Abstract
Rutin and chitosan could be utilized in the food industry owing to their antioxidant and antibacterial properties. This study was carried out to fabricate novel films using polycaprolactone (PCL-sole), PCL and chitosan (PCL-CS), PCL and rutin (PCL-R), and PCL, chitosan, and rutin (PCL-CS-R) through electros pinning method. Physical properties, in vitro antibacterial and antioxidant properties of the films, and their antibacterial activity on rainbow trout were further investigated. The PCL-CS, PCL-R, and PCL-CS-R had smaller fiber diameter and film thickness and lower viscosity while they showed higher surface tension, water contact angle, and conductivity and better antibacterial and antioxidant properties compared with PCL-sole film (P < 0.05). The PCL-CS-R film respectively decreased 17.45%, 19.27%, and 18.39% more populations of L. monocytogenes, S. aureus, and E. coli compared to PCL-sole film in the fish samples. Therefore, the PCL-CS-R film can be potentially used in active packaging because of its antioxidant and antibacterial activities.
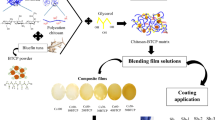
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidation deterioration and microbial growth have negative effects on nutritional value and food quality, such as color and texture (Salevic et al., 2019). Foodborne pathogens mainly cause food spoilage and deterioration (Huang et al., 2019). Antibacterial packaging systems are required to reduce microbial population and prevent oxidation deterioration. Some films and coatings have been used to retain food quality and stability (Azadbakht et al., 2018). Synthetic and natural antimicrobial materials have been employed in the preparation of films in packaging systems (Joerger, 2007). These films are deemed to have certain antibacterial and antioxidant properties and entail ethanol emission (Quintavalla and Vicini, 2002). Polymeric food packaging films have been largely used in the food industry owing to their properties, such as antimicrobial resistance, flexibility, strength, and stiffness, and they also act as barriers to the entrance of oxygen and moisture (Huang et al., 2019). Polycaprolactone (PCL) is utilized in packaging systems since it is cheap and has a high biocompatibility and low biodegradability and toxicity (Van der Schueren et al., 2013). PCL is simply processed because of its rheological and viscoelastic properties (Woodruff and Hutmacher, 2010). PCL has limitations in the preparation of the films due to its low hydrophobic properties (Prabhakaran et al., 2008). Adding the natural polysaccharide of chitosan (CS) into PCL solution improved the hydrophobic properties of PCL (Van der Schueren et al., 2013). CS is a copolymer of N-acetyl glucosamine and glucosamine units and has high biodegradability and biocompatibility and a low toxicity. CS and its derivations have also received a great deal of attention in the food industry owing to their antimicrobial properties (Stoica et al., 2013). Chitosan and PCL are biodegradable biomaterials approved by the Food and Drug Administration. Different studies have proposed the incorporation of different natural/bioactive compounds to modify the antimicrobial, antioxidant, barrier, thermal, and mechanical properties of the film (He et al., 2019; Rivero et al., 2010).
Rutin (quercetin-3-rhamnosyl-glucoside) is a flavonol, of which fruits and vegetables are rich sources (Agustin-Salazar et al., 2014). It is also known to have antibacterial and antioxidant properties (Almutairi et al., 2017).
Electrospinning is a simple and versatile technology for the production of nanofibrous films from polymers (He et al., 2019). It has also received significant attention in the food packaging industry (Aydogdu et al., 2019). The technology uses electrostatic force to generate an ultra-thin structure (Fabra et al., 2016).
Fish is more prone to lipid oxidation due to the high degree of unsaturated lipids in its structure (Tabatabaei Moradi et al., 2020). Rainbow trout (Oncorhynchus mykiss) is a fatty fish species susceptible to microbial spoilage and deterioration (Rezaei and Hosseini, 2008).
PCL, CS, and rutin are found to be appropriate for antibacterial and antioxidant properties. We attempted to fabricate a film that could be employed in the food industry by PCL, CS, and rutin. Accordingly, the objective of this study was to prepare a film by PCL, CS, and rutin via electrospinning and investigating the in-vitro antibacterial and antioxidant properties of the films and the antibacterial activity of the films on the rainbow trout.
