Abstract
The purpose of this paper is to investigate some quality attributes of low fat ice cream (LFIC) substituted with hulless barley flour (HBF) and barley ß-glucan (BBG). The methodology included in this paper is based on adding HBF (1, 2, 3 and 4 %) as a partial substitution of skim milk powder (SMP) and BBG (0.40 %) as a complete substitution of carboxy methyl cellulose (CMC). All mixes and resultant ice cream samples were evaluated for their physicochemical properties as well as the sensory quality attributes.The results indicated that substitution of SMP with HBF significantly increased total solids (TS), fat and crude fiber, while crude protein and ash significantly decreased in ice cream mixes. BBG exhibited the same manner of control. Specific gravity was gradually increased with adding HBFand BBG in the mixes and therefore the overrun percent was significantly changed in the resultant ice cream. Adding HBF in ice cream formula led to significant decrease in acidity with higher freezing point and the product showed higher ability to meltdown. BBG treatment showed the same trend of control. Values of flow time and viscosity significantly increased with increasing HBF in the ice cream mixes, but these values significantly decreased in BBG mix. The time required to freeze ice cream mixes was decreased with increasing the ratio of HBF but, increased in BBG treatment. The substitution of SMP with 1 and 2 % HBF significantly (P ≤ 0.05) enhanced sensory attributes of ice cream samples. While, BBG treatment achieved mild score and acceptability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Barley (Hordeum vulgare L.) is the fourth major cereal crop produced in the world (Damiran and Yu 2011; Tigre et al. 2014). The estimated total world barley production in 2011 is 130 million metric tones (FAOSTAT 2012). In Egypt, barley is qualified to be cultivated in wide areas, especially in the North Cost region and new reclaimed lands, because of its tolerance characteristics to biotic and abiotic stresses (Abdalla et al. 2008; Sary et al. 2014). Barley yields have tended to increase gradually over the past three decades from 2.92 t ha−1 in the eighties to 3.63 t ha−1 in 2008/09 (El-Banna et al. 2011).
Hulless barley (HB) differs from conventional barley in that the hulls firmly attached to the kernel and consequently is detached after thrashing, leading to a higher level of valuable nutrients and increased volume density (Sharifi et al. 2012). HB contained more protein and starch, total dietary fiber (TDF), total soluble ß-glucan, and pentosans than hulled barley varieties (Bhatty 1999; Juskiw et al. 2010). HB had higher concentration of limiting amino acids, lysine and theronine than wheat or hulled barley (Eticha et al. 2010). Moreover, starch is the abundant single component of HB, accounting for 60–75 % of grain on a dry weight basis (Bhatty and Rossnagel 1998; Zute et al. 2011). Components of HB carbohydrates include starch, cell-wall polysaccharides, ß-D-glucan, (1–3) ß- glucan and arabinoxylans (pentosans), cellulose and a number of simple sugars and oligosaccharides (BeMiller 1997; El Khoury et al. 2012). ß-Glucan positively affects serum cholesterol and glucose levels that can in turn impact cardio-vascular health and diabetes control, respectively. The USA Food and Drug Administration recently issued a health benefit endorsement for barley based on ß-glucan effects on lowering blood cholesterol. High fiber and/or other components also have a satiety effect, which can positively affect weight control, as well as speed up the passage of food in the colon (Baik and Ullrich 2008; Lee et al. 2010).
Nowadays, consumers have directed their interest towards reduced or low-fat products particularly, low-fat or non-fat dairy products due to potential health benefits and nutritional advantages. The low-fat market extension has forced dairy food manufacturers to devise products in order to satisfy demands of consumers. However, the retention of organoleptic characteristics in low-fat products is difficult, and they faced a lot of challenges in maintaining their quality (Liou 2006; Mahdian and Karazhian 2013).
Ice cream has a very complex structure, with multiple phases that can influence product textural quality and physical attributes including shape retention and structural collapse during melting. It is composed of a combination of milk and milk products, sugar, stabilizer and flavoring materials. This product is usually contains a higher ratios of milk solids particularly, solid non fat (SNF). Milk solids not fat (MSNF) is the solids of skim milk, and consists of protein, milk sugar and minerals. MSNF helps to make the ice cream more compact and smooth, improves body and texture, gives a higher overrun without snowy or flaky texture desirable, increases viscosity and resistance to melt, but also lowers the freezing point (Kilara and Chandan 2007; Awad 2007; Goff and Hartel 2013; Sonwae and Hembad 2014). Therefore, several resources of solids have been researched to substitute partially skim milk powder in ice cream industry products such as soy protein (Magdoub et al. 1992; Laan and Truelsen 2009; Pereira et al 2011; Ahanian et al. 2014), potato pulp (Das et al. 1989; Metwally 1994), Khoa (Rehman et al. 1994), total milk proteinate (Awad and Metwally 2000), sodium caseinate (Awad and Salama 2010; Supavititpatana and Kongbangkerd 2011) and rice flour either it can improve the acceptability properties of ice cream or as a cheap resources of ingredients to reduce the total cost of production (Awad 2007; Cody et al. 2007).
However, not many resources has been explored the utilization of HB and ß-glucan in ice cream manufacture even with those excellent multitude functional properties in foods like modification and improvement of the texture, sensory characteristics and shelf-life due to their water binding capacity, gel-forming ability, fat mimetic, anti sticking, anti clumping, textrising and thickening effects (Vaikousi and Biliaderis 2005; Soukoulis et al. 2009; Ahmad et al. 2012).
