Abstract
Despite significant advancement in the diagnostic and therapeutic aspects of breast carcinoma, the prognosis remains dismal. Recently, with advances in its understanding, various immune system-based management strategies have been developed. CTLA-4 suppresses lymphocyte reactivity, IL-2 secretion, and IL-2 receptor expression and triggers cell cycle arrest. PD-L1 inhibits the proliferation and cytotoxicity of T cells and inhibits release of cytokines. Hence, we planned to evaluate the immunoexpression of CTLA-4 and PD-L1 in invasive ductal carcinoma breast and seek correlation between their immunopositivity and the clinicopathological parameters. This was a retrospective study conducted on archival material of 50 cases of breast carcinoma tissue microarrays. Clinicopathological details were recorded. All cases were evaluated for immunohistochemical expression of CTLA-4 and PD-L1. Cytoplasmic expression of CTLA-4 and membranous expression of PD-L1 were considered positive and staining intensity was recorded as mild, moderate, and intense. Data was recorded and analyzed. Immunopositivity for CTLA-4 was seen in 92% of cases of breast carcinoma. CTLA-4 staining intensity showed significant association with TNM staging of breast carcinomas (p = 0.036). Age group of the breast carcinoma cases showed a statistically significant correlation with PD-L1 immunoexpression (p = 0.002). No significant correlation was found between all other clinicopathological characteristics and CTLA-4 or PD-L1 immunostaining. Our study shows that CTLA-4 is a more important immune checkpoint regulator in breast carcinomas in comparison to PD-L1. Thus, anti-CTLA-4 immunotherapy might prove to be of immense help in the treatment of invasive ductal carcinoma breast showing overexpression of CTLA-4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the fifth most common cause of death due to cancer and is currently the most common cancer occurring in women globally [1]. A significant increase in the incidence of breast cancer and associated morbidity and mortality has been reported in the Indian subcontinent [2]. Although significant advancement has taken place in the diagnostic and therapeutic aspects of breast carcinoma, yet the prognosis remains dismal. With the increasing incidence of resistance to traditional chemotherapeutic drugs and hormonal therapy, there is a need to identify new promising prognostic, predictive, and therapeutic biomarkers [3]. Recently, the focus of research in carcinomas has shifted to the crosstalk between cancer cells and the immune system. It is believed that immune evasion is one of the important mechanisms in the progression of cancer [4]. Amongst the immune checkpoint regulators, cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) have been found to be the most reliable targets [5].
Cytotoxic T lymphocyte antigen 4 (also known as CD152) is a negative regulator of the T cell activation [6]. It antagonizes signaling of CD28 by competing for the shared ligands, CD80 and CD86, which are present on antigen-presenting cells (APCs). These, in turn, suppress lymphocyte reactivity, IL-2 secretion, and IL-2 receptor expression and ultimately, trigger cell cycle arrest [7, 8]. In physiologic conditions, CTLA-4 has a role in the maintenance of peripheral tolerance [9]. On the other hand, in tumor patients who have suboptimal APC function, CTLA-4-mediated inhibition of T cell activation can prevent the development of antitumor T cell responses [10]. CTLA-4 has been implicated in immune dysregulation of various malignancies including esophageal cancer, breast cancer, lung cancer, and melanoma [11,12,13,14]. On the basis of this information, therapeutic strategies targeted toward blockade of CTLA-4 signaling have been developed in various cancers. Ipilimumab is a CTLA-4-targeting drug approved for treatment of various malignancies, while several others are under development [15].
Programmed cell death–ligand 1 (PD-L1) is a transmembrane protein commonly expressed on the surface of APCs and tumor cells. It specifically binds to its receptor, PD-1, which is expressed on the surface of immune-related lymphocytes, such as T cells, B cells, and myeloid cells, and also on tumor cell surface in some solid tumors and hematological malignancies [16,17,18,19,20]. The binding of PD-L1 to PD-1 activates the down-stream signaling of PD-1 receptor in T cells, thus inhibiting the proliferation and cytotoxicity of T cells along with inhibition of cytokine release by T cells. Besides preventing autoimmunity and chronic infection, this downregulation of immunity also protects many tumor cells from immune attack, resulting in tumor immune evasion [17]. Inhibition of either PD-1 or PD-L1 enhances T cell responses to cancer and this approach is known as PD-1/PD-L1-based immunotherapy. Immune blockade therapy targeting against PD-1/PD-L1 has been gaining interest in cancer treatment [21]. Six drugs targeting PD-1/PD-L1 are currently approved for treatment of various malignancies [15].
A single combination drug (ipilimumab plus nivolumab) targeting both CTLA-4 and PD-1 for treatment of metastatic melanoma, renal cell carcinoma, and colorectal cancer has also been approved with promising results [15].
