Abstract
The present study was aimed to evaluate the nutritional, antioxidant properties and inhibition of the key enzyme such as α-amylase and α-glucosidase from the fruits of Maesa indica. The results revealed that M. indica fruits possess an enormous amount of protein (45.68 mg/g), carbohydrates (25.12 mg/g) and mineral elements. The acetone extract were capable of hunting radicals by providing electrons and break chain reaction, especially in ABTS·+ (3719.23 µmol TE/g extract), OH· (66.50 %) and NO· (81.50 %) radical scavenging assays. The methanol extract showed a strong inhibition towards α-amylase and α-glucosidase (IC50 of 37.80 and 23.74 µg/mL, respectively). HPLC analysis enumerate that both extracts illustrates the presence of polyphenolic compounds namely quercetin, caffeic acid, rutin and chlorogenic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant kingdom is the major source for producing several health care products. Polyphenols are large group of phytochemicals found ubiquitously in the plant kingdom, especially in fruits and vegetables. Fruits and vegetables play a preventive role in the treatment of several acute and chronic diseases and helps to improve and/or develop several health care products (Tulipani et al. 2008). Nowadays, epidemiological reports strongly suggest that plant derived foods have health-promoting properties that depend on their nutritional value. Fruits have a relatively high proportion of several natural phytochemicals, which are important and exhibit several functional properties, such as radical scavenging ability, upholding blood glucose levels, prevent the cardiovascular ailments and several others (Hassan et al. 2011). Especially, the fruits like blackberries, highbush blueberries, murta, calafate, arrayán, chequén, and meli possess abundant nutritional properties and important dietary sources such as fiber, vitamins and minerals in addition to the phytochemical compounds which are essential for human health.
Diabetes mellitus is a complex syndrome characterized by hyperglycemia. It is primarily categorized into insulin dependent diabetes mellitus (type I diabetes) and non-insulin dependent diabetes mellitus (type II diabetes). Preventing type II diabetes is increasing worldwide. The important therapeutic approach in maintaining the postprandial hyperglycaemia is by hindering absorption of glucose by inhibition of carbohydrate-hydrolyzing enzymes, such as α-amylase and α-glucosidase. In this view, many exertions have been made to pursuit for more effective and safe inhibitors of α-amylase and α-glucosidase from natural products to develop physiological functional food to treat diabetes (Wang et al. 2010).
Maesa indica commonly known as wild berry belongs to the family Myrsinaceae. The fruits of M. indica are eaten as raw by Irula tribal of Kotagiri Hills (Chaudhuri 2007). Whole plant of M. indica excluding roots has spermicidal property (Pokharkar et al. 2010). M. indica have been reported to exhibit virucidal activity against Newcastle Disease Virus, Vaccinia Virus and Herpes Simplex Virus (Jassim and Naji 2003). North Indian people consume the leaves and fruits of M. indica in curries and are used in treatment of blood purification and anthelminthic ailments. Locally the plant is called as “Vavding”, the flavonoid compound quercetin is the major phytochemical constituent of this plant (Gaitonde and Naik 1989). Besides these reports of traditional usages, their nutritional and phytochemical properties still remains unexplored. Hence, the present investigation aimed to evaluate the nutritional analysis, antioxidant, anti-diabetic and phytochemical properties of M. indica fruits.
Materials and methods
Collection of plant materials
The fresh fruits of M. indica were collected from Kotagiri hills, Tamil Nadu, India, during the month of November. The collected plant material was identified and their authenticity was confirmed by Botanical Survey of India, Southern circle, Coimbatore, Tamil Nadu (BSI/SRC/5/23/2011-12/Tech.-1505). Freshly collected plant material was cleaned to remove adhering dust and dried under shade. The dried sample were powdered and used for further studies.