One reason could be that there is no information about the effects of HBF and BBG on the quality attributes of low fat ice cream mix (LFICM) and the resultant ice cream such as the physicochemical properties and the sensory characteristics. Therefore, this study was undertaken to provide such information.
Materials and methods
Source of barley grains
Hulless barley grains variety Giza 130 was obtained from Field Crops Research Institute, Agricultural Research Center (ARC), Giza- Egypt.
Source of ice cream ingredients
Raw buffalo’s milk (the most popular milk type in Egypt) was obtained from the herd of Faculty of Agriculture, Ain Shams University and used as an ingredient for preparing the low fat ice cream mixes (LFICM). Skim milk powder (SMP) made in USA was obtained from Domty for Milk and Food Company, Cairo, Egypt. Commercial grade cane sugar was purchased from Sugar and Integrated Industries Company, Giza, Egypt. High viscosity carboxymethyl cellulose (CMC) produced by TIC gums, MD, USA was used as a stabilizer. Vanilla was obtained from the local market and used to flavor the ice cream.
Preparation of hulless barley flour (HBF)
Hulless barley grains were laboratory milled to a fine powder using a hammer mill following by grinding into flour and sieving through 0.5 mm sieve. The obtained flour was packed into plastic container and kept at refrigerate temperature until used.
Extraction of barley ß-glucan (BBG)
BBG was extracted from HBF according to the method of Benito-Román et al. (2011) with small modifications according to lzydorczyk et al. (1998). HBF was weighted and added to an Erlenmeyer flask. Then water was added where, solid: liquid ratio 1:10 and pH of water being around 6. Then transferred to a water bath and the temperature was kept constant in a water bath 55 °C for 3 h and the flour suspended at high stirring rate. After the extraction process was finished, solid: liquid separation was done by centrifugation at 5500 rpm/4 °C for 10 min. ß-glucan was extracted from the clear supernatant after adjusting the concentration of absolute ethyl alcohol to 30 % and keeping the mixture overnight at 5 °C. ß-glucan precipitate was skimmed off then freeze dried.
Manufacture of low fat ice cream (LFIC)
LFIC batches were prepared from the fore mentioned ingredients with quantities calculated as shown in Table 1. The control mix was standardized to contain 4 % fat, 12 % soluble non fat (SNF), 15 % sugar and 0.40 % CMC according to Arbuckle (1986). HBF was used to replace 1, 2, 3 and 4 % of SMP added to raise the SNF in the mix as shown in Table 1. A 0.40 g ß- glucan was used to replace 0.40 g CMC. Mixes were heat treated up to 85 ± 1 °C for about 30 s, then rapidly cooled to 5 ± 1 °C and aged at same temperature for 4 h. After ageing, 0.01 %vanilla powder was directly added to the mixes before frozen in horizontal batch freezer (Taylor Co. USA). The frozen LFIC was drawn in plastic cups (120 ml) and hardened at −26 °C for 24 h before analyses. All treatments were of three replicates.
Chemical analysis
Moisture, crude protein, crude fiber, fat, and ash contents were determined in HBF, SMP, whole milk, fresh skim milk and LFICM according to the methods outlined in AOAC (2007). The lactose content of SMP, whole milk, fresh skim milk was determined as described by Nickerson et al. (1976). Total carbohydrates of HBF and ice cream mixes were calculated by differences.
Physicochemical analysis
-
1.
Specific gravity
Specific gravity of LFICM and the final frozen product were measured at 20 °C using pycnometer according to Winton (1958).
-
2.
Determination of viscosity
Viscosity was determined using a coaxial rotational viscometer (Rheotest II, Medingen, Germany) at shear rates ranging from 3.0 to 1312 s−1. The measuring device (S1) was used with a sample volume of 30 ml per run. All samples were adjusted to 20 ± 1 °C before loading in the viscometer device. Apparent viscosity was calculated at shear rate of 48.6 s−1.
-
3.
Calculation of the overrun
The overrun percent of the resultant low fat ice cream (LFIC) was calculated as mentioned by Muse and Hartel (2004) using the following equation.
\( \mathrm{Overrun}\ \left(\%\right)=\frac{\mathrm{weight}\ \mathrm{of}\ \mathrm{mix}-\mathrm{weight}\ \mathrm{of}\ \mathrm{ice}\ \mathrm{cream}}{\mathrm{weight}\ \mathrm{of}\ \mathrm{ice}\ \mathrm{cream}}\ \mathrm{x}\ 100 \)
-
4.
Determination of acidity
Titratable acidity of SMP, HBF and LFICM was determined in duplicate by titration with NaOH 0.1 N calculated as lactic acid percent according to Richardson (1986).
-
5.
Assessing pH Values
The pH values of SMP, HBF and LFICM were measured using a digital laboratory pH meter (HI 93 1400, Hanna instruments) with glass electrode.
-
6.
Determination of freezing point in LFICM
The freezing point was tested for LFICM (−°C) as mentioned in FAO Laboratory manual (1977).
-
7.
Determination of flow time
Flow time of the LFICM was measured as the time in seconds required to discharging a 50 ml pipette at 5 °C under atmospheric pressure according to Arbuckle (1986).
-
8.
