Abstract
Key nutritional factors were optimized for the maximum production of transgalactosylating β-galactosidase from Lactobacillus plantarum MCC2156. Galactose, yeast extract, sodium acetate and manganese sulphate were the most important nutrients affecting β-galactosidase production. Maximum β-galactosidase production (3015 miller units) was obtained by culturing L. plantarum in the optimized fermentation medium containing (w/v) galactose (4 %), yeast extract (2 %), sodium acetate (3 %) and manganese sulphate (0.075 %) with an optimum medium pH of 7.0, after 14 h of incubation at 35 °C. Further, permeabilization of L. plantarum cells using various chemical/ solvents for maximum β-galactosidase activity was performed for use as whole cell biocatalyst. Mixture of ethanol: n-butanol was found to effectively permeabilize the cells with maximum β-galactosidase activity under the following optimum conditions; 1: 1 mixture of ethanol (10 %, v/v): n-butanol (30 %, v/v) with a contact time of 10 min at 28 ± 2 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
β-Galactosidase (EC 3.2.2.23) is an industrially important enzyme that catalyzes the hydrolysis of lactose, a probable solution to the crystallization of lactose in frozen dairy products, whey disposal and lactose intolerance (Rao and Dutta 1977). In addition to hydrolysis, β-galactosidase also catalyses the transgalactosylation of lactose to produce galactooligosaccharides (GOS), which are well known for their potent prebiotic efficacy (Torres et al. 2010). GOS are stable in wide range of pH and temperature has low caloric value which in turn makes them useful as potential nutraceutical food ingredients (Crittenden and Playne 1996; Torres et al. 2010). Among various sources of β-galactosidases, intracellular β-galactosidase from probiotic lactic acid bacteria (LAB) such bifidobacteria and lactobacilli for lactose hydrolysis and GOS synthesis is of great industrial relevance mainly due to the ‘generally recognized as safe (GRAS)’ status of LAB (Hsu et al. 2007; Splechtna et al. 2006).
Intracellular β-galactosidase from probiotic Lactobacillus plantarum MCC2156 exhibited potent transgalactosylating efficacy for the synthesis of prebiotic GOS (Gobinath and Prapulla 2014). Downstream processing of intracellular enzymes involves cell disruptions, which could lead to low yields and decreased activity due to harsh conditions, additionally, adds to the cost of purification processes. Permeabilization of whole cells could be an effective alternative for circumventing the above mentioned disadvantages associated with the use of the intracellular enzymes. In this study, we investigated the effect of media components for improvement of transgalactosylating β-galactosidase production from L. plantarum MCC2156 and also the permeabilization conditions for L. plantarum MCC2156 cells for maximum β-galactosidase activity for further use as whole cell biocatalyst.
Materials and methods
Microorganism and media
Lactobacillus plantarum MCC2156, a kanjika isolate was cultured and maintained in Lactobacillus MRS Medium as reported earlier (Gobinath and Prapulla 2014).
Chemicals
Lactose, glucose, galactose, o-nitrophenyl-β-D-galactopyranoside (oNPG), o-nitrophenol (oNP), dimethyl sulphoxide (DMSO), Triton X-100, cetyltrimethylammonium bromide (CTAB) and sodium dodecyl sulphate (SDS) were procured from Sigma-Aldrich (St. Louis, MO, USA). All other solvents and chemicals used were of analytical grade.
Screening of different media components for production of β-galactosidase by L. plantarum MCC2156
The production of β-galactosidase from L. plantarum MCC2156 was evaluated in six different media (M1 to M6) based on MRS medium constituents (Table 1). Galactose and yeast extract were chosen as the carbon and nitrogen source, respectively, based on our earlier results (Gobinath and Prapulla 2014). Further, the effect of varying the concentration of the selected medium (M3) components (galactose, yeast extract, sodium acetate, metal ions); initial media pH (4.0–8.0) and incubation temperatures (25–45 °C) on β-galactosidase production was also studied. Each medium was inoculated with 10 % (v/v) inoculum of the freshly grown culture and then incubated at 37 °C, 16 h.
