and spectrometric profile (the wavelength dimension) in the other. Deconvolution aims to extract from the original data chromatographic and spectroscopic profiles of each individual component (Van Hoeylandt et al. 2014). Deconvolution has been successfully implemented in order to identifying or quantifying several compounds (Erny et al. 2015; Namara et al. 2007; Van Hoeylandt et al. 2014). This approach designated as HPLC–DAD with spectral deconvolution (HPLC–DAD/SD) which allowed quantifying each diterpene esters despite an important co-elution (Erny et al. 2015).
The chemical composition of roasted coffee, including diterpenes and diterpene esters exhibit different extraction rates according to coffee species (Bicho et al. 2011), genetic variability (Kitzberger et al. 2014) as well as climatic conditions and post-harvest techniques (Kitzberger et al. 2013). However, they may also be influenced by the choice of brewing technique and conditions (home brewing or industrial extraction) as diterpene extraction greatly varies among the different brewing methods (Gross et al. 1997; Moeenfard et al. 2015). Despite the high occurrence of diterpene esters in coffee, few reports are found in the literature regarding diterpene esters as they mainly focus on total diterpenes like total cafestol and kahweol. However, the transition of the cafestol esters into the espresso coffee was tested by other author (Kurzrock and Speer 2007). The content of 16-OMC esters in few coffee brews was also studied previously (Sehat et al. 1993). To the best of our knowledge, few reports exist on diterpene esters profile (Kurzrock and Speer 2007; Sehat et al. 1993) of commercial coffee available in retail outlets which may overshadowed by brewing procedures. Application of deconvolution for this purpose has not been reported elsewhere, as well.
Considering that diterpene esters have biological importance, investigation of their content in a wide range of coffee brews through a simple and inexpensive technique is relevant. Thus, it could be possible to identified not only coffee brews with higher diterpene esters content, but also evaluate the distribution of individual esters as well as unsaturation of coffee brews that may affect the health properties of final beverage. For these reasons, the aim of this study was to investigate diterpene esters profile of a range of classical and commercial coffee brews, in particular cafestol esters as the main diterpene present in coffee species, via HPLC–DAD/SD, which have been subjected to different preparation methods in order to understand the potential variation in the amount of diterpene esters consumed by coffee drinkers.
Materials and methods
Chemicals and reagents
Individual standards of cafestol and kahweol linoleate, oleate, palmitate and stearate were acquired from LKT lab (MN, USA). HPLC grade acetonitrile and methanol along with diethyl ether (purity of 99%) were purchased from VWR (BDH Prolabo, Belgium). Sodium chloride was supplied by Panreac Quimica (Spain). Filtered distilled water used for HPLC–DAD analysis was prepared by vacuum purification through 0.45 μm filter ionic exchange membranes.
Coffee samples
Roasted Arabica (100% C. arabica, 2.34% water content) packaged in protective atmosphere was kindly supplied by a local company in Porto, Portugal. Roasted beans were ground by means of a home grinder (Braun KSM 2 model 4041, Mexico) as the particle size distribution of roasted and ground Arabica was as follows: 51% > 500; 24%, 300–500; 13%, 212–300; 11% < 212 µm). These ground coffee was used to prepare classical brews (boiled, French, filtered and mocha). Various brands were also purchased randomly from local commerce in Porto, Portugal. Table 1 describes the coffee beans used for commercial coffee brew preparation.
Classical coffee brews preparation
Four different coffee samples were obtained using Arabica coffee with coffee/water ratio of 7.5 g/100 mL to uniformize the comparison of brewing techniques. The preparation modes were performed in duplicate and were as follows:
Boiled coffee
It was prepared by boiling 11.25 g roasted and ground coffee with 150 mL of distilled water for 10 min, followed by 2 min of settling time, followed by decanting the liquid. Individual cup size was 150 mL.
French press coffee
It was brewed by pouring 150 mL of boiled distilled water onto 11.25 g of roasted and ground coffee in glass French press pot, followed by stirring. After 2.5 min, the coffee brew was separated from ground coffee by pressing the plunger. Individual cup size was 150 mL.