Determination of Melting rate of the resultant ice cream
Melting rate of the resultant LFIC samples was determined as mentioned by Segall and Goff (2002). LFIC samples were allowed to melt at room temperature (23 ± 1 °C) and the melted portion was weighed every 10 min. The percent mass loss/min. in the linear region (slope) was used to compare the meltdown rate of different samples.
-
9.
Determination of Hardness of the frozen LFIC
Hardness of frozen LFIC was examined by adapted the method recommended by Bourne and Comstock (1986). Puncture test was based on using a (0.27) inch diameter of Chatillon Fruit and Vegetables Tester Precission Instrument model CATL 719–10. The yield point force was measured as lb./in2 in LFIC samples hardened in a deep freezer at −26 °C for 24 h before testing.
Sensory evaluation
Samples of LFIC after 24 h hardening at −18 °C were scored by a panel of 10 judges selecting in the basis of their consistency in scoring. The organoleptic properties were evaluated in a room lighted with several florescent lamp at a temperature adjusted to 20 °C. The samples were scored for flavor (45), body and texture (35), color (10) and melting quality (10) as suggested by Arbuckle (1986).
Statistical analysis
The obtained data from physicochemical properties and sensory evaluation were exposed to analysis of variance. Duncan’s multiple range tests at (P ≤ 0.05) level was used to compare between means. The analysis was carried out using the PRO ANOVA procedure of SAS Program (1996).
Results and discussions
Chemical composition of HBF, SMP, whole milk, fresh skim milk and LFICM
Table 2 exhibits the chemical composition of HBF, SMP, whole milk and fresh skim milk used in preparation of LFIC formula. Data in Table 2 revealed that the chmical composition of the above mensioned materials significantly varied. Moisture showed higher percentages in fresh skim milk and whole milk, respectively. Whole milk and HBF had a close fat percentage. SMP recorded the higher percentage of ash and crude protein followed by HBF, while fresh skim milk and whole milk exhibited the lower percentages. On the other hand, HBF exhibited the highest percentage of total carbohydrates followed by SMP, meanwhile fresh skim milk and whole milk exhibited the lower percentages. Also, HBF had a reasonable amount of crude fiber, but it seams to be absent in SMP, whole milk and fresh skim milk. SMP recorded the highest acidity percentage which in turn affects its pH value. Meanwhile, pH value of HBF is neutral.
Similar results regardingthe the chemical composition of SMP, HBF, whole milk and fresh skim milk were obtained by Reference Manual for U.S. Milk Powders (2005); Awad (2007); Ullrich et al. (2008); Sayed (2011); Hassanein and Moursy (2008); El-Deeb et al. (2014).
The development of structure in ice cream is often attributed to the macro molecules present in the ice cream mix- milk fat, protein, and complex carbohydrates (Junior and lannes 2011).
The chemical characteristics of LFICM is shown in Table 3. The data indicated that there was no significant difference in TS between BBG and control. In contrast, TS significantly increased with the steady increase of HBF. This significant increase in TS may reflected by the corresponding increase in total carbohydrates.
One of parameters that directly affects the quality of foods is total solid matters particularly, ice cream. It is directly related to ice crystal size distribution. Variations in solids content of just a few percent greatly influences ice crystal growth. If these are in excess then curded texture could result, while low contents resulted in ice crystal formulation and coarse texture (Abdullah et al. 2003; El Owni and Khater 2009; Amiri et al. (2014).
From the same Table, fat content showed no significant increase in BBG treatment, but a significant increase in fat content occurred by increasing the added ratio of HBF compared to control. Marshall (1991) demonstrated major challenges for manufacturing low-fat ice cream, which are related to providing creaminess and body, minimizing ice crystal formation, and finding good flavoring formulas. As increases in fat content have be shown to reduce ice crystal size and affect sensory evaluation by causing a lubricating sensation in the mouth (Keeney and Kroger 1974; Arbuckle 1986).
Crude fiber was absent in BBG treatment and significantly increased with increasing the added ratio of HBF and this is may be due the potentiality of HBF with crude fiber. Similar results obtained by Abou Zeid et al. (2015) whom studied the effect of substituting 10, 25, 50, 75, and 100 % SMP with chickpea flour on ice milk quality. The authors found that increasing chickpea flour caused a remarkable increase in crude fibers. Even though, there were a significant decrease in the percentage of crude protein and ash, but BBG treatment recroded the highiest contents with no signification with control and this is may be due to that the HBF has lower ash value compared to SMP as shown in Table 2.
Heikal (2009) studied the effect of a partly substitutions of fermented milk solids by HBF, data revealed that ash and protein, decreased by barley substitutions and especially during storage. Protein affects the quality of the ice cream where it provides a slight flavor, water holding and also affects the formation of size of air cells. Protein interacts at the oil water interface during homogenization to stabilize the fat emulsion and during freezing, proteins function to encapsulate the air cells and control destabilization of fat (Abdullah et al. 2003; Goff et al. 1989; Liou 2006).
BBG treatment and T1 recorded the same content of total carbohydrates. By increasing the ratio of HBF a significant increase in total carbohydrates was observed in LFICM. This is mainly due to the lower protein and ash, as well as higher carbohydrate contents of HBF compared to SMP as shown in Table 2. In ice creams, sugars are responsible for taste and an economic path to increase the solid content, but they also affect the freezing behavior and viscosity of processed mixes (Junior and lannes 2011). Increasing the sugar content of ice cream causes a smooth texture, because it lowers the freezing point, the amount of unfrozen material is increased, increased viscosity and free water contents declined (Marshall and Arbuckle, 1996).