The enzyme production was determined according to the method of Miller (1972) with slight modification. Culture broth (1 ml) was harvested (8000 × g, 10 min), cells were washed twice with 0.05 M sodium phosphate buffer (pH 7.0), suspended in 1 ml Z buffer (60 mM Na2HPO4.7H2O, 40 mM NaH2PO4.H2O, 10 mM KCl, 1 mM MgSO4.7H2O, 50 mM β-mercaptoethanol) and the Absorbance (A600) was recorded. SDS (0.1 %, w/v) 10 μl and 40 μl chloroform was added to the 1 ml of cell suspension and mixed thoroughly (IKA Vortex, IKA, Germany) for 10 min at 28 ± 2 °C, followed by the addition of oNPG (200 μl, 10 mM) and incubated at 37 °C for 15 min. Absorbance (A420) and (A560) were recorded after arresting the reaction by adding 0.5 ml of 1 M Na2CO3. The β-galactosidase activity (Miller units) was calculated according to the formula (1):
[A600 nm – Absorbance of cells before assay; A560 - Absorbance of cells debris after assay; A420− Absorbance of o-nitrophenol (ONP) released; T = time of the reaction (min); v = volume of original culture used (ml)].
Effect of chemicals/ solvents on cell permeabilization for effective β-galactosidase activity
The chemicals (CTAB, SDS, Triton X-100, DMSO, tween 80) and solvents (n-butanol, iso-propanol, acetone, ethanol and toluene) were evaluated for their permeabilization efficacy with reference to β-galactosidase activity. The selected mixtures of solvent (n-butanol and acetone, n-butanol and toluene, n-butanol and isopropanol, n-butanol and ethanol, ethanol and toluene, ethanol and isopropanol in addition, ethanol and acetone) have also been evaluated for their permeabilization efficacy. Cells were harvested (8000×g, 4 °C), washed twice with 0.05 M sodium phosphate buffer (pH 7.0) and the optical density was adjusted to 5.5–6.0 at A600 with the same buffer. Cells were permeabilized by adding equal volumes of 0.05 M sodium phosphate buffer (pH 7.0) containing different concentration of chemicals or solvents, mixed thoroughly for 5 min at 28 ± 2 °C. Effect of process parameters such as ratio of permeabilizing agents, contact time was evaluated. In addition, effect of temperature, pH and storage on permeabilized biocatalyst with reference to β-galactosidase activity was also studied.
Enzyme assay for permeabilized biocatalyst
The permeabilized biocatalyst suspension was centrifuged (8000×g 10 min, 4 °C) and β-galactosidase activity was determined both in pellet and cell free supernatant. To appropriately diluted permeabilized biocatalyst or cell free supernatant (50 μl), 750 μl of 0.05 M sodium phosphate buffer (pH 7.0) followed by 200 μl oNPG [10 mM in 0.05 M sodium phosphate buffer (pH 7.0)] were added and incubated in a temperature controlled shaking water bath (100 rpm) at 50 °C, 10 min. Reaction was arrested by adding 1 ml of 1 M sodium carbonate and release of oNP was measured at A420 nm. One unit of enzyme activity is defined as the amount of biocatalyst (permeabilized cells/ cell free supernatant) required to release 1 μmol of oNP per min under the above assay conditions.
Transgalactosylation efficacy of permeabilized biocatalyst using lactose
The transgalactosylation efficacy of permeabilized biocatalysts for the synthesis of GOS using lactose as substrate was carried out as described earlier (Gobinath and Prapulla 2014). The GOS synthesis was carried out by incubating lactose (40 %, w/v) in 0.05 M sodium phosphate buffer (pH 7.0) with 10 U of biocatalyst/ ml of reaction mixture at 50 °C, in a shaking water bath (100 rpm) for 12 h. The reaction was arrested by heating the reaction mixture for 10 min, at 95 °C. The reaction products were analysed by HPLC as described earlier (Gobinath and Prapulla 2014).
Statistical analysis
Statistical analysis was performed by one-way ANOVA using GraphPad InStat Version 3.06 (San Deigo, CA) and p < 0.05 was considered to be significant.
Results and discussion
In the present study, we investigated the effect of various nutritional factors and culture conditions on the trangalactosylating β-galactosidase production from L. plantarum MCC2156. In addition, permeabilization of L. plantarum MCC2156 for use as whole cell biocatalyst (β-galactosidase) was also evaluated.
Effect of media components on β-galactosidase production from L. plantarum MCC2156
Among the various media screened, β-galactosidase production was highest in medium 3 (M3) with defined components followed by M1, M2, M4, M5 and M6 (supplementary Fig. 1). Further, the concentration of individual components of M3 medium was varied independently and optimized for maximum β-galactosidase production. It is observed from the results that the β-galactosidase production increased with an increase in the concentration of galactose (Fig. 1), and yeast extract (Fig. 2) and sodium acetate (Fig. 3) and the optimum was found to be 4, 2 and 3 % (w/v), respectively, and a further increase in concentration of all these components did not significantly (p < 0.05) affect the β-galactosidase production.