Chemicals
The α-amylase and α-glucosidase respectively from porcine pancreas and rat intestine, 2,2-diphenyl-1-picryl-hydrazyl (DPPH), 2,2′aninobis(3-ethylbenzothiozoline-6-sulfonic acid) disodium salt (ABTS), quercetin, rutin, catechin and caffeic acid were purchased from Sigma Aldrich. All other reagents used were of analytical grade were procured from Himedia and Merck.
Successive solvent extraction
The powdered plant material was successively extracted by soxhlet extractor using petroleum ether, chloroform, acetone and methanol. Finally, the material was macerated using hot water. The extracts were concentrated by rotary vacuum evaporator (Yamato BO410, Japan) and the percentage yield was expressed in terms of dry weight of plant powder material.
Nutritional analysis
Proximate composition
The contents of moisture, ash and crude fiber was determined according to the methods defined in Association of Official Analytical Chemists (AOAC 1995). The protein, carbohydrate and starch content was estimated by Sadasivam and Manickam (2008). The results were expressed on dry weight basis.
Estimation of amino acids
Amino acids content of fruits were determined using the procedure of Ishida et al. (1981). Extracted samples were filtered through a 0.45 µm membrane filter (SFPV25X; Axiva Sichem Biotech, Delhi, India) and 20 µL of the filtrate was injected into an amino acid analyzer (HPLC-model LC 10 AS, Shimadzu, Mount holly, New Jersey) equipped with a Na type cation exchange column (f 5.0 mm × 190 mm) (ISC-07/S1504; Shimadzu Scientific Instruments) packed with the strongly acidic cation exchange resin styrene divinyl benzene copolymer with a sulphonic group. The instrument was equipped with a fluorescence detector (FL 6A; Shimadzu Scientific Instruments) and a Chrompac recorder (CR 6A; Shimadzu Scientific Instruments). The mobile phase of the system consisted of two buffers and a gradient system was used for effective separation of amino acids. The oven temperature was maintained at 60 °C with a total run time of 60 min. Amino acid analysis was performed using the non-switching flow method with fluorescence detection after post-column derivatization using o-phthalaldehyde. The imino groups of proline and hydroxyl proline are converted to amino groups using hypochlorite. An amino acid standard (Sigma Chemical Co., St. Louis, MO, USA) was also run to calculate the concentration of amino acids in the sample. The amount of each amino acid was expressed as mg/g of protein.
Mineral quantification
Amount of total nitrogen (N) content was estimated through micro Kjeldahl method; phosphorus (P) by treating the samples with ammonium molybdate and freshly prepared ascorbic acid and analyzed by spectrophotometer (Hitachi U-2001 Japan); Potassium (K), Sodium (Na), and Calcium (Ca) were determined by Flame Photometer by the method of Allen (1989). The microelements (Fe, CO, Cu, Mg, Mn and Zn) were determined through Atomic Absorption Spectrophotometer.
In vitro antioxidant studies
Quantification of total phenolics and tannins
The total phenolic content of different solvent extracts of M. indica fruits was determined by Folin ciocalteu method (Siddhuraju and Becker 2003). The same extract was used to estimate the tannins, after treatment with polyvinyl polypyrrolidone (PVPP). The amount of total phenolics and tannins were calculated as the Gallic acid equivalents (GAE).
Total flavonoid content
The total flavonoid content of fruit sample was assessed by the method described by Zhishen et al. (1999). The estimation was performed in triplicate analysis and the results were expressed as rutin equivalent (RE).
FRAP assay
The antioxidant capacities of fruit samples were estimated according to the procedure described by Pulido et al. (2000). Freshly prepared FRAP reagent was mixed with distilled water and 50 µL of test sample or methanol (for the reagent blank). The test samples and reagent blank were incubated at 37 °C for 30 min in a water bath. The absorbance readings were taken at 593 nm for the test samples and the results were calculated in ascorbic acid equivalents.