The substitution and the physicochemical properties of LFICM.
Table 4 represents the physicochemical properties of LFICM. Data indicated that the pH values slightly increased and titratable acidity values showed significant slight decrease with adding HBF in ice cream formula. These differences in acidity and pH values of ice cream mixes could be due to the lower acidity and higher pH values of HBF as compared to SMP in Table 2. Another reason, could be due to the increase of TS, where the acidity of ice cream is affected by solid matters. These findings are supported by Hagen and Narvhus (1999); Amiri et al. (2014). Regarding BBG treatment, there were no significant differences in pH value and acidity between BBG treatment and control with CMC. Segall and Goff (2002) reported that when values of the pH are comprised between 4 and 6, flocculation and creaming can occur. However, the same reduction in pH is desired, and thus the proteins get near to an isoelectric point and the repulsion between the groups with the same electric charge increases protein-protein interactions increasing the formation of a second layer around the fat globules increasing the emulsion stability. Specific gravity is the ratio of the density of water at 20 °C component or a material compared to the density of water and varies with varying mix composition. Specific gravity of LFICM was gradually increased with adding BBG and HBF in the mixes.
Freezing point of LFICM is highly dependant directly on, the soluble components like sweeteners and indirectly to, the ratio of fat and protein in the mix. Freezing points of LFICM showed no significant effect in BBG treatment, but significantly affected with adding HBF. Where, the mixes showed higher freezing points with increasing substitution of SMP with HBF. The obtained results are in line with Marshall et al. (2003) whom stated that freezing point is affected by the amount, type, and molecular weight of the solutes in the mix. Also, with Soukoulis et al. (2009) whom found a significant increase in freezing point of the ice ceam mix when 4 % apple fibre or 4 % inulin were added; caused by the enrichment of the aqueous phase in high molecular weight polysaccharides, due to the higher contents of water-soluble fibres.
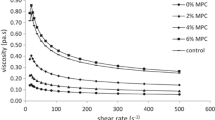
A certain level of viscosity is essential for proper whipping and retention of air cells in ice cream system (Marshall et al. 2003; Pinto and Dharaiya 2014). Substitution with BBG and HBF in LFICM has significantly affected the flow time and viscosity of the mixes. The data indicated that BBG treatment possessed the lowest flow time followed by control as well as viscosity value at shear rate of 48.6 s−1. Vaikousi and Biliaderis (2005) studied the processing and formulation effects on rheological behavior of barley ß-glucan aqueous dispersions. The authors found that viscosity reached higher values and the slopes of viscosity plots were considerably steeper for the samples containing only ß- glucan. On the other hand, when sucrose is present as co-solute the viscosity breakdown due to shearing is less for the respective cryostructurates. Also, Ghotra et al. (2009) found that the addition of xanthan (XAN) or CMC to aqueous dispersions of BBG improved the solution viscosities at high shear rates as compared to BBG alone, meaning that at particular shear rates (e.g. intestinal shear rates), blends of BBG with XAN or CMC will maintain higher viscosities than BBG alone.
Values of flow time and viscosity significantly increased with adding HBF in the formula; being the highest in T4. The significant differences in flow time and viscosity values of control and treatments with HBF could be due to the significant increase in TS; as total solids increased in food systems the fluidity of food decreases namely an increase in viscosity occurs (Amiri et al. 2014). Another reason, that the differences in composition of both materials that; HBF contains a high amount of dietary fibers which had gelatinize property and may absorb and bind greater amount of free water leads to increase the viscosity and flow time under the processing conditions of LFICM compared to substituted SMP which contains high amount of lactose. The results are in same the manner with El-Nagar et al. (2002) whom find an increase in the viscosities of the low-fat samples containing inulin which can be explained by the interactions of the dietary fibre and liquid components of the ice cream mixture. Also, Adapa et al. (2000) noted that ice cream mixtures containing carbohydrate based fat replacers exhibit a viscous behavior because of their ability to imbibe water, which in turn increases the viscosity of the system. Pinto and Dharaiya (2014) developed a low fat sugar free frozen dessert and the authors found that there was progressive increase in viscosity of mixtures with increase in level of maltodextrin and decrease in level of polydextrose. Addition of bulk fillers, in general resulted in increased viscosity of the mixtures.
The substitution and the physicochemical properties of LFIC.
The physicochemical properties of the resultant LFIC are presented in Table 5. Data indicated that the specific gravity of the LFIC was increased in the BBG treatment and also by increasing the added ratio of HBF at 3 and 4 %. Specific gravity depends on the formula components as well as the ability of mix to retain the air cells in ice cream system.
