Abstract
Lotus seed epicarp, a byproduct of lotus seed production process, is usually discarded as a waste. In this study, antioxidant and anti-α-amylase activities of freeze-dried water and various methanol extracts of lotus seed epicarp were evaluated. The extract obtained by 80% methanol exhibited the strongest DPPH and ABTS radical scavenging activity and ferric reducing power, as well as the greatest inhibitory potential on α-amylase. The excellent antioxidant and α-amylase inhibitory activities of 80% methanol extract might be attributed to its highest concentrations of total phenolics, flavonoids, and condensed tannins. The inhibition kinetic analysis revealed that the 80% methanol extract was a reversible and uncompetitive-type inhibitor of α-amylase. Furthermore, based on MALDI-TOF-MS and thiolysis-HPLC-ESI-MS, the main active components present in 80% methanol extract were identified to be B-type heteropolymeric condensed tannins built up of mixtures of propelargonidins, procyanidins, and prodelphinidins, with the predominance of procyanidins and epicatechin as the main constitutive units. The results obtained suggested that lotus seed epicarp could be exploited as a potential source of natural antioxidants and α-amylase inhibitors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antioxidants are substances that are able to delay or inhibit the oxidation process by terminating the oxidative chain reaction [1]. Increasing evidence has suggested that antioxidant supplementation or consumption of antioxidant-rich diets is associated with the reduction in the risk of some human diseases caused by oxidative damage [2, 3]. Alpha-amylase is a key enzyme involved in the digestion of carbohydrates [4]. Inhibition of α-amylase capable of controlling the levels of postprandial hyperglycemia [5, 6] is considered to be an effective strategy for the prevention or treatment of diabetes mellitus [5, 7]. Great attention has recently been paid to natural and effective α-amylase inhibitor, due to low toxicity and good health benefit [6, 8,9,10].
Nelumbo nucifera, Gaertn., commonly known as lotus, is a perennial aquatic plant of the Nymphaeaceae family. In China, it is mainly distributed in Hubei, Hunan, Jiangxi, Fujian, and Taiwan provinces [11]. Lotus seed epicarp, the inedible part of N. nucifera, is generated during the manufacture of lotus seeds. The annual production of lotus seed epicarp now exceeds 5000 tons in China [12], but most of them are discarded as a waste [13]. Previous studies have shown that lotus seed epicarp is rich in flavonoids [14, 15] and possesses potent antioxidant and anti-tyrosinase activities [13, 16, 17]. Qi and Zhou [18] demonstrated that lotus seed epicarp extracts can effectively delay lipid oxidation in homogenized pork and are recommended as natural antioxidant additive for sausage. Therefore, lotus seed epicarp may be a good candidate for further development of nutraceuticals and functional foods. Moreover, to our knowledge, the information on anti-α-amylase property of lotus seed epicarp has not previously been reported.
In the present study, methanol, 80% methanol, 20% methanol, and water were used as solvents for the extraction of lotus seed epicarp. The extracts obtained were used to evaluate their antioxidant and anti-α-amylase activities, as well as total phenolic, flavonoid, and condensed tannin concentrations. A structural identification of the active components that may be contributing to these functions were also performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and thiolysis coupled with reversed-phase high-performance liquid chromatography-electrospray ionization mass spectrometry (thiolysis-HPLC-ESI-MS) analyses.
Materials and Methods
Plant Materials and Chemicals
Fresh lotus seeds (N. nucifera) were purchased from a local market in Jingzhou city, Hubei province, People’s Republic of China. After being manually peeled, the lotus seed epicarp was immediately freeze-dried at − 56 °C for 72 h, then ground to pass through the 40-mesh sieve and stored at − 20 °C prior to extraction. The Folin-Ciocalteu reagent, gallic acid, corn starch (S4126), α-amylase (A3176, from porcine pancreas), catechin, epicatechin, gallocatechin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,4,6-tripyridyl-s-triazine (TPTZ), 2,5-dihydroxybenzoic acid (DHB), cesium chloride, and benzyl mercaptan were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Acetonitrile and trifluoroacetic acid (TFA) for HPLC analysis were of HPLC grade. All other reagents used were of analytical grade.