Mocha coffee
It was brewed using an aluminum mocha pot. Around 11.25 g roasted and ground coffee was placed in filter cup. Mocha pot was filled with 150 mL of cold distilled water. The pot was heated until the water reservoir was empty. Individual cup size was 60 mL.
Filtered coffee
22.5 g of roasted and ground coffee were put in a paper filter bag (N°2) and extracted with 300 mL of boiled distilled water by means of conventional percolation coffee machine KRUPS Aroma Café 5 (Germany). The brew dripped into a heated pot within 2–3 min. The individual cup size was 150 mL.
Commercial coffee brews preparation
Commercial coffee samples were brewed using various brands of different types of coffee purchased randomly from local commerce in Porto, Portugal. Thirteen coffee brews were prepared according to the manufacturers’ instructions. Coffee brews (three replicates for each sample) were prepared and stored at −22 °C until analysis, made in duplicates.
Capsule coffee
Extraction of each capsule was performed using an automatic coffee maker (KRUPS, XN2100, Germany) at a pressure of 19 bar by hot water (93 ± 2 °C). Amount of coffee in each capsule was as follows: A-type 1 (6.01 ± 0.01 g/capsule), A-type 2 (5.01 ± 0.06 g/capsule), A-type 3 (5.01 ± 0.03 g/capsule), A-type 4 (5.14 ± 0.02 g/capsule), A-type 5 (6.13 ± 0.11 g/capsule), B (5.71 ± 0.02 g/capsule). Each cup contained 40 mL of coffee brew.
Pod espresso
It was brewed using the SGL coffee machine, designed for pod. The size of a single serving was 40 mL derived from the brewing of a coffee pod (7.08 ± 0.15 g/pod).
Instant coffee
For this purpose, 2 g of commercial instant coffee powder was extracted with 150 mL of boiled distilled water. Regarding instant espresso, one pack contain 1.8 g of soluble coffee was dissolved in 50 mL of boiled distilled water.
Vending coffee
It was obtained from Necta Coffee Vending Machine (Necta Astro Double Brew) to draw a cup of coffee (30 mL).
Diterpene esters extraction
The compounds were extracted in duplicate according to a previously developed methodology (Moeenfard et al. 2015). Briefly, 2.5 mL of coffee brew was extracted using 5.0 mL of diethyl ether along with 0.5 mL of methanol in order to break the emulsion and create a neat interface between the aqueous and organic phases. Extraction procedure was repeated two times. The combined ether phase was washed with 5.0 mL of 2 M NaCl solution and after centrifugation (4000 rpm, 10 min), the cleaned ether phase was brought to dryness under N2 stream and stored at −22 °C prior to HPLC–DAD analysis.
HPLC–DAD analysis
Analysis was carried out in a Merck Hitachi Elite LaChrom (Tokyo, Japan) system equipped with a quaternary pump (L-2130), an L-2200 autosampler. Separation was achieved using a Purospher STAR LichroCART RP 18 end-capped (250 × 4 mm, 5 μm) column attached to a guard column (4 × 4 mm, 5 μm) of the same kind. The dried extract was dissolved in 0.5–5.0 mL of acetonitrile depending on diterpene esters content in coffee sample. Reconstituted samples were filtered through 0.45 μm filter membrane (PTFE, VWR, USA) and 20 µL was injected and analyzed using the isocratic elution of acetonitrile/isopropanol (70:30, v/v) at 0.4 mL/min. The detection was made using an L-2455 (Merck Hitachi) UV/vis spectrophotometry diode array detector in the range of 200–400 nm. Two detection wavelengths were also set, namely, 225 and 290 nm for cafestol esters and kahweol esters, respectively. EZChrom Elite 3.1.6 software was used for data acquisition and analysis. Target compounds were identified by comparing spectra and retention times with reference standard solutions. Diterpene esters contents were reported as mg/L ± standard deviation after applying the dilution or concentration factors.