The amount of air added to ice cream is known as overrun. Overrun percent in LFIC was significantly increased by adding HBF at 1 and 2 % as a substitution of SMP. But, significantly decreased in BBG treatment and HBF at 3 and 4 %. Changes in overrun percentages of LFIC treatments could be related to the different viscosity values. A certain level of viscosity in ice cream mix is needed for proper whipping and retention of air cells. Beyond that level with higher or lower viscosity values, the mix will be poor in whipping and retention of air cells, so the overrun will be reduced (Verma 2002; Marshall et al. 2003; Pinto and Dharaiya 2014). T3 and T4 contain a higher amount of crude fibers leads to very high viscosity level that the air cells can not be easy to incorporate into the mix during freezing process. Therefore, lowering the adding ratio in T1 and T2 treatments may help to lower the viscosity to suitable point and increase the overrun value.Similar results obtained by Abou Zeid et al. (2015) whom studied the effect of substituting 10, 25, 50, 75, and 100 % SMP with chickpea flour on ice milk quality. The authors found that the overrun increased significantly when chickpea flour increased up to 50 % while decreased significantly at level 75 and 100 %.
The time required for freezing ice cream mixes was increased in BBG treatment and this is due to the decrease in freezing point of the BBG mix as shown in Table 4. In contrast, the time required for freezing ice cream mixes was decreased with increasing the ratio of HBF. While control required 18.5 min. to be frozen, T4 with 4 % substitution of SMP by HBF took 14.0 min. The decrease in freezing time could be due to the increase in freezing point of the mixes as shown in Table 4.
Figure 1 represents the hardness values of the resultant LFIC. The Fig. explain a non significant decrease in BBG treatment. While, there was a significant increase with adding the ratio of HBF being highest with 4 % substitution of SMP. The significant increase in hardness of ice cream samples with HBF could be attributed to the higher dietary fiber contents which may act as stabilizing material and reduce the free water contents. Generally, the hardness values of ice cream are related to several factors such as TS, the freezing point, the amount and type of stabilizer, and thus the free water content, overrun percent and the consistency of the treatment (Awad and Metwally 2000; Marshall et al. 2003; Awad 2007).
Figure 2 depicts the melting rates of LFIC samples. It could be noticed that the melting rate was increased by increasing the adding ratio of HBF. This is due to the higher freezing points of these samples. On the other hand, BBG treatment showed the same trend of control because there were no significant changes in freezing point compared to control as shown in Table 4.
Sensory quality attributes of LFIC
The challenge in working with low fat ice cream is related to the fact that the fat globule network would either be disrupted or absent and this could seriously impact flavour and texture of the product. Where, milk fat interacts with other ingredients to develop the texture, mouthfeel, creaminess, and overall sensations of lubricity. Stabilizer had the ability to absorb and hold large amounts of bound water, which produces good body, smooth texture, slow melt down and heat shock in the resultant product. β-glucans serve as stabilisers and texture agents but, the product texture depends on the content of β-glucans (Keeney 1982; Junior and lannes 2011; Havrlentová et al. 2011; Mahdian and Karazhian 2013).
Sensory panel evaluation is an important indicator of potential consumer preferences. The sensory quality scors of LFIC samples are presented in Table 6. The substitution with BBG and 1 and 2 % HBF had no significant effects on flavor attribute of the ice cream, however increasing the ratio of HBF significantly affects the flavor of the product. The improved body and texture of the low-fat ice cream substituted with 1 and 2 % HBF may be associated with the reasonable increase of TS and hardness as shown in Tables 3 and 5. This is in line with Amiri et al. (2014) whom stated that total solid matters directly and effectively affect the texture of ice cream resulting in firm texture and improved mouth feel.
The substitution with BBG significantly decreased body and texture quality. Also, increasing the ratio of HBF in T3 and T4 produced ice cream with less quality. Where, the ice cream became rough, more harden and this is may be due to the excess in TS as shown in Table 3. Abdullah et al. (2003) reported that variations in solids content of just a few percent greatly influences ice crystal growth. If these are in excess then curded texture could result. The substitution with BBG and 1 and 2 % significantly improved the melting properties of the LFIC, while 3 and 4 % HBF substitution decreased the melting property scores and this is may be related to their viscosity values as shown in Table 5. Wang et al. (2013) stated that high viscosity means the cream hardly moves within the mouth and it may feel sticky on the palate because of its resistance to movement. However, low viscosity is easy to move within the mouth, and it may be perceived as watery immediately after the cream has liquefied.
Regarding color, The substitution with BBG and 1 and 2 % HBF significantly enhance the color acceptability compared to control. By increasing the ratio of HBF at 3 and 4 % the ice cream color became less whiter and less acceptable and this is may be due to the potential color of the HBF.
Substitution of SMP with HBF at 1and 2 % significantly enhanced the melting properties of the ice cream, in contrast by increasing the ratio of HBF at 3 and 4 % significantly lower the melting quality of the resultant ice cream.
Collectivelly, the substitution of SMP with 1 and 2 % HBF significantly enhanced the sensory attributes of ice cream samples. Where, the ice cream became less meltable, maintained flavor, improved body and texture more acceptable to panelists and with slight differences in the color. Our present findings are in accordance with Izydorczyk and Dexter (2008) whom stated that the barley enriched products have potential to exhibit acceptable sensory properties, especially if barley ingredients are incorporated at low to moderate levels whereas, it alters processing and handling of foods as well as their texture, color, flavor, and taste. In contrast, increasing the ratio of HBF in T3 and T4 produced ice cream with less quality. Where, the ice cream became rough, more harden, with flavor and color changes and less acceptable to the panelists. Regarding BBG treatment it seems to achieve mild score and acceptability with no significant changes in flavor but, it had less body, texture and melting quality compared to control. Abou Zeid et al. (2015) studied the effect of substituting 10, 25, 50, 75, and 100 % SMP with chickpea flour on ice milk quality. The authors found that substituting up to 50 % of SMP with chickpea flour would yield ice milk with the best organoleptic properties without adversely affecting during storage up to 4 weeks.