Preparation of Lotus Seed Epicarp Extracts
The freeze-dried powder (0.5 g) of lotus seed epicarp was extracted with 20 mL of methanol, 80% methanol, 20% methanol, and distilled water separately at room temperature for 24 h. The extracts were centrifuged at 4500 rpm for 10 min and collected. Each extraction process was repeated three times. The collected extracts were combined, concentrated with a vacuum evaporator, and dried with a freeze drier. Then, the freeze-dried methanol, 80% methanol, 20% methanol, and water extracts with the yields of 31.06, 34.23, 27.25, and 25.58% (w/w) were obtained, respectively. All these samples were stored at 4 °C in a fridge until determinations.
Determination of Phenolic Concentrations
Total phenolic concentrations (TPc) of the extracts were estimated by the Folin-Ciocalteu method described by Wei et al. [19]. Briefly, 0.2 mL aliquot of appropriately diluted extracts was added to a test tube containing 0.3 mL of distilled water. 0.5 mL of Folin-Ciocalteu reagent and 2.5 mL 20% Na2CO3 solution were added to the mixture and shaken vigorously. The absorbance at 725 nm was recorded after incubation for 40 min at room temperature. A mixture of distilled water and reagents was used as a blank. TPc of the extracts were expressed as milligram gallic acid equivalents.
Total flavonoid concentrations (TFc) of the extracts were measured according to the method described by Kim et al. [20] with some modifications. Briefly, 1 mL aliquot of appropriately diluted extracts was added to a 10-mL volumetric flask containing 4.0 mL distilled water. At zero time, 0.3 mL 5% NaNO2 was added to the flask. After 5 min, 0.3 mL 10% AlCl3·6H2O solution was added. At 6 min, 2 mL 1 M NaOH was added to the mixture. Immediately, the reaction flask was diluted to volume with the addition of 2.4 mL of distilled water and mixed thoroughly. Absorbance of the mixture, pink in color, was determined at 504 nm versus prepared distilled water blank. TFc of the extracts were expressed as milligram catechin equivalents.
Total condensed tannin concentrations (TCc) of the extracts were determined using the butanol-HCl method introduced by Terrill et al. [21]. Briefly, 1 mL aliquot of appropriately diluted extracts was transferred into test tubes. After addition of 6 mL 95% butanol-HCl reagent, test tubes were shaken vigorously and put into a water bath at 95 °C for 75 min. After cooling, the absorbance was recorded at 550 nm against a blank, containing 1 mL of distilled water instead of the extract. Purified lotus seed epicarp condensed tannins was used as the standard. The purification scheme established by Wei et al. [22] was used to obtain the purified condensed tannins from lotus seed epicarp.
Determination of Antioxidant Activity
DPPH radical scavenging activity of the extracts was evaluated according to the method of Brand-Williams et al. [23]. Briefly, 3.9 mL of DPPH solution (25 mg/L in methanol) was mixed with 0.1 mL of the sample. An equal amount of methanol and DPPH served as control. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. The absorbance at 517 nm was measured and the ability of the sample to scavenge the DPPH radical was calculated using the following equation: DPPH scavenging effect (%) = [(A1 − A2)/A1] × 100, where A1 is the absorbance of the control, and A2 is the absorbance of the sample. The IC50 value is the concentration of the sample that could scavenge 50% of the DPPH radicals.
ABTS radical scavenging activity of the extracts was measured as described by Re et al. [24] with slight modifications. Briefly, the ABTS radical was prepared from the reaction of 7 mM ABTS and 2.45 mM potassium persulfate. After incubation in the dark at room temperature for 16 h, the radical solution was further diluted with 80% ethanol to an absorbance of 0.70 ± 0.05 at 734 nm. 3.9 mL of diluted ABTS solution was mixed with 0.1 mL of the sample. The mixture was shaken vigorously and allowed to stand at room temperature for 6 min. The absorbance at 734 nm was recorded and the ability of the sample to scavenge the ABTS radical was calculated using the following equation: ABTS scavenging effect (%) = [(A1 − A2)/A1] × 100, where A1 is the absorbance of the control, and A2 is the absorbance of the sample. The IC50 value is the concentration of the sample that could scavenge 50% of the ABTS radicals.