Because kahweol esters can be adequately quantified using the chromatogram obtained at 290 nm or using deconvoluted chromatograms, these compounds were used to compare the analytical performance of deconvolution approach over the classical technique. Calibration curves were prepared by plotting the peak area against the corresponding concentrations by duplicate injections of 20 μL of standard solutions. Validation parameters, including linearity (expressed as coefficient of determination, R 2), precision (expressed as coefficient of variation, %CV), accuracy (expressed as percentage of recovery), sensitivity (expressed as slope of calibration curve), limit of detection (LOD) and limit of quantification (LOQ) were determined.
Deconvolution
After each run, data were exported as coma-separated values format by the acquisition software (EZChrom Elite 3.1.6). Those files were opened using Matlab R2013b and the chromatographic data loaded into a two-dimensional (2D) array. The Matlab function was programmed using Matlab R2013b and can be found in previous published study (Erny et al. 2015). Through applying spectral deconvolution to every spectrum, deconvoluted chromatograms were obtained that are specific to the cafestol and kahweol esters.
Statistical analysis
A one-way ANOVA analysis with a level of significance of 95% was performed to evaluate the differences among coffee brews in terms of cafestol and kahweol esters content. Differences were considered significant when p < 0.05. Data were reported as means ± standard deviations of samples submitted to duplicate extractions and injections. Statistical analysis was done by Matlab R2013b or Minitab 16 (2009) software. Graphs were plotted by means of Excel (2010) and Matlab R2013b.
Results and discussion
Method validation
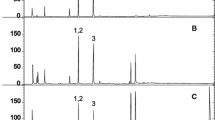
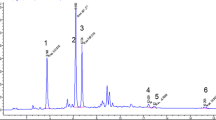
Before validation, individual standard of each diterpene esters (100 mg/L) was injected for HPLC–DAD analysis in order to obtain reliable spectra. Spectra of analytes of interest and background are illustrated in Fig. 2a–c. Through applying spectral deconvolution to every spectrum using Matlab R2013b, deconvoluted chromatograms were obtained (Fig. 2d). Although interfering substances were existed in the matrix, acceptable separation was achieved after deconvolution.
Spectra of a cafestol esters, b kahweol esters, c background, d deconvoluted chromatogram obtained using HPLC–DAD/SD analysis of a real coffee sample (espresso-bar) (1 kahweol linoleate, 2 kahweol oleate, 3 kahweol palmitate, 4 kahweol stearate, 5 cafestol linoleate, 6 cafestol oleate, 7 cafestol palmitate, 8 cafestol stearate). The chromatogram was recorded at 225 nm to allow the observation of all compounds of interest
Six standard solutions contain all diterpene esters were prepared in the range of 5–150 mg/L. Deconvoluted chromatograms and subsequently calibration curves were obtained. Satisfactory results were obtained within the linearity range (5–150 mg/L) for both analytical approaches (HPLC–DAD and HPLC–DAD/SD).
The calibration curves were linear and exhibited the coefficient of determination (R 2) higher than 0.99 (Table 2). The limits of detection (LOD) and of quantification (LOQ) were calculated based on a signal to noise ratio of three (S/N = 3) and ten (S/N = 10), respectively. Considering the deconvolution approach the LOD of cafestol and kahweol esters were in the range of 0.1–0.9 and 0.3–1.2 mg/L, respectively. Direct analysis of kahweol esters through classical approach by setting the wavelength at 290 nm, exhibited the LOD of 0.3–1.3 mg/L which was similar to those obtained by deconvolution technique. Regarding the LOQ, the results ranged from 0.3 to 2.9 mg/L for cafestol esters and 0.9–3.9 mg/L for kahweol esters analyzed by deconvolution method. Analysis of kahweol esters with classical approach revealed LOQ of 0.9–4.0 mg/L.