Therefore, immune checkpoint regulators CTLA-4 and PD-L1 have emerged as promising new targets for cancer therapy in different solid and hematological malignancies. In view of the increasing incidence and high mortality associated with breast cancer, there is a need to identify new prognostic biomarkers that can be used as potential therapeutic targets in future. Hence, in the present study, we evaluated the expression of CTLA-4 and PD-L1 in breast carcinoma to know whether anti-CTLA-4 and anti-PD-L1 immunotherapies can be used with efficacy in treatment of breast carcinomas showing overexpression of these immune modulators.
Materials and Methods
This analytical and observational study was conducted in Multi-disciplinary Research Unit (MRU) UCMS, Department of Pathology, and Department of Surgery, UCMS & GTB Hospital, Delhi, between January 2020 and October 2021. All 72 cases diagnosed as invasive ductal carcinoma breast on histopathologic examination with available representative tumor tissue in mastectomy specimens during the study duration were evaluated. Of 72 mastectomy specimens, 22 cases with insufficient tumor tissue in mastectomy specimens (< 500 cells on histology) and post chemo/radiotherapy cases were excluded. Clinicopathological details of all 50 cases included in the study were noted. Slides of these cases were reviewed for confirmation of diagnosis and other pathological findings.
Manual tissue microarray (TMA) blocks of tumor tissue taken from the corresponding mastectomy specimens were prepared. Each TMA had 12 tumor tissue cores and 2 control cores (one each for CTLA-4 and PD-L1). Immunohistochemistry using CTLA-4 (mouse monoclonal IgG1κ anti-CTLA-4, F-8; sc-376016, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and PD-L1 (rabbit monoclonal antibody anti-PD-L1 protein antibody, clone IHC411, GenomeMe, prediluted, ready to use) was done on lysinated slides prepared from the TMA blocks. Cytoplasmic immunoexpression of CTLA-4 in tumor cells and membranous immunoexpression of PD-L1 in > 1% tumor cells was considered positive. CTLA-4 and PD-L1 expression was evaluated semi-quantitatively using Tumor Proportion Score (TPS) based on percentage of positively stained viable tumor cells, staining localization (tumor cell cytoplasm in the case of CTLA-4 and complete or partial membranous in the case of PD-L1), and staining intensity, which was expressed as mild, moderate, and intense.
Data was entered in MS Excel sheet and analyzed using SPSS v20 software. For the association between immunoexpression of CTLA-4 and PD-L1 and clinicopathological parameters, Fisher’s exact test and Pearson chi-square test were used. p value less than 0.05 was considered significant.
Results
The age of the patients ranged from 27 to 75 years with the mean age of 48 years. The clinicopathological details of the patients are summarized in Table 1.
CTLA-4 Immunoexpression
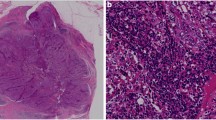
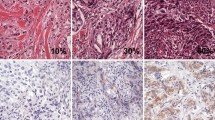
Out of 50 breast carcinoma cases, 46 (92.0%) showed cytoplasmic immunopositivity for CTLA-4 while the remaining 4 (8.0%) were negative. The percentage cell positivity for CTLA-4 in cases showing immunoexpression was found to be 100% in 36 cases, 90% in 4 cases, 80% in 2 cases, 60% in 1 case, 50% in 1 case, and 40% in 2 cases. Of 46 CTLA-4 positive cases of breast carcinoma, 17/46 (36.9%) showed mild intensity of CTLA-4 positivity, 21/46 (45.6%) showed moderate intensity staining, and intense immunostaining for CTLA-4 was found in 8/46 (17.4%) cases (Fig. 1).
CTLA-4 immunopositivity was correlated with patient age, menopausal status, tumor size, tumor grade, presence of in situ component, lymph node status, TNM staging, and molecular subtyping (Table 2). None of the clinicopathological parameters studied showed statistically significant correlation with CTLA-4 immunoexpression in breast carcinoma. However, statistically significant correlation was found between staining intensity of CTLA-4 and TNM staging of breast carcinoma cases (p = 0.038) (Table 3).
PDL-1 Immunoexpression
Out of 50 breast carcinoma cases, 4 (8.0%) showed membranous immunopositivity for PDL-1, while the remaining 46 (92.0%) were negative. Of these PD-L1-positive cases of breast carcinoma, 3/4 (75.0%) showed moderate intensity of PD-L1 positivity (Fig. 2). Strong intensity of PD-L1 positivity was found in 1/4 (25%) and none of the cases revealed mild immunostaining for PD-L1. Immunoexpression of PD-L1 was correlated with patient age, menopausal status, tumor laterality, tumor size, tumor grade, presence of in situ component, lymph node status, TNM staging, and molecular subtyping. Age group of the breast carcinoma cases showed a statistically significant correlation with PD-L1 immunoexpression (p = 0.002) while all other clinicopathological characteristics did not reveal any statistically significant correlation with PD-L1 immunostaining and these findings are shown in detail in Table 4.
Discussion
Breast carcinoma is a leading cause of cancer death in the female population. Despite numerous currently available treatment modalities, there is a need to identify newer treatment modalities of breast carcinoma in view of tumor heterogeneity and the changes in its intrinsic nature during metastasis.