Ferric reducing antioxidant power (FRAP) of the extracts was determined as described in our previous paper [22]. Briefly, 0.1 mL of the sample or methanol (the blank control) was mixed with 3 mL of freshly prepared FRAP reagent, which consisted of 300 mM acetate buffer (pH 3.6), 10 mM TPTZ in 40 mM hydrochloric acid, and 20 mM ferric chloride in the ratio of 10:1:1 (v/v/v). The mixture was shaken vigorously and allowed to stand at room temperature for 10 min. The absorbance of reaction mixture was measured by using a spectrophotometer at 593 nm. The FRAP value was expressed in millimole ascorbic acid equivalents (mmol AAE/g extract).
Determination of Anti-α-amylase Inhibitory Activity
The α-amylase inhibitory activity of the extracts was performed using the method reported by Ranilla et al. [7] and Fu et al. [25] with slight modifications. In brief, 0.2 mL of sample solution at different concentrations was pre-mixed with 0.2 mL of α-amylase solution (0.1 mg/mL, dissolved in 0.02 M sodium phosphate buffer, PH 6.9 with 6.7 mM NaCl). Following incubation at 37 °C for 10 min, 0.6 mL of 1% (w/w) starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 6.7 mM NaCl) was added to each tube. The reaction mixture was carried out at 37 °C for 3 min and the reaction was stopped by adding 1.0 mL of dinitrosalicylic acid color reagent. The test tubes were then placed in a water bath at boiling point for 8 min and cooled down to room temperature. The reaction mixture was then diluted with 3 mL of distilled water and the absorbance was recorded at 540 nm. The inhibitory activity of the sample against α-amylase was calculated as follows: α-amylase inhibitory activity (%) = [(A1 − A2)/A1] × 100, where A1 is the absorbance of control (buffer in place of sample) and A2 is the absorbance in the presence of sample. The IC50 value is the concentration of the sample that could inhibit 50% of the α-amylase activity.
MALDI-TOF-MS Analysis of Active Components
The MALDI-TOF-MS spectra of the extracts were recorded on a Bruker Reflex III instrument (Bruker, Germany). The method described by Wei et al. [19] was applied. The irradiation source was a pulsed nitrogen laser with a wavelength of 337 nm, and the duration of the laser pulse was 3 ns. In the positive reflectron mode, an accelerating voltage of 20.0 kV and a reflectron voltage of 23.0 kV were used. The DHB was used as the matrix, and Cs+ was selected as the cationization reagent. The sample solutions (10 mg/mL), the cesium chloride solution (1.52 mg/mL), and the matrix solution (10 mg/mL) were mixed at a volumetric ratio of 1:1:3. The mixture (1 μL) was then spotted to the steel target.
Thiolysis-HPLC-ESI-MS Analysis of Active Components
Thiolysis was carried out by the method described by Li et al. [26] and Wei et al. [19]. Thiolytic products of the extracts were analyzed by reversed-phase HPLC-ESI-MS, which was performed using an Agilent 1200 system (Agilent, USA) interfaced to a QTRAP 3200 (Applied Biosystems, USA) with a Hypersil ODS column (4.6 mm × 250 mm, 5 μm) (Elite, China). Two solvents, namely A = 0.5% (v/v) TFA in aqueous and B = CH3CN, were used. The gradient condition was 0–45 min, 12–80% B; 45–50 min, 80–12% B. The flow rate was set at 1 mL/min and the chromatogram was detected at 280 nm.
Statistical Analysis
Data were expressed as the mean of three replicate determinations plus or minus the standard deviation (SD). One-way analysis of variance (ANOVA) and the Student-Newman-Keuls test were used to examine the difference between samples (P < 0.05). The correlation coefficients of the antioxidant and anti-α-amylase activities versus the total phenolic, flavonoid, and condensed tannin concentrations of tested samples were carried out using linear regression analysis. All statistically analyses were done with SPSS 16.0 for windows.
Results and Discussion
TPc, TFc, and TCc of the Extracts
Phenolic compounds are considered to be the major contributor to the biological activity of plant extracts. It is thus worth determining TPc, TFc, and TCc in the extracts from lotus seed epicarp. TPc and TFc were expressed as the authentic standards gallic acid and catechin equivalents, respectively. While for quantifying TCc, the condensed tannins purified from lotus seed epicarp was selected as the standard. In this study TCc was measured by the butanol-HCl assay based on the acid-catalyzed oxidative depolymerization of condensed tannins to produce colored anthocyanidins [27, 28]. According to the report by Schofield et al. [28], anthocyanidin color yields are influenced by the type of condensed tannins being assayed. Herein, condensed tannins from the plant material are recommended as the standards under study [29, 30].