Method and instrumental precisions were obtained through the coefficient of variation of repeated analysis at intra-day and inter-day conditions, respectively. Intra-day precision (repeatability) was evaluated through six replicate analysis in the same day. In the inter-day variation studies (reproducibility), an extract of the same sample was analyzed three times on three consecutive days. Considering deconvolution, the average coefficient of variation of kahweol esters was 3.0 (min 0.9, max 6.3) and 1.8% (min 0.4%, max 4.8%) for intra and inter-day precision, respectively. Regarding cafestol esters, intra-day precision of 1.6% (min 0.3%, max 2.9%) and inter-day precision of 1.5% (min 0.8%, max 4.6%) were obtained. Average intra-day precision of 1.5% (min 0.6%, max 2.0%) and inter-day precision of 0.5% (min 0.1%, max 1.7%) were obtained for kahweol esters in classical approach, as well.
The accuracy of both analytical methods was studied through recovery test. For this purpose, coffee sample (capsule B) was spiked with known quantity of each diterpene esters at two spiking levels (50 and 100 mg/L). The average recovery (%) was reported as the mean ratio between the obtained and expected concentration of each diterpene esters in spiked sample. In terms of deconvolution approach, the mean recovery ranged 87–103% and 91–97% for cafestol esters and kahweol esters, respectively. Regarding classical approach, the mean recovery ranged from 92 to 101% for kahweol esters which was almost similar to those obtained by deconvolution technique.
Comparison of deconvolution and classical approach
Looking at both analytical approaches (deconvolution and classical), Fig. 3 demonstrated the applicability of deconvolution for quantification of diterpene esters in variety of coffee brews. Setting the wavelength at 290 nm allowed baseline separation of kahweol esters while such wavelength does not exist for measuring cafestol esters. Therefore, the following wavelength (290 nm) was used to compare deconvolution approach with classical separation in HPLC–DAD. Considering all coffee brews in the present study, no significant differences (p ≥ 0.05) were observed between deconvolution and classical approach and perfect agreement was achieved for all kahweol esters among these two analytical techniques. Accordingly, deconvolution method was then successfully extended to quantify cafestol esters in coffee brews. These results demonstrated the advantageous of using this low-cost and accurate analytical technique over other methods like LC–MS for quantification of cafestol and kahweol esters in variety of coffee samples.
Concentration of kahweol esters in various types of coffee brews obtained from HPLC–DAD and HPLC–DAD/SD measurement at 290 nm. One-way ANOVA indicated that for each coffee sample, there was no significant differences (p ≥ 0.05) among values obtained from classical (HPLC–DAD) or deconvolution (HPLC–DAD/SD) approach. Since, in some samples the diterpene esters concentrations were below the LOQ, their bar were not shown in the respective figure
Diterpene esters content in coffee brews
Even potentially small health benefits or risks associated with coffee intake may have important public health implications due to its widespread consumption. Therefore, the aim of this part of the study was to provide information about some diterpene esters of coffee brews that are directly available to the consumer. For this purpose, the average concentrations of four cafestol esters and four kahweol esters were quantified in some traditional and commercial coffee brews through an inexpensive and accurate method and the results were summarized in Tables 3 and 4, respectively.
A large variability was found among diterpene esters content in coffee brews. In general, boiled coffee was the most concentrated brew in terms of total cafestol esters (232 ± 9 mg/L) and total kahweol esters (1016 ± 29 mg/L) content followed by French press coffee. While, mocha and espresso brews had an intermediate contribution to cafestol esters intake with values of 36 ± 3 and 10–54 mg/L, respectively. They also were consider as an intermediate source of kahweol esters (123 ± 2 mg/L of mocha and 40–403 mg/L of espresso brews).
The most widespread espresso brews obtain from bar machines and single-dose capsules, designed in large numbers because of their commercial popularity (Parenti et al. 2014). Over the last few decades, capsule and pod received substantial attention in market share as 14 billion units of capsule and pods has been sold in 2005 (Parenti et al. 2014). According to our results, among espresso brews, espresso-bar was the most concentrated one in terms of total cafestol esters (54 ± 2 mg/L), followed by vending (43 ± 4 mg/L) and capsule B (41 ± 4 mg/L). Capsule B which composed of 100% Arabica coffee, presented the highest total kahweol esters concentration (403 ± 11 mg/L), as well.