Conclusion
It could be concluded that the results of this study lead to a valuable addition in ice cream processing where, low fat ice cream could be produced with high quality by partial substitution of SMP with HBF at 1 and 2 % levels. Particularly, for whom concern about the negative health effects of fat and health functionality of HBF and its ß-glucans. Regarding BBG treatment, further work needs to be conducted to determine the optimum levels of addition in LFICM formulation, which leads to acceptable physicochemical and sensory quality attributes.
References
Abdalla KS, Abdallah M, Fahmy AH (2008) Development of Egyptian barley with HMW-GS Dy10 gene of wheat. Arab J Biotechnol 11:59–168
Abdullah M, Saleem AM, Zubair H, Saeed HM, Kousar S, Shahid M (2003) Effect of skim milk in soymilk blend on the quality of ice cream. Pak J Nut 2:305–311
Abou Zeid NA, Zedan AN, El-Sisi AS, Ali AOM (2015) Effect of replacing skim milk powder (SMP) with chickpea flour on ice milk quality. Minufiya J Agric 40:1–14
Adapa S, Dingeldein H, Schmidt KA, Herald TJ (2000) Rheological properties of ice cream mixes and frozen ice creams containing fat and fat replacers. J Dairy Sci 83:2224–2229. doi:10.3168/jds.S0022-0302(00)75106-X
Ahanian B, Pourahmad R, Mirahmadi F (2014) Effect of substituting soy milk instead of skim milk on physicochemical and sensory properties of sesame ice cream. Advances Enviro Biol 8:9–16
Ahmad A, Munir B, Abrar M, Bashir S, Adnan M, Tabassum T (2012) Perspective of β-glucan as functional ingredient for food industry. J Nutr Food Sci 2:133. doi:10.4172/2155-9600.1000133 Published March 12, 2012
Amiri HS, Nateghi L, Berenji S (2014) Effect of date syrup as a substitute for Sugar on physicochemical and sensory properties of ice cream. IJB 5:80–88
Arbuckle WS (1986) Ice cream, 4th ed. AVI Publishing Company, Inc. Westport, Connecticut pp 326–362
AOAC (2007) AOAC Official Methods of Analysis, 18th.ed. AOAC, Washington, DC
Awad RA (2007) Performance of rice flour in ice cream manufacture. Proc 10th. Egyptian Conf Dairy Sci Technol 517– 534. ISBN 978–92–5–105817–6 Record Number 20083078741
Awad RA, Metwally AI (2000) Evaluation of total milk proteinate as a milk solids source in ice cream manufacture. Annals Agric Sci, Ain Shams Univ Cairo 45:603–618. ISSN 0570–1783 Record Number 20013015317
Awad RA, Salama WM (2010) Using sodium caseinate and butter milk powder in production of low and free fat ice milk. In: Proc 11th. Egyptian Conf Dairy Sci Technol 523–535
Baik B, Ullrich SE (2008) Barley for food: characteristics, improvement, and renewed interest: a review. J Cereal Sci 48:233–242. doi:10.1016/j.jcs.2008.02.002
BeMiller JN (1997) Starch modification: challengers and prospects. Starch/Starke 49:127–131
Benito-Román O, Alonso E, Lucas S (2011) Optimization of the ß-glucan extraction conditions from different waxy barley cultivars. J Cereal Sci 53:271–276. Available online 10 February 2011
Bhatty RS (1999) The potential of hull-less barley. Cereal Chem 76:589–599
Bhatty RS, Rossnagel BG (1998) Comparison of pearled and unpearled Canadian and Japanese barleys. Cereal Chem 75:15–25. doi:10.1094/CCHEM.1998.75.1.15
Bourne, MC, Comstock SH (1986) Effect of temperature on firmness of thermally processed fruits and vegetables. J Food Sci 51:531–533. doi:10.1111/j.1365-2621.1986.tb11179.x Article first published online: 25 AUG 2006
Cody TL, Olabi A, Pettingell AG, Tong PS, Walker JH (2007) Evaluation of rice flour for use in vanilla ice cream. J Dairy Sci 90:4575–4585. doi:10.3168/jds.2006-531 Available online 16 February 2010
Damiran D, Yu P (2011) Protein molecular spectral features in hull-less barley varieties and their relation to protein and energy values. In: Proc Soils Crops Conf. Canada, Saskatoon, Saskatchewan, Canada
Das TC, Rao MR, Krishnaiah N, Suphakar K (1989) Ice cream made by incorporation of different level of potato pulp. Indian J Dairy Sci 42:295–299
El-Banna MN, Nassar MA, Mohamed MN (2011) Evaluation of 16 barley genotypes under calcareous soil conditions. Egyptian J Agric Sci 3:105–121. doi:10.5539/jas.v3n1p105
El-Deeb AM, Hassan NSY, Hassanein AM (2014) Preparation and properties of flavored fermented beverage based on partial or complete replacement of milk with quinoa seeds water extract (QSWE). Int J Dairy Sci 9:96–105. doi:10.3923/ijds.2014.96.105
El Khoury D, Cuda C, Luhovyy BL, Anderson GH (2012) Beta glucan: health benefits in obesity and metabolic syndrome. J Nutr Metab 851362. doi:10.1155/2012/851362