Metal chelating activity
The ferrous ion chelation of fruit extracts was estimated by the method of Dinnis et al. (1994). Briefly, 50 µL of FeCl2 was added to 1 mL of different concentration of the extract. The reaction was initiated by the addition of 0.2 mL of ferrozine solution. The mixture was vortexed and left for 10 min at room temperature. The absorbance of the solution was measured at 562 nm against deionized water which was used as blank. All the reagents without addition of sample extract were used as negative control. BHT was taken as standard and the results of metal chelating activity of fruit extract were expressed as EDTA equivalence.
Phosphomolybdenum assay
The antioxidant activity of samples was evaluated by the phosphomolybdenum method Prieto et al. (1999). An aliquot of 50 μl of sample solution (1 mM in dimethyl sulphoxide) was combined in a 4 mL vial with 1 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction mixer was incubated in a water bath at 95 °C for 90 min. After the samples had cooled to room temperature, the absorbance of the mixture was measured at 765 nm against a blank. The results were expressed as grams of ascorbic acid equivalents per gram extract (AAE).
ABTS radical cation scavenging activity
The total antioxidant activity of the plant extract was measured by ABTS·+ radical cation decolorization assay according to the method of Re et al. (1999). The unit of total antioxidant activity (TAA) is defined as the concentration of Trolox having equivalent antioxidant activity expressed as µM/g sample extract as dry matter.
DPPH radical scavenging activity
The antioxidant capacity of fruit extracts was examined in terms of contribution of hydrogen atoms or free radical scavenging ability, using the stable radical 2,2-diphenyle-2-picrylhydrazyl (DPPH·), according to the method of Blois (1958). IC50 values of the extract i.e., concentration of extract necessary to decrease the initial concentration of DPPH by 50 % was calculated.
Superoxide radical scavenging activity
The assay was based on the capacity of the plant extract to inhibit formazan formation by scavenging superoxide radicals generated in riboflavin-light-NBT system (Beauchamp and Fridovich 1971). The inhibition percentage of superoxide radical generation was calculated as following formula.
Hydroxyl radical scavenging activity
The scavenging activity of different solvent extract of fruit of M. indica and the reference standard rutin and BHT was calculated according to the procedure of Klein et al. (1981). The inhibition percentage of superoxide radical generation was calculated as per the formula (1).
Nitric oxide radical scavenging activity
The nitric oxide radical scavenging activity of different solvent extract of M. indica fruit extract was measured according to the method of Sreejayan Rao (1997). The inhibition percentage of superoxide radical generation was calculated as per the formula (1).
Scavenging of hydrogen peroxide radicals
The hydrogen peroxide scavenging ability of fruit extract was determined according to the method of Ruch et al. (1989). The percentage inhibition of hydrogen peroxide scavenging activity was calculated from the formula (1).
α-Amylase inhibition assay
Different concentrations of (50–250 µL) of the fruit extracts (1 mg/mL DMSO) and 500 µL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) having porcine pancreatic α-amylase enzyme (0.5 mg/mL) were incubated at 25 °C for 10 min. After that incubation 500 µL of 1 % starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) was added to the reaction mixture. Subsequently, the reaction mixture was incubated at 25 °C for 10 min after that 1.0 mL of dinitrosalicylic acid (DNS) was added. Finally the reaction was stopped by 5 min boiling water bath incubation and cooled to room temperature. The reaction mixture was diluted with addition of 10 mL distilled water, and absorbance was measured at 540 nm (Worthington 1993). The mixture of all other reagents and the enzyme with the exception of the test sample was used as a control, acarbose used as a standard and the results of α-amylase inhibition activity was expressed as in terms of IC50.