Despite the similarity of the coffee/water ratio (7.5 g/100 mL) of boiled coffee in comparison to other classical brews, the prolonged contact time of ground coffee with the surrounding water (10 min for boiled and around 2.5–3.0 min for French press coffee) may justify the higher concentration of diterpene esters in boiled and French press coffee compared with other brews. Besides that, as other authors stated (Gross et al. 1997) the higher content of diterpenes in boiled and French press may be attributed to the presence of high amount of fine coffee particles as the main source of diterpene esters, in the respective brews. Sehat et al. (1993) reported the highest concentration of 16-OMC esters as one of the main esters existing in Robusta beans in Scandinavian boiled coffee and espresso coffee.
One the other hand, brews that were made by filtration or instant coffees showed significantly (p < 0.05) lower diterpene esters content than other samples. Concerning filtered coffee, probably diterpene esters were retained on filter paper (Silva et al. 2012). The low concentration of diterpene esters in instant coffees may be attributed to additional thermal extraction treatment at high temperature after roasting (Vignoli et al. 2011). The presence of negligible amount of 16-OMC esters in filtered coffee was also reported previously (Sehat et al. 1993).
The volume of coffee brew per cup varies from person to person, depending on the cultural and personal preferences of each consumer. A cup of boiled or mocha coffee may range from 150–190 mL to 40–120 mL, respectively (Alves et al. 2010). Accordingly, the big cup size of boiled and French press makes them a rich source of these compounds. On the other hand, espresso coffees are very small beverages ranging between 20 and 35 mL in Italy and up to 70 mL in Portugal (Alves et al. 2010). Therefore, the concentration of diterpene esters in espresso coffees are balanced with the limited volume of a serving cup, which resulted in a moderate diterpene esters intake in comparison to French press or boiled coffee. On the other hand, instant and filtered coffees are light brews and despite the high volume of consumption they might not be an important contributor to diterpene esters consumption.
One the other hand, it seems that individual diterpene esters were not affected by brewing procedure as among kahweol esters, the kahweol palmitate (2–409 mg/L) was the main compound in all samples, followed by kahweol linoleate (20–381 mg/L), oleate (5–176 mg/L) and stearate (1–50 mg/L). The order was in agreement with other report (Kurzrock and Speer 2007). Higher amounts of cafestol palmitate (1–103 mg/L) and stearate (2–62 mg/L) were also observed compared to cafestol linoleate (0.5–46 mg/L) and cafestol oleate (0.5–20 mg/L). The average percentage of cafestol esters distribution in coffee samples were slightly different from those that were previously reported (Kurzrock and Speer 2007; Speer and Kölling-Speer 2006) as in the present work, the highest percentage of individual cafestol esters has been attributed to cafestol palmitate, whereas cafestol stearate, linoleate and oleate were in the next order. This discrepancy could probably be due to the different numbers of diterpene esters quantified. It should be noted that, despite the variety of diterpene esters reported for coffee, few of them are considered key esters (Speer and Kölling-Speer 2006). Among them, 16-OMC are dedicated only to Robusta coffee. Others, like dehydrocafestol or dehydrokahweol esters are formed during coffee bean roasting. However, cafestol and kahweol esters, mainly palmitate, linoleate, oleate and stearate are the most abundant diterpene esters in particular in Arabica coffee beans (Speer and Kölling-Speer 2006) which gained also the main proportion in coffee blends.
Comparison of coffee brews according to the fatty acid composition of their diterpene esters
Even though individual cafestol and kahweol esters vary significantly between samples, differences among the ratio of total unsaturated diterpene esters to total diterpene esters were small. The values for the ratio of diterpene esters esterified with unsaturated fatty acids (DE-UFA) to total diterpene esters (TDE) content as a measure of their unsaturation ranged from 47 to 52%, with the lowest value for capsule A-type 2 and the highest value for the capsule B (Fig. 4). Generally, coffee brews sample presented approximately 49 ± 2% unsaturated cafestol and kahweol esters content (esters of oleic acid, 18:1 cis-9 and linoleic acid, 18:2 cis, cis-9,12). Fatty acids of coffee lipid extract may present either free or esterified with glycerol or diterpenes alcohols. It is noteworthy to mention that these results were based on the determination of the eight diterpene esters of Arabica coffee so they include only four esters of cafestol and another four for kahweol. Accordingly, it seems that brewing method does not modify the distribution of diterpene esters as both saturated and unsaturated diterpene esters presented the similar extraction rate.