Effect of galactose concentration on the production of β-galactosidase from L. plantarum MCC2156. Cultivation medium contains 1 % yeast extract, 1 % sodium acetate, 0.05 % MgSO4.7H2O, 0.001 % MnSO4.H2O and different galactose concentrations. Activity measured after 16 h cultivation at 37 °C and expressed in miller units. a,b,cDifferent alphabets above each concentration indicate significant difference (p < 0.05)
Effect of yeast extract concentration on the production of β-galactosidase from L. plantarum MCC2156. Cultivation medium contains 4 % galactose, 1 % sodium acetate, 0.05 % MgSO4.7H2O, 0.001 % MnSO4.H2O and different yeast extract concentrations. Activity measured after 16 h cultivation at 37 °C and expressed in miller units. a,b,c,dDifferent alphabets above each concentration indicate significant difference (p < 0.05)
Effect of sodium acetate concentration on the production of β-galactosidase from L. plantarum MCC2156. Cultivation medium contains 4 % galactose, 2 % yeast extract, 0.05 % MgSO4.7H2O, 0.001 % MnSO4.H2O and different sodium acetate concentrations. Activity measured after 16 h cultivation at 37 °C and expressed in miller units. a,b,c,dDifferent alphabets above each concentration indicate significant difference (p < 0.05)
The role of carbon source on the induction of β-galactosidase production vary among microorganism and in our previous study, β-galactosidase production by L. plantarum MCC2156 was highest when galactose was used as carbon source (Gobinath and Prapulla 2014) which is in accordance with other reports (Kim and Rajagopal 2000; Laxmi et al. 2011). Not only the type of carbon source, but also the concentration of the chosen carbon source plays an important role in β-galactosidase production by microorganisms (Fiedurek and Szczodrak 1994; Inchaurrondo et al. 1998). Our results evidenced that the β-galactosidase production significantly (p < 0.05) increased with an increase in the concentration of galactose up to 6 % (w/v) (Fig. 1). However an increase in the galactose concentration beyond 6 % resulted in a decrease in β-galactosidase production which could be due to substrate inhibition. This observation is in accordance with earlier reports (Sriphannam et al. 2012; Murad et al. 2011; Hsu et al. 2005).
Nitrogen source is not only essential for the growth of microorganism but may also affect the biosynthesis of β-galactosidase production (Hsu et al. 2005; Rao and Dutta 1977). Similar to the effect of varying concentration of galactose, β-galactosidase production significantly (p < 0.05) increased upon increasing the yeast extract concentration up to 2 % and further increase did not have any significant effect (Fig. 2). This observation corroborates with the earlier reports of Murad et al. (2011) and Hsu et al. (2005).
From our results, sodium acetate containing medium (M3) was found to have a significant effect on the enzyme production when compared to ammonium citrate (M2) and di-potassium hydrogen phosphate (M4) containing media (supplementary Fig. 1). The β-galactosidase production significantly (p < 0.05) increased with an increase in sodium acetate concentration upto 3 % and further increase did not result in any significant change (Fig. 3).
Metal ions in the growth medium not only serve as co-factors in various enzymatic reactions but are also important in the regulation of the enzyme production (Priyadarshini et al. 2012). The effect of different metals ions on β-galactosidase production have been carried out. β-Galactosidase production was observed to be significantly (p < 0.05) higher when L. plantarum MCC2156 was grown in the medium containing metal ions (Mg2+, Mn2+, Ca2+, K+ and Na+) as against the production in the medium with no metal ions (supplementary Table 1) which indicates the need of metal ions for the regulation of the β-galactosidase production. Among the metal ions, the presence of Mn2+ appears to have a pronounced effect on β-galactosidase production as compared to other metal ions. β-galactosidase production improved significantly (p < 0.05) upon increasing the Mn2+ concentration up to 0.075 % and further increase did not result in added advantage (Fig. 4). Rao and Dutta (1977) and Kumar et al. (2012) have reported the positive effect of metal ions on β-galactosidase production.