α-Glucosidase inhibition assay
Various concentrations of fruit extracts (50–250 µL) (1 mg/mL DMSO) and 100 µL of rat intestinal α-glucosidase (0.5 mg/mL) in 0.1 M phosphate buffer (pH 6.9) solution were incubated at 25 °C for 10 min. Then, 50 µL of 5 mM p-nitrophenyl-a-d-glucopyranoside in 0.1 M phosphate buffer (pH 6.9) solution was added. Reaction mixtures were incubated at 25 °C for 5 min and absorbance was taken at 405 nm by spectrophotometer (Apostolidis et al. 2007). The mixer of all other reagents and the enzyme with the exception of the test sample was used as a control; acarbose used as a standard and the results of α-amylase inhibition activity was expressed as in terms of IC50.
HPLC analysis
The acetone and methanol extract of fruits were subjected to HPLC analysis along with standard chemical markers on High Performance Liquid Chromatography system (Beckman, USA) equipped with UNIPOINT system software and the chromatographic separations were performed using a C18 Kromacil Column (250 × 4.6 mm), at a flow rate of 1 mL/min. Twenty microliters of extracts (2 mg/mL in methanol) and phenolic standard quercetin were prepared to obtain a final concentration of 100 µg/mL in methanol. They were loaded, injected by auto sampler and eluted through the column with a mobile phase system consisting of water: 0.4 % acetic acid: methanol: acetonitrile (70:20:5:5). The sample was monitored with UV detection at 280 nm at ambient temperature. Peak purity was checked by the software contrast facilities and the phenolic and flavonoid compounds were identified (Vinholes et al. 2011).
Statistical analysis
The results were statistically analyzed and expressed as mean (n = 3) ±standard deviation. Values are analyzed by Duncan’s multiple test range (One Way ANNOVA by statistical software SPSS 20 version) and p < 0.05 as considered significant value.
Results and discussion
Nutritional analysis
The proximate compositions of fruits of M. indica are shown in Table 1. Moisture content of fruit was found to be 80.95 %. The ash content was also determined as 85.5 mg/g. The crude protein and carbohydrate was found to be 45.6 and 25.12 mg/g, respectively. The obtained results proved that M. indica fruit can supply more balanced nutrients when compared to the other berries. The high protein content serves as a good supplement to cereals grains or as a meat substitute for human health.
Amino acids and minerals
The results of amino acids analysis are shown in Table 2. The results showed that the fruit exhibited higher cysteine (76.77 mg/g protein) and histidine (1.44 mg/g protein) content, when compared to other amino acids. Amino acids play a vital role as intermediates in plant and animal metabolism, and join together to form proteins. Most of the proteins are broken down by enzymes into amino acids and Absorbed from the small intestine. Nine amino acids, called essential, must come from the diet, including arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, tryptophan, and valine. Arginine, methionine, and phenylalanine are considered essential for reasons not directly related to lack of metabolic pathway, but because the rate of their synthesis is insufficient to meet the needs of the body (Spallholz et al. 1999). Histidine is considered an essential amino acid in children. Many of the fruits and vegetables contain all essential amino acids which required for humans (Young and Pellett 1994). The presence of one or more essential amino acids in adequate amounts increased the nutritive value of the protein. Hence, M. indica fruit as a source of amino acids can usually be assessed by comparison with FAO/WHO/UNU suggested pattern of essential amino acids which will serve as a better pathway for nutritional supplement.
The mineral content of M. indica fruits are shown in Table 3. N, K, Ca, Fe, Sl and Na content was 6412, 1300, 13,800, 800, 420 and 120 rpm, respectively. Generally, the elements like K and Ca are mostly present in all the berries like raspberry, blackberry, mulberry and strawberry (Pavlovic et al. 2013). Ca has been reported to be effective in building of skeletal structures and muscle functioning which was beneficial to human body so that they were thought to be used as food materials useful in health. From the results M. indica fruits were found to be good sources of K, Na, N and Ca. The intake of such micronutrient-rich foods helps to build a strong immune system, thereby helping the body to absorb, utilize and digest required body nutrients (Njoku and Ohia 2007). Higher amount of dietary K and Ca in humans plays a protective role against hypertension, stroke, cardiac dysfunctions, renal damage, hypercalcaemia, kidney stones, and osteoporosis (Demigne et al. 2004).