Materials and methods
Materials
Rutin (Cat No: 207671-50-9 SIGMA), PCL (Mn = 80,000, Milwaukee, WI, USA), and chitosan were bought from Sigma-Aldrich Company. Listeria monocytogenes (ATCC 19118), Staphylococcus aureus (ATCC 191186358), and Escherichia coli O157:H7 (ATCC 10536) were prepared from the culture collection of the IROST (Tehran, Iran). Brain Heart Infusion agar (BHI) was obtained from Merck Company (Munich, Germany).
Preparation of films
The films were prepared from PCL incorporated with CS (PCL-CS), rutin (PCL-R), and CS + R (PCL-CS-R). The PCL films without CS and rutin were prepared and considered as control films (PCL-sole). To prepare the PCL-R film, 6 g PCL was added into 94 mL dichloromethane, and the obtained solution was stirred for 6 h at room temperature. Subsequently, 5 g rutin was added into the solution and stirred for 24 h. To prepare the PCL-CS films, PCL and CS solutions were mixed in a ratio of 75:25 and stirred for 12 h. The PCL-CS-R solution was prepared by adding 5 g rutin into the PCL-CS solution and stirring it for 24 h. The solutions were then transferred into a plastic syringe connected to a thin needle. The syringe was connected to a high voltage generator, and electrospinning was performed to optimize the fibers. All the utilized ratios and concentrations were tested, and the best ratios and concentrations were used.
Characterization of the films
Scanning electron microscopy (SEM)
Surface morphology of the prepared films was investigated by SEM (Mira3, FEG, Tescan, Czech Republic). In order to increase the conductivity, the samples were primarily fixed on the brass stub, sputtered coated with gold, and ultimately investigated.
Characterization of the solutions
Viscosity, surface tension, and conductivity were analyzed by viscometer, tensiometer, and conductivity meter, respectively.
Film thickness
The film thickness was examined via a digital micrometer with an accuracy of 0.001 mm at five random points per film.
Water contact angle
An optical tensiometer was used to assess the contact angle of water on the film surface through employing 5 droplets (5 µL) of ultrapure water in different parts per sample (22 mm × 55 mm).
Antibacterial analyses
Disc diffusion method was utilized to evaluate the antibacterial activity of the films against L. monocytogenes, S. aureus, and E. coli as previously reported by Shahbazi (2017). The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) were assessed as reported by Rostami et al. (2012).
Antioxidant activity
Antioxidant activity was assessed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution. To investigate the antioxidant activity, 1.9 mL of DPPH solution was added into a vial containing 1 mg of film samples. The inhibition rate was examined by the absorbance at 517 nm at room temperature for 0.5, 6, 12, and 24 h.
Fish samples
Twenty fresh rainbow trout (Oncorhynchus mykiss), with a mean weight of 600 ± 30 g, were prepared from a local market in Kermanshah city (Iran) and washed to remove the remaining blood and slimes. The fillets were prepared with at a size of 21 × 9 cm2 and a weight of 100 ± 10 g. The samples were uniformly inoculated with 105 CFU g/L of a pure culture of L. Monocytogenes, S. aureus, and E. coli by a swab. Afterwards, they were placed between films, kept in barrier packaging bags, and sealed under vacuum by a sealing machine. The samples were kept in a refrigerator at 4 °C for 14 days. Following the storage period, all the films were removed and the samples were tenfold diluted in sterile PBS (pH 7.4). The samples were homogenized for 2 min at 260 rpm, and 100 µL of the sample was placed on a nutrient agar medium. The plates were incubated for 48 h at 37 °C, and the bacteria count was reported as colony-forming unit (CFU) g/L as per sample.
Data analysis
The data were analyzed by the analysis of variance (ANOVA) pathway of SPSS software (version 23) for five replications per sample. P < 0.05 was considered as statistically significant. The graphs were illustrated by Graph Pad Prism Software (Version 6.07).