El-Nagar G, Glowers G, Tudorica CM, Kuri V, Brennan CS (2002) Rheological quality and stability of yog-ice cream with added inulin. Int J Dairy Technol 55:89–93. Article first published online: 30 MAY 2002
El Owni OAO, Khater ZKO (2009) Chemical composition of ice cream produced in Khartoum State Sudan. Pak J Nutr 8:158–160. doi:10.3923/pjn.2009.158.160
Eticha F, Grausgruber H, Berghoffer E (2010) Multivariate analysis of agronomic and quality traits of hull-less spring barley (Hordeum vulgare L.). J Plant Breed Crop Sci 2:81–95
Fagan CC, Donnell CP, Cullen PJ, Brennan CS (2006) The effect of dietary fiber inclusion on milk coagulation kinetics. J Food Eng 77:261–268. doi:10.1016/j.jfoodeng.2005.06.030 Available online 8 August 2005
FAOSTAT (2012) Food outlook global market analysis. Available on line :http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor
FAO (1977) Reg. Dairy Devel. Training Center, Near East. Lab. Manual, FAO, Rome, Italy
Ghotra BS, Vasanthan T, Temelli F (2009) Rheological properties of aqueous blends of high purity barley ß-glucan with high purity commercial food gums. Food chem 117:417–425. doi:10.1016/j.foodchem.2009.04.027 Available online 19 April 2009
Goff HD, Kinsella JE, Jordan WK (1989) Influence of various milk protein isolates on ice cream emulsion stability. J Dairy Sci 72:385–397. doi:10.3168/jds.S0022-0302(89)79120-7
Goff HD, Hartel RW (2013) Ice cream the 7th.ed. Springer Bussiness New York 313–352
Hagen M, Narvhus JA (1999) Production of ice cream containing probiotic bacteria. Milchwissenchaft 54:265–268
Hassanein AM, Moursy SM (2008) Production of youghort fortified by hull-less barley flour. Egyptian J Agric Res 86:643–653
Havrlentová M, Petruláková Z, Burgárová A, Gago F, Hlinková A, Šturdík E (2011) Cereal β-glucans and their significance for the preparation of functional foods: a review. Czech J Food Sci 29:1–14
Heikal FM (2009) Studies on biological functional effects of the alternative yoghurt rich in dietary fiber and β-glucan. Ph.D thesis Mansoura Univ Fac Specific Education Dep Home Economics Egypt
Junior ES, Lannes SC (2011) Effect of different sweetener blends and fat types on ice cream properties. Food Sci Tech (Campinas) 31:217–220. doi:10.1590/S0101-20612011000100033
Juskiw PE, Helm JH, Salmon DF (2010) Breeding of feed grains for Western Canada: invited reiew. Amer J Plant Sci Biotechnol 5:64–77
Keeney PG (1982) Development of frozen emulsions. Food Technol 36:65–70
Keeney PG, Kroger M (1974) Frozen dairy products. In: fundamentals of dairy chemistry, 2nd ed. Webb BH, Johnson AH, Alford JA (ed). Avi Pub. Co, Inc, Westport, Connecticut, pp 897–899
Kilara A, Chandan RC (2007) Ice cream and frozen desserts In: Handbook of food products manufacturing. Hui YH (ed), Copyright 2007 John Wiley & Sons, Inc, Chap 74:594–633. doi:10.1002/9780813804033 Published Online: 27 MAR 2009
Laan D, Truelsen A (2009) Soy milk substitution in ice cream: effects on texture, color, and taste. 453:1–10. www.Cfs.purdue.edu
Lee NY, Kim Y, Choi I, Cho S, Hyun J, Choi J, Park K, Kim K, Lee M (2010) Biological activity of barley (Hordeum vulgare L.) and barley byproduct extracts. Food Sci Biotechnol 19:785–791. doi:10.1007/s10068-010-0110-2
Liou BK (2006) Sensory analysis of low fat strawberry ice creams prepared with different flavor chemicals and fat mimetics. Ph.D thesis, Food Sci Dep Fac Graduate School, Univ Missouri-Columbia. http://hdl.handle.net/10355/4497
Izydorczyk MS, Dexter JE (2008) Barley ß-glucans and arabinoxylans: molecular structure, physicochemical properties, and uses in food products: a review Food Res Int 41:850–868. doi:10.1016/j.foodres.2008.04.001 Available online 22 April 2008
lzydorczyk MS, Macri LJ, MacGregor AW (1998) Structure and physicochemical properties of barley non-starch polysaccharides 1. water extracted ß-glucans and arabinoxylans. Carbohydrate Polym 35: 249– 259
Magdoub MN, Fayed EO, Nargis HM, Salem MM (1992) Use of soy protein in ice cream making. Egyptian J Food Sci 20:245–251
Mahdian E, Karazhian R (2013) Effects of fat replacers and stabilizers on rheological, physicochemical and sensory properties of reduced-fat ice cream. J Agr Sci Techol 15:1163–1174
Marshall RT (1991) Fat in ice cream. Dairy Field 74:32
Marshall RT, Arbuckle WS (1996) Ice cream 5th ed. Indian diet Prof Int Soy protein food conf., Chapman and Hall, Int, Thomson Pub. USA, pp70
Marshall RT, Goff HD, Hartel RW (2003) Ice cream, 6th edn. Kluwer Academic/Plenum Publisher, NY