Quantification of total phenolics, tannins and flavonoids
Total phenolics, tannins and flavonoids content of different extracts of fruits of M. indica are shown in Table 4. Among all the different extracts, acetone extract of fruit contained total phenolic and tannin content of 6.53 g GAE/100 g extract and 3.21 g GAE/100 g extract, respectively. The chloroform extracts of fruit exhibited higher flavonoid content (21.33 g RE/100 g extract) compared to the other solvent extracts. Previous reports from the other berry fruits such as blackberry, highbush blueberries, murta, calafate, arrayán, chequén, and meli contained lower amount of phenolics and flavonoid compounds when compared to fruits of M. indica (Ramirez et al. 2015). Polyphenols (phenolics, tannins and flavonoids), extensively dispersed in plants have gained much attention due to their anti-mutagenic, antitumor, antioxidant, anti-diabetic, and free radical scavenging abilities which potentially have beneficial implications for human health (Davis et al. 2009). Polyphenolic compounds are secondary metabolites widely distributed in fruits and vegetables, mostly represented by flavonoids and phenolic acids. These antioxidant compounds donate an electron to the free radical and convert it into an innocuous molecule. Hence from these concepts it could be suggested that the fruits of M. indica can serve as a new source for potential health benefits.
In vitro antioxidant assays
The result showed (Table 4) that the ferric reducing ability of fruit acetone extract was higher (621.56 mM/g) and the least was of petroleum ether (260 mM/g). The method of metal chelating activity is based on chelation of Fe2+ ions by the reagent ferrozin, which is quantitative formation of a complex with Fe2+ ions (Dinnis et al. 1994). The metal chelating ability of different solvent extracts of M. indica fruit are shown in Table 4. Among all the extracts tested, acetone and methanol extracts showed better chelating ability. The metal chelating capacity of acetone extract of fruit was found to be 12.44 g EDTA equivalents/100 g extract. These results showed that the extracts could chelate irons and the values are substantial. The phosphomolybdenum method is based on the reduction of Mo (VI) to Mo (V) by the antioxidant compounds and the formation of green phosphate/Mo(V) complex. The total antioxidant capacity of different solvent extracts of fruit of M. indica are shown in Table 4. Among different solvent extracts analyzed, the better antioxidant capacity was shown by methanol extract of fruit (676 mg AA equivalents/g extract). The lower antioxidant capacity was found for petroleum ether extract of fruit (131 mg AA equivalents/g extract). Acetone extract of M. indica fruit show maximum capability of ferric reducing, chelating metal ions and phosphomolybdenum reduction activities. The results of other berry fruits showed the minimum amount of activity especially, the fruits of Vaccinium corymbosum (Highbush blueberries), Luma chequén, Luma apiculata, Ugni molinae, Royle berry and Amomyrtus meli shows minimum amount of ferric reducing property when compared to this M. indica fruits (Ramirez et al. 2015; Pradhan and Saha 2015).
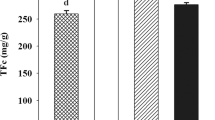
All the radical scavenging assays such as ABTS·+, DPPH·, superoxide, hydroxyl, nitric oxide and hydrogen peroxide radicals shows the same results. The results of ABTS·+ scavenging activities of different solvents of fruit extracts of M. indica are shown in Table 4. The acetone extracts of fruit showed higher radical scavenging activity (3719.23 µmol TE/g extract) as compared to the other solvent extracts. All the extracts of M. indica were analyzed with DPPH radicals and results are shown in Fig. 1. The IC50 of methanol extract of fruit was found to be 68.82 µg/mL followed by petroleum ether and chloroform extracts were exhibit 69.69 and 69.49 µg/mL respectively. The results of superoxide, hydroxyl radical, nitric oxide and hydrogen peroxide radicals scavenging activities of fruit of M. indica are shown in Fig. 2. Superoxide radical scavenging activity was found to be higher in hot water extract of fruit 32.0 %. Meanwhile, the acetone extract of fruit showed the better hydroxyl radical scavenging activity 66.5 % when compared to other solvent extracts. The nitric oxide scavenging activity of M. indica was higher in both methanol (88.50 %) and acetone extract of fruit (81.50 %) while acetone extract exhibited higher percentage (50.78 %) of hydrogen peroxide radical scavenging activity. All the results were compared with the reference compounds BHT and rutin.