As can be seen from the Fig. 1, the TPc, TFc, and TCc of the extracts from lotus seed epicarp varied obviously from 449.31 ± 8.18 to 535.21 ± 10.06 mg/g, 259.19 ± 6.16 to 306.80 ± 6.77 mg/g, and 154.80 ± 7.12 to 265.10 ± 7.83 mg/g, respectively. The highest values of TPc, TFc, and TCc were found in 80% methanol extract, whereas the lowest were detected in water extract. The TPc, TFc, and TCc of lotus seed epicarp extracts were arranged in the same order of 80% methanol extract > 20% methanol extract > methanol extract > water extract (P < 0.05). Results obtained indicated that lotus seed epicarp contained remarkable levels of phenolics, flavonoids, and condensed tannins. The increase of methanol concentration in water was significantly influent in the extraction of phenolic compounds and the 80% methanol would be a good choice for the extraction of lotus seed epicarp. Some previous studies also found that the mixtures of alcohol and water are more suitable for extracting phenolic compounds than the corresponding mono-component solvent system [31,32,33].
Antioxidant Activity of the Extracts
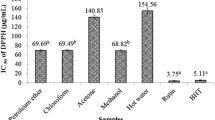
The extracts from lotus seed epicarp exhibited concentration-dependent antioxidant activities as evaluated by employing three in vitro antioxidant models, including DPPH, ABTS, and FRAP (Fig. 2a–c). The antioxidant activities of the extracts were expressed using IC50 or FRAP values. The IC50 values of water extract, 20% methanol extract, 80% methanol extract, and methanol extract of lotus seed epicarp for the DPPH assay were 159.35 ± 0.54, 138.47 ± 0.32, 110.95 ± 0.28, and 140.47 ± 1.12 μg/mL, and for the ABTS assay were 105.78 ± 0.47, 90.06 ± 0.45, 80.69 ± 0.52, and 91.12 ± 0.57 μg/mL, respectively (Fig. 3a, b). The lower IC50 value, the higher is the free radical scavenging power. Evidently, 80% methanol extract exhibited the strongest DPPH and ABTS radical scavenging activity in comparison to other extracts. In addition, among all the extracts tested, 80% methanol extract also showed the highest ferric reducing activities with the FRAP value of 4.04 ± 0.07 mmol AAE/g, successively followed by methanol extract, 20% methanol extract, and water extract, with FRAP values of 3.66 ± 0.04, 3.46 ± 0.03, and 2.86 ± 0.02 mmol AAE/g, respectively (Fig. 3c). Result obtained on the basis of FRAP method was similar to the findings of DPPH and ABTS radical scavenging assays.
DPPH radical scavenging activity (IC50), ABTS radical scavenging activity (IC50), ferric reducing antioxidant power (FRAP), and α-amylase inhibitory activity (IC50) of extracts from lotus seed epicarp: water extract (WE), 20% methanol extract (20% ME), 80% methanol extract (80% ME), and methanol extract (ME)
Furthermore, the correlations of TPc, TFc, and TCc against the antioxidant activities of the extracts from lotus seed epicarp based on the DPPH, ABTS, and FRAP assays were also estimated. It was found that the correlation coefficients were all satisfactory with R2 values ranging from 0.686 to 0.910 (Table 1), implying that the TPc, TFc, and TCc in lotus seed epicarp extracts might contribute to their radical scavenging activities and ferric reducing powers. Comparable correlations also existed between antioxidant activities and total phenolics, flavonoids, or condensed tannins of the extracts from the leaf and fruit of Averrhoa carambola [34] and the flower of Litchi chinensis [35].