Metwally AI (1994) The use of red sweet potato in ice cream manufacture. Egyptian J Dairy Sci 22:59–66
Muse MR, Hartel RW (2004) Ice cream structural elements that affect melting rate and hardness. Amer Dairy Sci Asso 87:1–10. doi:10.3168/jds.S0022-0302(04)73135-5 Available online 20 March 2010
Nickerson TA, Vujicic IF, Lin AY (1976) Colorimetric estimation of lactose and its hydrolytic products. J Dairy Sci 59:386–390. doi:10.3168/jds.S0022-0302(76)84217-8
Pereira GD, Resende JV, Abreu LR, Giarola OT, Perrone IT (2011) Influence of the partial substitution of skim milk powder for soy extract on ice cream structure and quality. Eur Food Res Technol 232:1093–1102. doi:10.1007/s00217-011-1483-z
Pinto S, Dharaiya CN (2014) Development of a low fat sugar free frozen Dessert. Inter J Agri Sci 4:90–101
Reference Manual for U.S. Milk Powders (2005) Arlington, VA: U.S. Dairy Export Council 41
Rehman SU, Upadhyay KG, Pandya AJ (1994) Evaluation of Khoa as a milk solids source in ice cream. J Dairy Res 61:143–149. doi:10.1017/S0022029900028132 Published online: 01 June 2009
Richardson HG (1986) Standard methods for the examination of dairy products, 15th edn. Amr. Public Health Assoc, Washington
Sharifi SD, Dari FS, Yaghobfar A (2012) Effects of inclusion of hull-less barley and enzyme supplementation of broiler diets on growth performance, nutrient digestion and dietary metabolizable energy content. J Central Euro Agric 13:193–207. doi:10.5513/JCEA01/13.1.1035
Sary GA, El-Deepah HR, El-Gizawy NK, Gobarah ME, Tawfik MM, Khedr HH (2014) Impact of organic manures and foliar spraying with micronutrients on growth, yield and yield components of barley grown in newly reclaimed sandy soil. Am-Euras J Agric Environ Sci 14:1130–1140. doi:10.5829/idosi.aejaes.2014.14.11.12442
SAS (1996) SAS/STAT1 User’s Guide, Version 8. SAS Inst, Inc, Cary, NC
Sayed AM (2011) Biochemical and technological studies on barley talbina. Fac Agric Dep Food Tech Assiut Univ Egypt, M.Sc thesis
Segall KI, Goff HD (2002) A modified ice cream processing routine that promotes fat destabilization in the absence of added emulsifier. Inter Dairy J 12:1013–1018. doi:10.1016/S0958-6946(02)00117-6
Sonwae R, Hembad S A (2014) Sensorial Quality of dietic soft serve ice cream prepared by using different proportions of maltodextrin. Int J Curr Res Aca Rev 51–55. www.ijcrar.com
Soukoulis C, Lebesi D, Tzia C (2009) Enrichment of ice cream with dietary fibre: effects on rheological properties, ice crystallisation and glass transition phenomena. Food Chem 115:665–671. doi:10.1016/j.foodchem.2008.12.070 Available online 30 December 2008
Supavititpatana P, Kongbangkerd T (2011) The effect of partial replacement of non-fat dry milk with sodium caseinate on qualities of yogurt ice cream from coconut milk. Int Food Res J 18:439–443
Tigre W, Worku W, Haile W (2014) Effects of nitrogen and phosphorus fertilizer levels on growth and development of barley (Hordeum vulgare L.) at Bore District, Southern Oromia, Ethiopia. Amer J Life Sci 2: 260– 266. doi:10.11648/j.ajls.20140205.12 Published online September 30, 2014 (http://www.sciencepublishinggroup.com/j/ajls)
Ullrich SE, Baik Z, Axtell Q, Nair S (2008) Barley for food:traits and improvement. Proc 10th. Inter Barley Genetics Sympo Alex Egypt 563– 588
Vaikousi H, Biliaderis CG (2005) Processing and formulation effects on rheological behavior of barley ß-glucan aqueous dispersions. Food Chem 91:505–516. doi:10.1016/j.foodchem.2004.04.042 Available online 11 September 2004
Verma RB (2002) Technological studies on manufacture of frozen dairy desserts using artificial sweeteners. Ph.D. thesis submitted to NDRI Deemed Univ Karnal. http://krishikosh.egranth.ac.in/handle/1/18196
Wang C, He X, Huang Q, Fu X, Liu S (2013) Physicochemical properties and application of micronized cornstarch in low fat cream. J Food Eng 116:881–888 doi:10.1016/j.jfoodeng.2013.01.025 Available online 4 February 2013
Winton AL (1958) Analysis of foods, 3rd edn. Wiley, New York, p. 6
Zute S, Berga L, Belicka I (2011) Chemical composition of hulless barley genotypes from Latvian breeding program. Proc 6th.Inter Cong Flour-Bread Croatian Cong Cereal Techno, Opatija, Croatia, 497–504
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Haleem, A.M.H., Awad, R.A. Some quality attributes of low fat ice cream substituted with hulless barley flour and barley ß-glucan. J Food Sci Technol 52, 6425–6434 (2015). https://doi.org/10.1007/s13197-015-1755-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-1755-x