Superoxide, hydroxyl, nitric oxide and hydrogen peroxide radical scavenging activities of M. indica fruit extract. Values are mean of different aliquot determination (n = 3) ±standard deviation. Mean values followed by different superscript letters indicate significant statistical difference (p < 0.05)
Similar observations specifying that the dry heated samples showed high radical scavenging activity in fruits and leaves (Saravanan and Parimelazhagan 2014). Hagerman et al. (1998) have conveyed that the high molecular weight phenolics have more ability to diminish free radicals (ABTS·+) and that effectiveness depends on the molecular weight, the number of aromatic rings and nature of hydroxyl groups substitution than the specific functional groups. An earlier study have stated that higher amount of phenolics rich fruits scavenge the DPPH radicals, superoxide, hydroxyl, nitric oxide and hydrogen peroxide radicals (Saravanan and Parimelazhagan 2014; Ozarda et al. 2015). The hydrogen molecules from phenolics and flavonoids of the plant extracts possibly react with the fenton reaction and scavenge the hydroxyl radicals. In the meantime, hydrogen peroxide developed from the cellular membrane is removed by three important antioxidant enzymes namely catalases, glutathione peroxidases, and peroxiredoxins. It can be inferred that the fruit extract possibly produce antioxidant enzymes and may have played a potential role in cell membrane and hunted hydrogen peroxide radicals. Previous reports (Joseph et al. 2014) revealed that all the berry fruits such as bilberry (Vaccinium myrtillus), black currant (Ribes nigrum), black raspberry (Rubus occidentalis), blueberry (Vaccinium corymbosum), chokeberry (Aronia melanocarpa), cranberry (Vaccinium macrocarpon), sea buckthorn berry (Hippophae rhamnoides), strawberry (Fragaria × ananassa), wolfberry (Lycium barbarum) possess enormous amount of flavonoid like rutin, quercetin and anthocyanin compounds with potential radical scavenging, antioxidant and anti-inflammatory properties. Hence, the present results also confirmed that the fruits of M. indica are a good source of natural phenolic and flavonoid compound with antioxidative property.
α-Amylase and α-glucosidase inhibition assays
Diabetes mellitus, insulin secretion deficiency causes severe chronic diabetic condition. The IC50 values for α-amylase and α-glucosidase of methanol extracts of M. indica were 37.80 and 41.10, respectively while of aceton extract was 23.74 and 37.72 µg/mL respectively (Fig. 3). Both the extracts inhibit the key enzymes significantly when compared to the other solvents, meanwhile the both extracts expressed better activity which is comparable with standard acarbose. During the hydrolysis of carbohydrates the key enzymes α-amylase and α-glucosidase inhibition is used as a therapeutic approach for controlling postprandial hyperglycemia. In the interim the inhibition of these enzymes reduced the glucose absorption rate and concentration of postprandial serum glucose. This effect delayed the degradation of starch and oligosaccharides, which in turn caused a decrease in the absorption of glucose and consequently inhibited the increase in postprandial blood glucose (Lee et al. 2010). Several other berries have also shown promising activity via various mechanism of action earlier (Yang and Kortesniemi 2015). Hence, from the results, the consumption of M. indica fruit could effectively reduce the levels of α-amylase and α-glucosidase and significantly decrease the postprandial increase of blood glucose level.