Inhibitory α-Amylase Activity of the Extracts
Inhibitory effects of the extracts from lotus seed epicarp against α-amylase are presented in Fig. 4a. It was found that all the extracts with inhibitory potential on α-amylase behaved in a concentration-dependent manner. At a concentration of 12 μg/mL, water extract, 20% methanol extract, 80% methanol extract, and methanol extract inhibited α-amylase by 14, 44, 91, and 84%, respectively. The IC50 values of lotus seed epicarp extracts vary significantly, as displayed in Fig. 3d. A lower IC50 value represents a higher anti-α-amylase activity. Obviously, 80% methanol extract possessed the strongest α-amylase inhibitory activity with the lowest IC50 value of 7.81 ± 0.08 μg/mL, followed by methanol extract, 20% methanol extract, and water extract, with IC50 values of 8.39 ± 0.13, 13.34 ± 0.27, and 30.03 ± 1.83 μg/mL, respectively. The results suggested that it is likely to use 80% methanol extract of lotus seed epicarp as α-amylase inhibitor to treat diabetes mellitus. In addition, from the correlation analysis, the TPc, TFc, and TCc in the extracts contributed to the α-amylase inhibitory activity at P < 0.01 or P < 0.05 level (Table 1). Herein, phenolic compounds would probably play an important role in the anti-α-amylase activity of lotus seed epicarp extracts. Similar relationships have also been reported by Wang et al. [36], who found that α-amylase inhibitory activity of tea fruit peel extracts significantly correlated with their concentrations of total phenolics and flavonoids.
a Inhibitory activities of lotus seed epicarp extracts against α-amylase: water extract (WE), 20% methanol extract (20% ME), 80% methanol extract (80% ME), and methanol extract (ME). b Inhibitory mechanism and c inhibitory type of 80% methanol extract against α-amylase. The concentrations of the 80% methanol extract for curves 1–4 were 0, 5.0, 10.0, and 15.0 μg/mL, respectively
The inhibitory mechanism of 80% methanol extract on α-amylase activity was also determined. As observed in Fig. 4b, the plots of the enzyme activity versus the concentrations of the enzyme at varying concentrations of 80% methanol extract yielded a suite of straight lines, which all passed through the original point and brought about a decrease in the slope of the line with increasing the 80% methanol extract concentration. The consequence indicated that the inhibitory mechanism of 80% methanol extract of lotus seed epicarp on the activity of α-amylase belonged to be reversible [25, 37]. Moreover, the inhibitory mode of 80% methanol extract on α-amylase action was further subjected to analysis by Lineweaver-Burk plot. The double-reciprocal image of 1/v against 1/[S] at varying concentrations of 80% methanol extract gave a family of parallel lines (Fig. 4c). According to the report by Hargrove et al. [37], 80% methanol extract from lotus seed epicarp was an uncompetitive-type inhibitor of α-amylase.
Identification of Active Components in the Extract
In the present study, due to 80% methanol extract from lotus seed epicarp exhibited the strongest antioxidant capacity and inhibitory activity against α-amylase, the active components present were further investigated by MALDI-TOF-MS and thiolysis-HPLC-ESI-MS methods. Figure 5a shows the positive-ion MALDI-TOF mass spectrum of 80% methanol extract recorded as CS+ adducts in the reflectron mode. The active components were characterized by mass spectrum with a series of periodic peaks separated by 288 Da. On the basis of the report by Reed et al. [38], it was equivalent to one (epi)catechin unit between each polymer. From the detail of 4-mers region of the spectrum (Fig. 5b), the major peaks were accompanied by peaks that were 16 Da smaller or larger. They might be attributed to the coexistence of (epi)afzelechin and (epi)gallocatechin units [38]. Furthermore, the mass signals at the distance of 132 and 152 Da following the main set of peaks were also detected (Fig. 5c). The former might arise from two possibilities: the substitution of a pentoside [38] or synchronous attachment of two Cs+ and loss of a proton [M + 2Cs – H]+ [19, 39]. And the latter was likely due to the addition of one galloyl group at the heterocyclic C-ring [34, 38]. The spectra did not contain the characteristic ions with 2 Da multiples lower than those described peaks for heteropolyfalvan-3-ols. It could be concluded that the nature of interflavan bonds were composed solely of B-type linkages. These results suggested that the major active components present in 80% methanol extract were built up of mixtures of propelargonidins, procyanidins, and prodelphinidins, with the predominance of procyanidins.