Effect of MnSO4.H2O concentration on the production of β-galactosidase from L. plantarum MCC2156. Cultivation medium contains 4 % galactose, 2 % yeast extract, 3 % sodium acetate and different MnSO4.H2O concentrations. Activity measured after 16 h cultivation at 37 °C and expressed in miller units. a,b,c,dDifferent alphabets above each concentration indicate significant difference (p < 0.05)
Effect of initial medium pH, incubation temperature on β-galactosidase production and time course studies
The effect of initial medium pH (4.0 to 8.0) on β-galactosidase production was studied and the maximum enzyme production was observed between pH ranges of 6.0 to 7.5. Many researchers have reported that an initial media pH in the range of 6.0 to 7.0 to be best for β-galactosidase production from lactic acid bacteria (Murad et al. 2011; Rao and Dutta 1977; Hsu et al. 2005). Higher the initial medium pH (8.0) resulted in decreased enzyme production by L. plantarum MCC2156.
Maximum β-galactosidase production was observed when L. plantarum was grown at incubation temperature of 35 °C. Β-Galactosidase production was notably lower than that of maxima when the incubation temperature was either lower or higher than 35 °C. Hsu et al. (2005) and Murad et al. (2011) have reported that the optimum incubation temperature was found to be in the range of 30 to 40 °C for maximum enzyme production from lactic acid bacteria. The same trend was observed for β-galactosidase production by L. plantarum MCC2156.
Under optimized medium conditions (w/v) [galactose (4 %), yeast extract (2 %), sodium acetate (3 %), manganese sulphate (0.075 %)] with an initial medium pH of 7.0, at 35 °C, nearly a two fold increase in β-galactosidase production from L. plantarum MCC2156 was observed during the time course studies. A progressive increase in β-galactosidase production was observed with an increase in incubation time and reached maximum by 14–16 h, after which it decreased (Fig. 5). During the β-galactosidase production, the growth increased gradually and reached maximum after 14 h of incubation, whereas, the pH of the medium declined from 7.0 to 3.9.
Permeabilization of L. plantarum MCC2156 for maximum β-galactosidase activity
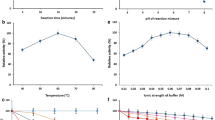
The process of permeabilization is useful in facilitating the utilization of intracellular enzymes or transforming microbial cells into biocatalysts (Somkuti et al. 1998). Surfactants, detergents or organic solvents such as toluene, ethanol, acetone, CTAB and SDS are the most commonly used permeabilizing agents (Kumari et al. 2012; Panesar et al. 2007; Krishnan et al. 2000; Sekhar et al. 1999; Somkuti et al. 1998). In our study, among the various chemicals/ solvents tested individually, maximum permeabilization with reference to β-galactosidase activity was achieved with organic solvents (Fig. 6a). Among solvents used, ethanol and n-butanol showed higher (p < 0.05) permeabilization in terms of β-galactosidase activity. Further, among the combination of selected solvents tested, ethanol (40 %, v/v): n-butanol (40 %, v/v) and n-butanol (40 %, v/v): acetone (40 %, v/v) in the ratio of 1:1 resulted in a significant (p < 0.05) increase in β-galactosidase activity of 530 ± 15 U/gDW and 512 ± 11 U/gDW, respectively (Fig. 6b). This result correlates with the earlier studies on the use of mixture of organic solvents on yeast cells permeabilization for β-galactosidase activity (Kumari et al. 2011; Siso et al. 1992). Based on the results, combination of ethanol and n-butanol were chosen for further experiments. The effect of varying concentration of these two solvents was studied. Amongst the different concentrations of ethanol and n-butanol mixture studied, mixture of ethanol (10 %, v/v) and n-butanol (30 %, v/v) in the ratio of 1:1 was found to effectively permeabilize the whole cells resulting in maximum β-galactosidase activity (574 ± 9 U/gDW) with a short span of 10 min contact time (Fig. 7). The results also indicated that an increase in contact time with the permeabilizing agents decreased the β-galactosidase activity, which could be due to the partial inactivation of enzyme due to longer exposure to solvents (Kumari et al. 2012; Krishnan et al. 2000; Sekhar et al. 1999).
a Screening of different chemicals and solvents on permeabilization of L. plantarum MCC2156 cells for maximum whole cell β-galactosidase activity. b Mixture of selected organic solvents on permeabilization of L. plantarum MCC2156 cells for maximum whole cell biocatalytic activity. Five minutes incubation with vortex at 28 ± 2 °C. Activity expressed per gram dry weight (U/gDW) basis. a,b,c,d,e,fDifferent alphabets indicate significant difference (p < 0.05)
Effect of pH and temperature on activity and stability of whole cell biocatalyst (β-galactosidase activity)
The effect of pH on β-galactosidase activity was studied using various buffers in the range from pH 3.0 to 9.0 and the optimum pH was found to be 7.0. The pH stability was determined by incubating the permeabilized biocatalyst at various buffers pH range of 3.0 to 9.0 at 28 ± 2 °C for 60 min. Samples were taken every 15 min and the residual activity (%) was measured. β-Galactosidase was found to stable in the pH range of 5.0–8.0 even after 60 min incubation (Fig. 8) but 56 % activity was lost at pH 9.0 and the activity was completely lost at pH 3.0 and 4.0 within 10 min.