HPLC analysis
The results of HPLC analysis indicated that the acetone and methanol extracts from the fruits of M. indica showed the presence of various polyphenolic and flavonoid compounds such as chlorogenic acid, rutin, quercetin and catechin, which was confirmed from their retention time when compared with the standards (RT 18.040, 41.959 and 47.452 min for 18.237, 43.750 and 45.489 min compounds respectively) (Fig. 4 A, B).
Gaitonde and Naik (1989) previously reported that the leaves of M. indica and M. macrophylla contained the natural bioactive compound of quercetin. It has been reported that quercetin, quercetin derivatives and phenolic compounds chlorogenic acid and caffeic acid greatly reduces oxidative damages and chronic diseases like diabetic, cardiovascular, proliferative and inflammatory related diseases (Zhang et al. 2008). Further, all the berry fruits such as lingonberry (Vaccinium vitis-idaea), bilberry (Vaccinium myrtillus), cranberries (Vaccinium oxycoccos), black currant (Ribes nigrum), black raspberry (Rubus occidentalis), blueberry (Vaccinium corymbosum), chokeberry (Aronia melanocarpa), cranberry (Vaccinium macrocarpon), sea buckthorn berry (Hippophae rhamnoides), strawberry (Fragaria × ananassa), wolfberry (Lycium barbarum) possess enormous amount of flavonoid like rutin, quercetin and anthocyanin compounds with potential radical scavenging, antioxidant, anti-inflammatory and anti-diabetic properties (Joseph et al. 2014; Yang and Kortesniemi 2015). The present HPLC result proved the fruit extracts of M. indica possess flavonoid, phenolics and their derivatives. Hence, it can endorse that the significant radical scavenging, antioxidant and anti-diabetic effects of the fruit extract may be due to the quercetin like flavonoid and other phenolic compounds observed in the present study.
Conclusion
Maesa indica is used in folk medicine to treat various diseases. The present study revealed that the fruit extracts of M. indica showed significant nutritional, radical scavenging, antioxidant and anti-diabetic properties. It can be concluded that M. indica fruit is a prominent source to inhibit the key enzyme linked to type 2 diabetes (α-amylase and α-glucosidase) and as natural nutritional supplements.
References
Allen SE (1989) Chemical analysis of ecological material, 2nd edn. Blackwell Scientific Publications, London
Apostolidis E, Kwon YI, Shetty K (2007). Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov Food Sci Emerg Technol 8:46–54
AOAC (1995) Official methods of analysis. Method 1094, 16th edn. Association of Official Analytical Chemists, Arlington
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Chaudhuri AB (2007) Endangered medicinal plants. Daya Publishing House, Deva Ram Park, Tri Nagar, New Delhi, pp 102, 175
Davis JM, Murphy EA, Carmichael MD, Davis B (2009) Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol 296: R1071–1077
Demigne C, Sabboh H, Remesy C, Meneton P (2004) Protective effects of high dietary potassium: nutritional and metabolic aspects. J Nutr 134:2903–2906
Dinnis TCP, Madeira VMC, Almeida LM (1994) Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169
Gaitonde RV, Naik PL (1989) Estimation of Quercetin in the Leaves of Maesa indica wall by a new colorimetric method. Curr Sci 58:982–983
Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL (1998) High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J Agric Food Chem 46:1887–1892
Hassan FA, Ismail A, Hamid AA, Azlan A, Al-sheraji SH (2011) Characterisation of fibre-rich powder and antioxidant capacity of Mangifera pajang K. fruit peels. Food Chem 126:283–288
Ishida Y, Fujita T, Asai K (1981) New detection and separation method for amino acids by high-performance liquid chromatography. J Chromatogr A 204:143–148
Jassim SAA, Naji MA (2003) Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol 95:412–427