Results and discussion
SEM results
Figure 1 presents the surface morphology results of the films. The SEM micrograph of PCL-sole shows smooth, thin, and coarse fibers in PCL-sole films. Meanwhile, the PCL-R and PCL-CS-R films had grains on their fibers, implying the presence of rutin on the PCL surface. As observed, PCL-CS-R films contain more irregular grains. The SEM micrographs illustrate a mesh network for the PCL-CS film. The network is not clear in the PCL-CS-R films as the rutin covers the mesh. The increased number of grains in the PCL-CS-R films might be attributed to the better compatibility and strong adhesion between rutin and CS. Similar to our findings, Narasagoudr et al. (2020) reported a good compatibility between the active compound of plants and CS owing to the strong intermolecular interactions between their functional groups.
Physical properties
Table 1 shows the results related to the physical properties of the films. The PCL-R, PCL-S, and PCL-CS-R films showed a lower viscosity compared to PCL-sole (P < 0.05) and the lowest viscosity belonged to PCL-CS-R films. The viscosity of PCL, PCL-CS, PCL-R, and PCL-CS-R films were 2041.2 cP, 1870.15 cP, 1841.40 cP and 1825.31 cP, respectively. In line with the present study, Salevic et al. (2019) reported that adding plant essential oils into PCL solution decreased the viscosity. Li et al. (2017) reported increased viscosity in films with PCLs higher than 19 wt%. The reduction in the viscosity of the PCL-CS, PCL-R, and PCL-CS-R films might be ascribed to their molecular weights (Salevic et al., 2019). It was expected that PCL-CS-R would have lower viscosity compared to PCL-CS and PCL-R films because the molecular weight is lower in PCL-CS-R films. Higher ratios of chitosan and rutin in PCL-CS-R films might be required.
Addition of CS and rutin into the PCL solution decreased the surface tension and increased the conductivity compared with PCL-sole (P < 0.05). The conductivity of PCL, PCL-CS, PCL-R, and PCL-CS-R films was 0.02 µS/cm, 0.05 µS/cm, 0.07 µS/cm, and 0.09 µS/cm, respectively. Our findings also showed that the surface tension of PCL, PCL-CS, PCL-R, and PCL-CS-R films was 29.20 mN/m, 27.31 mN/m, 26.59 mN/m, and 23.12 mN/m, respectively. Similar to our findings for the surface tension and conductivity, Salevic et al. (2019) reported that adding plant extracts into the PCL solution was able to increase the conductivity and reduce the surface tension in the films. The increase in the conductivity and the surface tension might be associated with the viscosity and mobility of the charged species (Tampau et al., 2017). Seemingly, the combination of the CS and rutin reduces the surface tension and increases the conductivity possibly due to their structures.
The PCL-R and PCL-CS-R films had smaller fiber diameter and film thickness compared with the PCL-sole and PCL-CS films (P < 0.05). This means that adding the CS into the films did not have a significant effect on the fiber diameter and thickness. The fiber diameter was 206.71 µm, 209.21 µm, 173.21 µm, and 156.00 µm for PCL, PCL-CS, PCL-R, and PCL-CS-R, respectively. The film thickness was 0.09 mm, 0.082 mm, 0.078 mm, and 0.071 mm in the PCL, PCL-CS, PCL-R, and PCL-CS-R films, respectively. The results of the fiber diameter and film thickness were parallel, such that the highest and the lowest fiber diameter and film thickness belonged to the PCL and PCL-CS-R films, respectively. In accordance with our research, different studies showed that adding plant active compounds into fibers decreased the fiber diameter (Figueroa-Lopez et al., 2018; Tampau et al., 2018). Salevic et al. (2019) also reported that the incorporation of the essential oils into PCL films reduced the film thickness. The decrease in the fiber diameter might be attributed to the decreased viscosity and surface tension and the increased conductivity (Neo et al., 2012), which is consistent with the current study. Jin et al. (2019) showed that the fiber diameter was dependent on the concentration of the viscosity in the electrospinning method.