The effect of temperature on β-galactosidase activity was studied in the range of 30–60 °C and the optimum temperature for β-galactosidase activity was found to be 50 °C. Temperature stability was determined by incubating the enzyme in 0.05 M sodium phosphate buffer (pH 7.0) at different temperatures. Samples were taken every 15 min and the residual activity was measured. The enzyme was stable in the range of 30–45 °C but slight decrease (17 %) in the activity was observed at 50 °C after 60 min. The enzyme was not stable both at 55 and 60 °C but the inactivation was observed to be rapid at 60 °C (Fig. 9).
Permeabilized L. plantarum MCC2156 was kept at 4 °C and 28 ± 2 °C for a period of 60 days to evaluate the storage stability. Samples were withdrawn every 24 h, centrifuged and suspended in 0.05 M sodium phosphate buffer (pH 7.0) and the β-galactosidase activity was determined as mentioned in the enzyme assay section. β-Galactosidase was stable and there was no apparent loss in activity at 4 °C but 50 % loss in activity was observed after 45 days of storage at 28 ± 2 °C.
Synthesis of GOS
Transgalactosylation efficacy of permeabilized biocatalyst was carried out using lactose (40 % w/v) as substrate, which resulted in an yield of 34 % (w/w) GOS at 12 h of reaction accounting to about 80 % of lactose conversion. The GOS mixture was composed (w/w) of non-lactose disaccharides (12–13 %), trisaccharides (16–17 %) and tetrasaccharides (3–5 %) which is comparable to our earlier findings (Gobinath and Prapulla 2014). The results indicated that the permeabilization did not have any adverse effect on either transgalactosylating or hydrolysing efficacy of β-galactosidase from L. plantarum MCC2156.
In conclusion, the present communication details the optimization of nutritional parameters for β-galactosidase production by potent probiotic L. plantarum MCC2156 and permeabilization of L. plantarum for use as whole cell biocatalyst. Nearly two fold increases in intracellular β-galactosidase activity was obtained after optimization. A mixture of ethanol (10 %, v/v): n-butanol (30 %, v/v) in the ratio of 1:1 with a contact time of 10 min at 28 ± 2 °C was found to be most effective for the permeabilization of L. plantarum with maximum β-galactosidase activity. The permeabilized biocatalyst yielded 34 % (w/w) GOS from 40 % (w/v) of initial lactose concentration corresponding to about 80 % lactose conversion. The results obtained have been encouraging and may thus be a solution to the disadvantages associated with intracellular enzyme such as extraction and purification. The study could further lead to the development of a low-cost and simple technology for the production of galacto-oligosaccharides, a prebiotic of great demand.