The fatty acids are found to distributed differently among various lipid classes of coffee oil and the only common feature being that palmitic (16:0) and linoleic acids (cis 9,12–18:2) are the dominant fatty acids components in all classes (Nikolova-Damyanova et al. 1998). As reported by Nikolova-Damyanova et al. (1998), in lipid classes of crude beans of Catuaí Vermalho (C. arabica L.), terpenes and sterol esters were the most saturated and free fatty acids and triacylglycerol were the most unsaturated classes (Nikolova-Damyanova et al. 1998).
Several epidemiological studies suggested the controlled intake of saturated fatty acids in particular lauric, myristic and palmitic acids due to their effects on increasing the blood cholesterol concentration (Williams 2000). Considering the various effects of diterpenes on human health, in order to reinforce the beneficial properties, we should favor coffee with moderate content of diterpenes and high proportion of unsaturated fatty acid. Although, more experiments require, probably selection of type of bean or roasting degree may allowed that. Besides that, provide information about the content of saturated or unsaturated fatty acids may be used for study the effect of coffee oil on quality of final product.
Conclusion
Diterpene intake through coffee brews is substantially dependent on the method of brew preparation. HPLC–DAD measurement combined with spectral deconvolution allowed us to determine four cafestol esters and four kahweol esters in several coffee brews, simultaneously.
This method can be applied routinely for determination of these compounds in a large monitoring scheme of coffee brews allowing a high sample throughput. With the results obtained, in general, boiled and French press were the most concentrated brews in terms of diterpene esters content, whereas mocha and espresso had an intermediate contributions to diterpene esters intake. Only negligible amount of diterpene esters were effectively extracted to the filtered and instant coffee. Regarding kahweol esters, the palmitate ester was the main compound in all samples, followed by linoleate, oleate and stearate. Higher amounts of cafestol palmitate and stearate were observed compare to linoleate and oleate. Among these eight diterpene esters studied, diterpenes esterified with unsaturated fatty acids were included almost half (47–52%) of the total diterpene esters content. Considering the significant consumption of coffees in the world, they could be account as important source for natural biological active compounds such as diterpene esters. As a consequence, although various effects of coffee consumption on human health has been raised, these data provide new information regarding the diterpene esters that are directly available for consumption which may have a large influence on the potential health of coffee intake.
References
Alves RC, Casal S, Oliveira MBPP (2010) Tocopherols in coffee brews: influence of coffee species, roast degree and brewing procedure. J Food Compost Anal 23:802–808. doi:10.1016/j.jfca.2010.02.009
Bicho NC, Leitão AE, Ramalho JC, Lidon FC (2011) Identification of chemical clusters discriminators of the roast degree in Arabica and Robusta coffee beans. Eur Food Res Technol 233:303–311. doi:10.1007/s00217-011-1518-5
Cavin C, Holzhaeuser D, Scharf G, Constable A, Huber WW, Schilter B (2002) Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol 40:1155–1163. doi:10.1016/S0278-6915(02)00029-7
Dias RCE, Alves ST, Benassi MDT (2013) Spectrophotometric method for quantification of kahweol in coffee. J Food Compost Anal 31:137–143. doi:10.1016/j.jfca.2013.04.001
Erny GL, Moeenfard M, Alves A (2015) Liquid chromatography with diode array detection combined with spectral deconvolution for the analysis of some diterpene esters in Arabica coffee brew. J Sep Sci 38:612–620. doi:10.1002/jssc.201401095