Based on the results, adding CS and rutin increased the contact angle compared to PCL-sole. The contact angle was 74.12°, 78.21°, 99.21°, and 109.70° in the PCL, PCL-CS, PCL-R, and PCL-CS-R films, respectively. A contact angle of \(\Theta\) < 65° indicates hydrophilicity while a contact angle of \(\Theta\) > 65° shows hydrophobicity (Vogler, 1998), so PCL-sole films have weak hydrophobicity. Similar to our results for hydrophobicity, Figueroa-Lopez et al. (2018) reported weak hydrophobicity in PCL-sole films. Jaramillo et al. (2015) observed that adding plant extracts into the films increased hydrophobicity because they increased the roughness in the films, which is in line with the present study.
In-vitro antibacterial properties of the films
The data associated with the antibacterial properties of the films are presented in Fig. 2. The results showed that the PCL films incorporated with CS and rutin had a higher inhibition zone and lower MIC and MBC values against L. monocytogenes, S. aureus, and E. coli (P < 0.05). Chitosan is an antibacterial compound because of its interaction with bacterial cell wall, cell membrane, and cytoplasmic constituents via electrostatic interactions (Duan et al., 2019). Rutin is also an antibacterial compound that functions by preventing DNA isomerase (Bernard et al., 1997). Previous studies have reported the antibacterial activity of rutin (Araruna et al., 2012) and the films prepared from CS (Dutta et al., 2009). The highest antibacterial activity was observed in the PCL-CS-R films, which could be attributed to the positive interactive effects between CS and rutin for their antibacterial properties.
In-vitro antioxidant properties of the films
Antioxidant properties of the films are shown in Fig. 3. Based on the results, the PCL-CS, PCL-R, and PCL-CS-R films revealed higher antioxidant properties compared to PCL-sole; meanwhile, PCL-sole film had a constant antioxidant activity during a 24-h period, which might be attributed to its poor antioxidant properties. The films showed better antioxidant properties over longer periods. Rutin is an antioxidant compound that has shown antioxidant properties through preventing lipid peroxidation and increased endogenous antioxidant defense enzymes (Ganeshpurkar and Saluja, 2016). Chitosan has also shown antioxidant activity by the production of highly deacetylated products and scavenging activities (Yen et al., 2008). The PCL-CS-R film had the highest antioxidant activity that could be attributed to the interactive effects between CS and rutin for their antioxidant properties.
Antibacterial effects of the films on fish samples
The results of the antibacterial properties of the films on fish samples are shown in Table 2. Similar to the results of the in-vitro section, the highest antibacterial effect belonged to PCL-CS-R whereas the lowest antibacterial activity was detected in the PCL-sole films (P < 0.05). Our findings also showed that the PCL-CS, PCL-R, and PCL-CS-R films respectively decreased L. monocytogenes population by 4.60%, 12.80%, and 17.45% more compared to the PCL-sole films. The S. aureus population showed that the PCL-CS, PCL-R, and PCL-CS-R films respectively reduced its population by 6.02%, 14.45%, and 19.27% more in comparison to PCL-sole films. The PCL-CS, PCL-R, and PCL-CS-R films respectively decreased the E. coli population by 5.74%, 12.64%, and 18.39% more compared to the PCL-sole films. The results also indicated the better antibacterial activity in the PCL-R film on fish samples compared to PCL-CS films. This is another piece of evidence for the positive interactive effects between CS and rutin on the PCL-CS-R films. The mechanism of action related to the antibacterial activity of CS and rutin was previously discussed. The results confirmed that PCL-CS-R films had antibacterial activity on the fish samples and under in-vitro conditions. Our findings further showed that electrospinning method is an efficient method for the preparation of films in food packaging. The films prepared by electrospinning method have a large surface, a small diameter, and a porous structure that help to produce simple and applicable films in the food industry (He et al., 2019).
In conclusion, we fabricated the films by adding the CS and rutin into the PCL structure by the help of the electrospinning method. The PCL-CS-R films had the highest contact angle and conductivity and the lowest fiber diameter, surface tension, and film thickness. They also showed the highest antioxidant and antibacterial properties on the fish samples under in-vitro conditions. This is a preliminary study which can pave the way for future studies. Based on the results, the PCL-CS-R film could be used to retain the quality of rainbow trout fish.