Structural identification of the active components present in 80% methanol extract from lotus seed epicarp. a MALDI-TOF-MS positive reflectron mode mass spectrum of 80% methanol extract. b and c Enlarged spectra of tetramer and pentamer series showing different chemical constitutions. d Reversed-phase HPLC chromatogram of thiolytic products of 80% methanol extract. Peak numbering: 1, gallocatechin; 2, catechin; 3, epicatechin; 4, (epi)afzelechin; 5, (epi)gallocatechin benzylthioether; 6, (epi)gallocatechin-3-O-gallate benzylthioether; 7, epicatechin benzylthioether; 8, (epi)afzelechin benzylthioether; 9, benzyl mercaptan; 10, thiolytic byproduct
Reversed-phase HPLC chromatogram of thiolytic products of 80% methanol extract is shown in Fig. 5d. Peak identification was performed by comparing retention times and mass spectra with those of reference standards or literature data [40, 41]. The dominant peak observed was identified to be epicatechin benzylthioether (peak 7). Seven other smaller peaks for gallocatechin (peak 1), catechin (peak 2), epicatechin (peak 3), (epi)afzelechin (peak 4), (epi)gallocatechin benzylthioether (peak 5), (epi)gallocatechin-3-O-gallate benzylthioether (peak 6), and (epi)afzelechin benzylthioether (peak 8) were also detected. These chromatographic observations suggested that the active components present in 80% methanol extract predominately consisted of procyanidins together with a few propelargonidins and prodelphinidins. Epicatechin was the main constitutive units. These results agreed well with the findings revealed by MALDI-TOF-MS analysis.
Conclusions
In conclusion, increase of methanol concentration in water was significantly influent in the extraction of phenolic compounds from lotus seed epicarp. Among all the extracts examined, 80% methanol extract exhibited the strongest antioxidant and α-amylase inhibitory activities, accompanied with the highest concentrations of total phenolics, flavonoids, and condensed tannins. The major active components present in 80% methanol extract were predominantly composed of procyanidin-type condensed tannins, consisting essentially of epicatechin units linked by B-type interflavan bonds. The present study implied that the lotus seed epicarp, usually discarded as a waste, could be effectively used to develop functional foods and potential antidiabetic agents in the future.
References
Yanishlieva, N. V., Marinova, E., & Pokorny, J. (2006). Natural antioxidants from herbs and spices. European Journal of Lipid Science and Technology, 108(9), 776–793.
Mattei, J., Malik, V., Wedick, N. M., Hu, F. B., Spiegelman, D., Willett, W. C., & Campos, H. (2015). Reducing the global burden of type 2 diabetes by improving the quality of staple foods: the global nutrition and epidemiologic transition initiative. Globalization and Health, 11(1), 23.
Shao, Y. F., & Bao, J. S. (2015). Polyphenols in whole rice grain: genetic diversity and health benefits. Food Chemistry, 180, 86–97.
Tsujita, T., Shintani, T., & Sato, H. (2013). α-Amylase inhibitory activity from nut seed skin polyphenols. 1. Purification and characterization of almond seed skin polyphenols. Journal of Agricultural and Food Chemistry, 61(19), 4570–4576.
Tarling, C. A., Woods, K., Zhang, R., Brastianos, H. C., Brayer, G. D., Andersen, R. J., & Withers, S. G. (2008). The search for novel human pancreatic α-amylase inhibitors: high-throughput screening of terrestrial and marine natural product extracts. Chembiochem, 9(3), 433–438.
Sudha, P., Zinjarde, S. S., Bhargava, S. Y., & Kumar, A. R. (2011). Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complementary and Alternative Medicine, 11, 5.
Ranilla, L. G., Kwon, Y. I., Apostolidis, E., & Shetty, K. (2010). Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresource Technology, 101(12), 4676–4689.
Li, Q., Chen, J., Li, T., Liu, C. M., Zhai, Y. X., McClements, D. J., & Liu, J. Y. (2015). Separation and characterization of polyphenolics from underutilized byproducts of fruit production (Choerospondias axillaris peels): inhibitory activity of proanthocyanidins against glycolysis enzymes. Food and Function, 6(12), 3693–3701.
Saeidnia, S., Ara, L., Hajimehdipoor, H., Read, R. W., Arshadi, S., & Nikan, M. (2016). Chemical constituents of Swertia longifolia Boiss. with α-amylase inhibitory activity. Research in Pharmaceutical Sciences, 11(1), 23–32.