References
Crittenden RG, Playne MJ (1996) Production, properties and applications of food-grade oligosaccharides. Trends Food Sci Technol 7:353–361
Fiedurek J, Szczodrak J (1994) Selection of strain, culture conditions and extraction procedures for optimum production of β-galactosidase from Kluyveromyces fragilis. Acta Microbiol Pol 43:57–65
Gobinath D, Prapulla SG (2014) Permeabilized probiotic Lactobacillus plantarum MCC2156 as a potential source of β-galactosidase for prebiotic galactooligosaccharides synthesis. Biotechnol Lett 36:153–157
Hsu CA, Yu RC, Chou CC (2005) Production of β-galactosidase by Bifidobacteria as influenced by various culture conditions. Int J Food Microbiol 104:197–206
Hsu CA, Lee SL, Chou CC (2007) Enzymatic production of galactooligosaccharides by β-galactosidase from Bifidobacterium longum BCRC 15708. J Agric Food Chem 55:2225–2230
Inchaurrondo VA, Flores MV, Voget CE (1998) Growth and β-galactosidase synthesis in aerobic chemostat cultures of Kluyveromyces lactis. J Ind Microbiol Biotechnol 20:291–298
Kim JW, Rajagopal SN (2000) Isolation of isolation and characterization of β-galactosidase from Lactobacillus crispatus. Folia Microbiol 45(1):29–34
Krishnan S, Gowda LR, Karanth NG (2000) Studies on lactate dehydrogenase of Lactobacillus plantarum spp. involved in lactic acid biosynthesis using permeabilized cells. Process Biochem 35:1191–1198
Kumar DJM, Sudha M, Devika S, Balakumaran MD, Ravi Kumar M, Kalaichelvan PT (2012) Production and optimization of β-galactosidase by Bacillus Sp. MPTK 121, isolated from dairy plant soil. Ann Biol Res 3(4):1712–1718
Kumari S, Panesar PS, Bera MB, Singh B (2011) Permeabilization of a yeast cells for β-galactosidase activity using mixture of organic solvents. Asian J Biotechnol 3(4):406–414
Kumari S, Panesar PS, Bera MB, Panesar R (2012) Permeabilization of a newly isolated Kluyveromyces sp. for the preparation of whole cell biocatalysts with β-galactosidase activity. Inter J Food Nutr Sci 2(1):22–26
Laxmi NP, Mutamed MA, Nagendra PS (2011) Effect of carbon and nitrogen sources on growth of Bifidobacterium animalis Bb12 and Lactobacillus delbrueckiis sp. bulgaricus ATCC 11842 and production of β-galactosidase under different culture conditions. Int Food Res J 18:373–380
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Murad HA, Refaea RI, Aly EM (2011) Utilization of UF-permeate for production of β-galactosidase by lactic acid bacteria. Pol J Microbiol 60(2):139–144
Panesar R, Panesar PS, Singh RS, Kennedy JF, Bera MB (2007) Production of lactose-hydrolyzed milk using ethanol permeabilized yeast cells. Food Chem 101:786–790
Priyadarshini SRB, Mugeraya G, Sandhyavali MS (2012) Effect of media constituents on microbial enzyme activity. Inter J Pharma Chem Biol Sci 2(3):236–241
Rao MVR, Dutta SM (1977) Production of beta-galactosidase from Streptococcus thermophilus grown in whey. Appl Environ Microbiol 34:185–188
Sekhar S, Bhat N, Bhat SG (1999) Preparation of detergent permeabilized Bakers’ yeast whole cell catalase. Process Biochem 34:349–354
Siso MIG, Cerdan E, Picos MAF, Ramil E, Belmonte ER, Torres AR (1992) Permeabilization of Kluyveromyces lactis cells for milk whey saccharification: a comparison of different treatments. Biotechnol Tech 6:289–292
Somkuti GA, Dominiecki ME, Steinberg DH (1998) Permeabilization of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus with ethanol. Curr Microbiol 36:202–206
Splechtna B, Nguyen TH, Steinbock M, Kulbe KD, Lorenz W, Haltrich D (2006) Production of prebiotic galacto-oligosaccharides from lactose using β-galactosidases from Lactobacillus reuteri. J Agric Food Chem 54:4999–5006
Sriphannam W, Unban K, Ashida H, Yamamoto K, Khanongnuch C (2012) Medium component improvement for β-galactosidase production by a probiotic strain Lactobacillus fermentum CM33. Afr J Biotechnol 11(51):11242–11251
Torres DPM, Goncalves MPF, Teixeira JA, Rodrigues LR (2010) Galacto-oligosaccharides: production, properties, applications, and significance as prebiotics. Compr Rev Food Sci F 9:438–454
Acknowledgments
Duraiswamy Gobinath is thankful to Council of Scientific and Industrial Research, India for the award of senior research fellowship. Authors thank The Director, CFTRI for supporting the work.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
1. Optimization of nutritional parameters for the maximum production of transgalactosylating β-galactosidase from generally regarded as safe bacteria (GRAS) Lactobacillus plantarum MCC2156 for galactooligosaccharides synthesis.
2. Optimization of whole cell permeabilization as an effective alternative to the utilization of intracellular enzyme for use of whole cell as biocatalyst (β-galactosidase).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(DOC 27 kb)
Supplementary Table 1
(DOC 32 kb)
Rights and permissions
About this article
Cite this article
Gobinath, D., Prapulla, S.G. Transgalactosylating β-galactosidase from probiotic Lactobacillus plantarum MCC2156: production and permeabilization for use as whole cell biocatalyst. J Food Sci Technol 52, 6003–6009 (2015). https://doi.org/10.1007/s13197-014-1656-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1656-4