References
Agustin-Salazar S, Medina-Juarez LA, Soto-Valdez H, Manzanares- Lopez F, Gamez-Meza N. Influence of the solvent system on the composition of phenolic substances and antioxidant capacity of extracts of grape (Vitis vinifera L.). Australian Journal of Grape and Wine Research 20: 208-13 (2014)
Almutairi MM, Alanazi WA, Alshammari MA, Alotaibi MR, Alhoshani AR, Al-Rejaie SS. Neuro-protective effect of rutin against Cisplatin-induced neurotoxic rat model. BMC Complementary and Alternative Medicine 17: 472 (2017)
Araruna MK, Brito SA, Morais-Braga MF, Santos KK, Souza TM, Leite TR, Costa JG, Coutinho HD. Evaluation of antibiotic & antibiotic modifying activity of pilocarpine & rutin. Indian Journal of Medical Research 135: 252-254 (2012)
Aydogdu A, Yildiz E, Ayhan Z, Aydogdu Y, Sumnu G, Sahin S. Nanostructured poly(lactic acid)/soy protein/HPMC films by electrospinning for potential applications in food industry. European Polymer Journal 112: 477-486 (2019)
Azadbakht E, Maghsoudlou Y, Khomiri M, Kashiri M. Development and structural characterization of chitosan films containing Eucalyptus globulus essential oil: Potential as an antimicrobial carrier for packaging of sliced sausage. Food Packaging and Shelf Life 17: 65-72 (2018)
Bernard FX, Sable S, Cameron B, Provost J, Desnottes JF, Crouzet JF, Blanche F. Glycosylated flavones as selective inhibitors of topoisomerase IV. Antimicrobial Agents and Chemotherapy 41: 992-998 (1997)
Duan C, Meng X, Meng J, Khan IH, Dai L, Khan A, An X, Zhang J, Huq T, Ni Y. Chitosan as a preservative for fruits and vegetables: a review on chemistry and antimicrobial properties. Journal of Bioresources and Bioproducts 4: 11-21 (2019)
Dutta PK, Tripathi S, Mehrotra GK, Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chemistry 114: 1173-1182 (2009)
Fabra MJ., López-Rubio A, Lagaron JM. Use of the electrohydrodynamic process to develop active/bioactive bilayer films for food packaging applications. Food Hydrocolloids 55: 11-18 (2016)
Figueroa-Lopez K, Castro-Mayorga J, Andrade-Mahecha M, Cabedo L, Lagaron J. Antibacterial and barrier properties of gelatin coated by electrospun polycaprolactone ultrathin fibers containing black pepper oleoresin of interest in active food biopackaging applications. Nanomaterials 8: 199-208 (2018)
Ganeshpurkar A, Saluja AK. The pharmacological potential of rutin. Saudi Pharmaceutical Journal 23: 14-21 (2016)
He L, Lan W, Ahmed S, Qin W, Liu Y. Electrospun polyvinyl alcohol film containing pomegranate peel extract and sodium dehydroacetate for use as food packaging. Food Packaging and Shelf Life 22: 100390 (2019)
Huang T, Qian Y, Wei J, Zhou C. Polymeric antimicrobial food packaging and its applications. Polymers 11: 560 (2019)
Jaramillo CM, González Seligra P, Goyanes S, Bernal C, Famá L. Biofilms based on cassava starch containing extract of yerba mate as antioxidant and plasticizer. Starch-Starke 67: 780-789 (2015)
Jin S, Sun T, Fan Y, Wang L, Zhu M, Yang J, Jiang W. Synthesis of freestanding PEDOT:PSS/PVA@Ag NPs nanofiber film for high-performance flexible moelectric generator. Polymers 167: 102-108 (2019)
Joerger RD. Antimicrobial films for food applications: A quantitative analysis of their effectiveness. Packaging Technology and Science 20: 231-273 (2007)
Li W, Shi L, Zhang X, Liu K, Ullah I, Cheng P. Electrospinning of polycaprolactone nanofibers using H2O as benign additive in polycaprolactone/glacial acetic solution. Journal of Applied Polymer Science 143: 45578 (2017)
Narasagoudr SS, Hegde VG, Vanjeri VN, Chougale RB, Masti SP. Ethyl vanillin incorporated chitosan/poly (vinyl alcohol) active films for food packaging applications. Carbohydrate Polymers 116049 (2020)