Joseph SV, Edirisinghe I, Burton-Freeman BM (2014) Berries: anti-inflammatory Effects in humans. J Agric Food Chem 62:3886–3903
Klein SM, Cohen G, Cederbaum AI (1981) Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochemistry 20:6006–6012
Lee SH, Park MH, Heo SJ, Kang SM, Ko SC, Han JS, Jeon YJ (2010) Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem Toxicol 48:2633–2637
Njoku PC, Ohia CC (2007) Spectrophometric estimation studies of mineral nutrient in three cocoyam cultivars. Pak J Nutr 6:616–619
Ozarda O, Demirkoz AB, Özdemir M (2015) Sensory characteristics and antioxidant capacity of red raspberry extract as a preservative in fruity flavoured beverages. J Food Sci Technol. doi:10.1007/s13197-015-1763-x
Pavlovic AV, Dabic DC, Momirovic NM, Dojcinovic BP, Milojkovic-Opsenica DM, Tesic Z, Natic MM (2013) Chemical composition of two different extracts of berries harvested in Serbia. J Agric Food Chem 61:4188–4194
Pokharkar RD, Saraswat RK, Kotkar S (2010) Survey of plants having antifertility activity from western ghat area of Maharastra state. J Herb Med Toxicol 4:71–75
Pradhan PC, Saha S (2015) Anthocyanin profiling of Berberis lycium Royle berry and its bioactivity evaluation for its nutraceutical potential. J Food Sci Technol. doi:10.1007/s13197-015-2117-4
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48:3396–3402
Ramirez JE, Zambrano R, Sepúlveda B, Kennelly EJ, Simirgiotis MJ (2015) Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem 17:106–114
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med 26:1231–1237
Ruch RJ, Cheng SJ, Klaunig JE (1989) Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogen 10:1003–1008
Sadasivam S, Manickam A (2008) Biochemical methods, 3rd edn. New Age International (P) Limited, New Delhi, pp 8–12
Saravanan S, Parimelazhagan T (2014) In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci Hum Wellness 3:56–64
Siddhuraju P, Becker K (2003) Studies on antioxidant activities of mucuna seed (Mucuna pruriens var utilis) extract and various non-protein amino/imino acids through in vitro models. J Sci Food Agric 83:1517–1524
Spallholz JE, Boylan LM, Driskel JA (1999) Nutrition: chemistry and biology, 2nd edn. CRC Press, Boca Raton
Sreejayan Rao MN (1997) Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol 49:105–107
Tulipani S, Mezzetti B, Capocasa F, Bompadre S, Beekwilder J, de Vos CHR, Battino M (2008) Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J Agric Food Chem 56:696–704
Vinholes J, Grosso C, Andrade PB, Gil-Izquierdo A, Valentão P, de Pinho PG, Ferreres F (2011) In vitro studies to assess the antidiabetic, anti-cholinesterase and antioxidant potential of Spergularia rubra. Food Chem 129:454–462
Wang H, Du YJ, Song HC (2010) α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem 123:6–13
Worthington V (1993) Alpha amylase. Worthington Biochemical Corp, Lakewood
Yang B, Kortesniemi M (2015) Clinical evidence on potential health benefits of berries. Curr Opin Food Sci 2:36–42
Young VR, Pellett PL (1994) Plant proteins in relation to human protein and amino acid nutrition. Am J Clin Nutr 59(5 Suppl):1203S–1212S
Zhang Y, Seeram NP, Lee R, Feng L, Heber D (2008) Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J Agric Food Chem 56:670–675
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig 4
HPLC Analysis of M. indica fruit extracts. (A) HPLC profiles of fruit acetone extract at 280 nm. (B) HPLC profiles of fruit methanol extract at 280 nm (DOC 731 kb)
Rights and permissions
About this article
Cite this article
Shanmugam, S., Baby, J. ., Chandran, R. et al. Maesa indica: a nutritional wild berry rich in polyphenols with special attention to radical scavenging and inhibition of key enzymes, α-amylase and α-glucosidase. J Food Sci Technol 53, 2957–2965 (2016). https://doi.org/10.1007/s13197-016-2263-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2263-3