Zhang, Y., Wong, A. I. C., Wu, J. E., Karim, N. B. A., & Huang, D. J. (2016). Lepisanthes alata (Malay cherry) leaves are potent inhibitors of starch hydrolases due to proanthocyanidins with high degree of polymerization. Journal of Functional Foods, 25, 568–578.
Wu, J. Z., Zheng, Y. B., Chen, T. Q., Yi, J., Qin, L. P., Rahman, K., & Lin, W. X. (2007). Evaluation of the quality of lotus seed of Nelumbo nucifera Gaertn from outer space mutation. Food Chemistry, 105(2), 540–547.
Chen, X., & Zhou, J. (2011). A study on the chemical composition of lotus seed epicarp. Transactions of the Chinese Society for Agricultural Machinery, 29, 139–141.
Kredy, H. M., Huang, D. H., Xie, B. J., He, H., Yang, E. N., Tian, B. Q., & Xiao, D. (2010). Flavonols of lotus (Nelumbo nucifera, Gaertn.) seed epicarp and their antioxidant potential. European Food Research and Technology, 231(3), 387–394.
Huang, D. H., Hu, C. L., Husam, M. C., Xie, B. J., He, H., & Yang, E. N. (2009). Antioxidant activity and structure of flavonoids from epicarp of Nelumbo nucifera gaertn. Food Science and Technology, 30(23), 209–213.
Chen, S., Fang, L. C., Xi, H. F., Guan, L., Fang, J. B., Liu, Y. L., Wu, B. H., & Li, S. H. (2012). Simultaneous qualitative assessment and quantitative analysis of flavonoids in various tissues of lotus (Nelumbo nucifera) using high performance liquid chromatography coupled with triple quad mass spectrometry. Analytica Chimica Acta, 724(8), 127–135.
Zhou, D. L., Gao, J. H., Yang, H. J., Chen, A. J., & Mai, Z. L. (2011). Analysis on the nutrient components of lotus seed shell and study on the antioxidation activity of flavonoid. Journal of Anhui Agricultural Sciences, 39(7), 3968–3970.
Liu, Y., Ma, S. S., Ibrahim, S. A., Li, E. H., Yang, H., & Huang, W. (2015). Identification and antioxidant properties of polyphenols in lotus seed epicarp at different ripening stages. Food Chemistry, 185 159–164.
Qi, S., & Zhou, D. (2013). Lotus seed epicarp extract as potential antioxidant and anti-obesity additive in Chinese Cantonese sausage. Meat Science, 93(2), 257–262.
Wei, S. D., Lin, Y. M., Liao, M. M., Zhou, H. C., & Li, Y. Y. (2012). Characterization and antioxidative properties of condensed tannins from the mangrove plant Aegiceras corniculatum. Journal of Applied Polymer Science, 124(3), 2463–2472.
Kim, D. O., Jeong, S. W., & Lee, C. Y. (2003). Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chemistry, 81(3), 321–326.
Terrill, T. H., Rowan, A. M., Douglas, G. B., & Barry, T. N. (1992). Determination of extractable and bound condensed tannin concentrations in forage plants, protein concentrate meals and cereal grains. Journal of the Science of Food and Agriculture, 58(3), 321–329.
Wei, S. D., Zhou, H. C., & Lin, Y. M. (2010). Antioxidant activities of extract and fractions from the hypocotyls of the mangrove plant Kandelia candel. International Journal of Molecular Sciences, 11(10), 4080–4093.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT- Food Science and Technology, 28(1), 25–30.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9-10), 1231–1237.
Fu, C. L., Yang, X. N., Lai, S. J., Liu, C., Huang, S. R., & Yang, H. S. (2015). Structure, antioxidant and α-amylase inhibitory activities of longan pericarp proanthocyanidins. Journal of Functional Foods, 14, 23–32.
Li, C. M., Leverence, R., Trombley, J. D., Xu, S., Yang, J., Tian, Y., Reed, J. D., & Hagerman, A. E. (2010). High molecular weight persimmon (Diospyros kaki L.) proanthocyanidin: a highly galloylated, A-linked tannin with an unusual flavonol terminal unit, myricetin. Journal of Agricultural and Food Chemistry, 58(16), 9033–9042.