Neo YP, Ray S, Jin J, Gizdavic-Nikolaidis M, Nieuwoudt MK, Liu D, Quek SY. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chemistry 136: 1013-1021 (2012)
Prabhakaran MP, Venugopal JR, Chyan TT, Hai LB, Chan CK, Lim AY. Electrospun biocomposite nanofibrous scaffolds for neural tissue engineering. Tissue Engineering Part A 14: 1787-1797 (2008)
Quintavalla S, Vicini L. Antimicrobial food packaging in meat industry. Meat Science 62: 373-380 (2002)
Rezaei M, Hosseini S. Quality assessment of farmed rainbow trout (Oncorhynchus mykiss) during chilled storage. Journal of Food Science 73: H93-H96 (2008)
Rivero S, Garcia M, Pinotti A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohydrate Polymers 82: 270-276 (2010)
Rostami H, Kazemi M, Shafiei S. Antibacterial activity of Lavandula officinalis and Melissa officinalis against some human pathogenic bacteria. Asian Journal of Biochemistry 7: 133-142 (2012)
Salevi´c A, Prieto C, Cabedo L, Nedovi´c V, Lagaron JM. Physicochemical, antioxidant and antimicrobial properties of electrospun poly("-caprolactone) films containing a solid dispersion of sage (Salvia officinalis L.) extract. Nanomaterials 9: 270 (2019)
Shahbazi Y. Antibacterial and antioxidant properties of methanolic extracts of apple (Malus pumila), grape (Vitis vinifera), pomegranate (Punica granatum L.) and common Fig (Ficus carica L.) fruits. Pharmaceutical Sciences 23: 308–315 (2017)
Stoica P, Chifiriuc MC, Râpă M, Bleotu C, Lungu L, Vlad G, Grigore R, Bertesteanu S, Stavropoulou E, Lazăr V. Fabrication, characterization and bioevaluation of novel antimicrobial composites based on polycaprolactone, chitosan and essential oils. Romanian Biotechnological Letters 20: 10521-10535 (2013)
Tabatabaei Moradi L, Sharifan A, Larijani K. The effect of multilayered chitosan–pectin–Mentha piperita and lemon essential oil on oxidation effects and quality of rainbow trout fillet (Oncorhynchus mykiss) during refrigeration at 4±1˚C storage. Iranian Journal of Fisheries Sciences 19: 2544-2559 (2020)
Tampau A, Gonzalez-Martinez C, Chiralt A. Carvacrol encapsulation in starch or PCL based matrices by electrospinning. Journal of Food Engineering 214: 245-256 (2017)
Tampau A, González-Martínez C, Chiralt A. Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-("-caprolactone) nanofibres: Application in starch multilayer films. Food Hydrocolloids 79: 158-169 (2018)
Van der Schueren L, De Meyer T, Steyaert I, Ceylan O, Hemelsoet K, Van Speybroeck V, De Clerck K. Polycaprolactone and polycaprolactone/chitosan nanofibres functionalised with the pH-sensitive dye Nitrazine Yellow. Carbohydrate Polymers 91:284-293 (2013)
Vogler EA. Structure and reactivity of water at biomaterial surfaces. Advances in Colloid and Interface Science 74: 69-117 (1998)
Woodruff MA, Hutmacher DW. The return of a forgotten polymer-Polycaprolactone in the 21st century. Progress in Polymer Science 35: 1217-1256 (2010)
Yen MT, Yang JH, Mau JL. Antioxidant properties of chitosan from crab shells. Carbohydrate Polymers 74: 840-844 (2008)
Acknowledgements
This work was supported by Razi University.
Author information
Authors and Affiliations
Contributions
All the authors had similar contribution for conducting and writing article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piri, H., Moradi, S. & Amiri, R. The fabrication of a novel film based on polycaprolactone incorporated with chitosan and rutin: potential as an antibacterial carrier for rainbow trout packaging. Food Sci Biotechnol 30, 683–690 (2021). https://doi.org/10.1007/s10068-021-00898-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-021-00898-9