Gross G, Jaccaud E, Huggett AC (1997) Analysis of the content of the diterpenes cafestol and kahweol in coffee brews. Food Chem Toxicol 35:547–554
Kitzberger CSG, Scholz MBDS, Pereira LFP, Vieira LGE, Sera T, Silva JBGD, Benassi MDT (2013) Diterpenes in green and roasted coffee of Coffea arabica cultivars growing in the same edapho-climatic conditions. J Food Compos Anal 30:52–57. doi:10.1016/j.jfca.2013.01.007
Kitzberger CSG, Scholz MBDS, Benassi MDT (2014) Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions. Food Res Int 61:61–66. doi:10.1016/j.foodres.2014.04.031
Kurzrock T, Speer K (2007) Diterpenes and diterpene esters in coffee. Food Rev Int 17:433–450. doi:10.1081/fri-100108532
Moeenfard M, Silva JA, Borges N, Santos A, Alves A (2015) Quantification of diterpenes and their palmitate esters in coffee brews by HPLC–DAD. Int J Food Prop 18:2284–2299. doi:10.1080/10942912.2014.933351
Mussatto SI, Machado EMS, Martins S, Teixeira JA (2011) Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol 4:661–672. doi:10.1007/s11947-011-0565-z
Namara KM, Howell J, Huang Y, Robbat A Jr (2007) Analysis of gin essential oil mixtures by multidimensional and one-dimensional gas chromatography/mass spectrometry with spectral deconvolution. J Chromatogr A 1164:281–290. doi:10.1016/j.chroma.2007.07.042
Nikolova-Damyanova B, Velikova R, Jham GN (1998) Lipid classes, fatty acid composition and triacylglycerol molecular species in crude coffee beans harvested in Brazil. Food Res Int 31:479–486
Novaes FJM, Oigman SS, de Souza ROMA, Rezende CM, de Aquino Neto FR (2015) New approaches on the analyses of thermolabile coffee diterpenes by gas chromatography and its relationship with cup quality. Talanta 139:159–166. doi:10.1016/j.talanta.2014.12.025
Parenti A, Guerrini L, Masella P, Spinelli S, Calamai L, Spugnoli P (2014) Comparison of espresso coffee brewing techniques. J Food Eng 121:112–117. doi:10.1016/j.jfoodeng.2013.08.031
Sehat N, Montag A, Speer K (1993) Lipids in the coffee brew. In: 15th international colloquium on the chemistry of coffee. ASIC, Paris, pp 869–872
Silva JA, Borges N, Santos A, Alves A (2012) Method validation for cafestol and kahweol quantification in coffee brews by HPLC–DAD. Food Anal Methods 5:1404–1410. doi:10.1007/s12161-012-9387-5
Speer K, Kölling-Speer I (2006) The lipid fraction of the coffee bean. Braz J Plant Physiol 18:201–216
Van Hoeylandt T, Chen K, Du Prez F, Lynen F (2014) Deconvolution of overlapping spectral polymer signals in size exclusion separation-diode array detection separations by implementing a multivariate curve resolution method optimized by alternating least square. J Chromatogr A 1342:63–69. doi:10.1016/j.chroma.2014.03.052
Vignoli JA, Bassoli DG, Benassi MT (2011) Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: the idnfluence of processing conditions and raw material. Food Chem 124:863–868. doi:10.1016/j.foodchem.2010.07.008
Williams CM (2000) Dietary fatty acids and human health. Anim Res 49:165–180
Acknowledgements
This work was financially supported by: project UID/EQU/00511/2013-LEPABE (Laboratory for Process Engineering, Environment, Biotechnology and Energy—EQU/00511) by FEDER funds through Programa Operacional Competitividade e Internacionalização—COMPETE2020 and by national funds through FCT—Fundação para a Ciência e a Tecnologia through SFRH/BD/79318/2011. This work was also funded by FEDER funds through the Operational Programme for Human Potential and by National Funds through FCT—Foundation for Science and Technology under the project IF/00528/2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Rights and permissions
About this article
Cite this article
Moeenfard, M., Erny, G.L. & Alves, A. Variability of some diterpene esters in coffee beverages as influenced by brewing procedures. J Food Sci Technol 53, 3916–3927 (2016). https://doi.org/10.1007/s13197-016-2378-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2378-6