Grabber, J. H., Zeller, W. E., & Mueller-Harvey, I. (2013). Acetone enhances the direct analysis of procyanidin- and prodelphinidin-based condensed tannins in lotus species by the butanol-HCl-iron assay. Journal of Agricultural and Food Chemistry, 61(11), 2669–2678.
Schofield, P., Mbugua, D. M., & Pell, A. N. (2001). Analysis of condensed tannins: a review. Animal Feed Science and Technology, 91(1-2), 21–40.
Hummer, W., & Schreier, P. (2008). Analysis of proanthocyanidins. Molecular Nutrition and Food Research, 52(12), 1381–1398.
Wolfe, R. M., Terrill, T. H., & Muir, J. P. (2008). Drying method and origin of standard affect condensed tannin (CT) concentrations in perennial herbaceous legumes using simplified butanol-HCl CT analysis. Journal of the Science of Food and Agriculture, 88(6), 1060–1067.
Singh, P. P., & Chauhan, A. S. M. S. (2009). Activity guided isolation of antioxidants from the leaves of Ricinus communis L. Food Chemistry, 114(3), 1069–1072.
Inglett, G. E., Chen, D. J., Berhow, M., & Lee, S. Y. (2011). Antioxidant activity of commercial buckwheat flours and their free and bound phenolic compositions. Food Chemistry, 125(3), 923–929.
Tan, K. W., & Kassim, M. J. (2011). A correlation study on the phenolic profiles and corrosion inhibition properties of mangrove tannins (Rhizophora apiculata) as affected by extraction solvent. Corrosion Science, 53(2), 569–574.
Wei, S. D., Chen, H., Yan, T., Lin, Y. M., & Zhou, H. C. (2014). Identification of antioxidant components and fatty acid profiles of the leaves and fruits from Averrhoa carambola. LWT- Food Science and Technology, 55(1), 278–285.
Liu, S. C., Lin, J. T., Wang, C. K., Chen, H. Y., & Yang, D. J. (2009). Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chemistry, 114(2), 577–581.
Wang, Y. F., Huang, S. R., Shao, S. H., Qian, L. S., & Xu, P. (2012). Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: antioxidant activity and inhibitory potential against glucosidase and amylase in vitro. Industrial Crops and Products, 37(1), 520–526.
Hargrove, J. L., Greenspan, P., Hartle, D. K., & Dowd, C. (2011). Inhibition of aromatase and α-amylase by flavonoids and proanthocyanidins from Sorghum bicolor bran extracts. Journal of Medicinal Food, 14(7-8), 799–807.
Reed, J. D., Krueger, C. G., & Vestling, M. M. (2005). MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry, 66(18), 2248–2263.
Wei, S. D., Zhou, H. C., Lin, Y. M., Liao, M. M., & Chai, W. M. (2010). MALDI-TOF MS analysis of condensed tannins with potent antioxidant activity from the leaf, stem bark and root bark of Acacia confusa. Molecules, 15(6), 4369–4381.
Zhou, H. C., Lin, Y. M., Wei, S. D., & Tam, N. F. Y. (2011). Structural diversity and antioxidant activity of condensed tannins fractionated from mangosteen pericarp. Food Chemistry, 129(4), 1710–1720.
Song, W., Zhu, X. F., Ding, X. D., Yang, H. B., Qin, S. T., Chen, H., & Wei, S. D. (2017). Structural features, antioxidant and tyrosinase inhibitory activities of proanthocyanidins in leaves of two tea cultivars. International Journal of Food Properties, 20(6), 1348–1358.
Funding
This work was supported by the National Natural Science Foundation of China (31700360), the Hubei Provincial Scientific Research Project in Environmental Protection (2017HB10), the WEL Visiting Fellowship Program of Xiamen University (WEL201706), the Engineering Research Center of Ecology and Agricultural Use of Wetland of Yangtze University (KF201505, KF201704), and the Yangtze Youth Fund of Yangtze University (2015cqn60, 2016cqn41).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, H., Sun, K., Yang, Z. et al. Identification of Antioxidant and Anti-α-amylase Components in Lotus (Nelumbo nucifera, Gaertn.) Seed Epicarp. Appl Biochem Biotechnol 187, 677–690 (2019). https://doi.org/10.1007/s12010-018-2844-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2844-x