In the present study, CTLA-4 cytoplasmic positivity was found in 92% of cases. In previous studies, CTLA-4 positivity in tumor cells of breast carcinoma cases has been reported to be between 70 and 100% [22, 23].
The intensity of CTLA-4 immunoexpression in our study showed significant association with the tumor stage (p = 0.036), hence indicating that higher expression is associated with poorer prognosis. Similarly, Mao et al. in their study on 60 breast carcinoma cases found that patients with greater levels of CTLA-4 mRNA were of significantly higher clinical stage. They also found significant association between axillary lymph node involvement and CTLA-4 levels. While in our study, no significant association was found between axillary lymph node involvement and CTLA-4 immunoexpression. This could be explained by the greater sensitivity of mRNA detection methods [22].
No association was found between CTLA-4 immunopositivity and patient age, tumor size, menstrual status, lymph node status, presence of in situ component, tumor grade, tumor stage, ER/PR/Her-2/neu status, or molecular subtype. Yu et al. in their study on 130 breast carcinoma status also did not find any correlation between expression of CTLA-4 in tumor cells and age, grade, stage, and ER/PR/Her-2/neu/Ki-67 status. However, unlike our findings, they reported that significantly higher expression of CTLA-4 was found in post-menopausal cases. This could be explained by the larger sample size, inclusion of other histological tumor types including invasive lobular, medullary, and mucinous and other demographic and population-based differences [12].
Of the 12 TNBC cases in our study, 11 showed CTLA-4 expression, of which 5 showed mild, 5 showed moderate, and 1 showed intense staining. Out of the 25 luminal cases, only 3 did not show CTLA-4 expression. The majority (40%) showed moderate staining intensity. All of the Her-2/neu-enriched cases showed CTLA-4 immunopositivity. But none of these findings was statistically significant. Kim et al., on the other hand, found that higher tumor cell expression of CTLA4 was significantly associated with negative hormone receptor expression, which is not in line with the present study findings [24]. Hence, further population-specific evaluation in a larger sample size is required for validation of these findings.
We found only 8% positivity of PD-L1 in breast carcinoma cases. No association was found between PD-L1 immunoexpression and menopausal status, tumor laterality, tumor size, tumor grade, presence of in situ component, lymph node status, TNM staging, and molecular subtyping. The reported positivity of PD-L1 in literature has ranged between 19 and 50%; however, these include mRNA-based studies on large sample sizes with various tumor histological types, mainly including TNBCs [25,26,27]. Further, PD-L1 has also been reported to have an association with greater tumor size, higher grade, ER-negative, PR-negative, Her-2/neu-positive status, higher proliferation, and basal and Her-2/neu-enriched subtype, which is contrary to our study results. The reason for this may also be the same as mentioned above [25]. In our study, 1 TNBC and 1 luminal case showed intense PDL-1 immunopositivity, while 2 Her-2/neu-enriched cases showed moderate intensity of PDL-1 expression. PD-L1 immunopositivity is known to be seen mainly in TNBCs [25] and our sample size included only a small number of such cases, hence explaining the probable discrepancy in our study results. In our study, PD-L1 immunoexpression showed significant association with patient age (p = 0.002). We could not find any other study in literature that has found such an association.
Despite the small number of studies on the expression of CTLA-4 in breast carcinomas, there has been evidence of the association of CTLA-4 expression in the breast cancer microenvironment and its association with poor prognosis, regardless of the molecular subtype [28]. Recent evidence also suggests that anti-PD1/PD-L1 agents show promising results when delivered as monotherapy or in combination with conventional treatment options in breast cancer. A combination of these two biomarkers has been found to be appealing due to the underlying rationale suggesting a synergistic mechanism of their actions [29]. Thus, CTLA-4 expression in breast cancer has immense clinical significance and must be considered a promising therapeutic target for immunotherapy. Further research is needed to allow for development of effective synergistically acting CTLA-4- and PDL-1-based therapeutic agents.
Limitations
Our study was a retrospective study limited by a small sample size and lack of uniformity of tumor molecular subtypes. Further, we could not evaluate the combined positive score in our samples as we had only representative limited available tumor tissue in the TMA. There was also a lack of follow-up of our patients after surgery due to the outbreak of the COVID-19 pandemic. Hence, disease-free survival and overall survival could not be calculated.
Conclusions
This study results show immunopositivity for CTLA-4 in a significant number of breast carcinomas (92%). However, PD-L1 immunostaining was identified in only few cases of invasive ductal carcinomas (8.0%), thus indicating CTLA-4 to be more important immune checkpoint regulator in breast carcinomas in comparison to PD-L1. The study findings suggest that anti-CTLA-4 immunotherapy might prove to be of immense help in the treatment of CTLA-4-positive invasive ductal carcinoma breast. However, more studies with a larger sample size are required to validate our study results and trial of anti-CTLA-4 immunotherapy in invasive ductal carcinoma breast to see the patient outcome.
Data Availability
All data underlying the findings are fully available.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Ali I, Wani WA, Saleem K (2010) Cancer scenario in India with future perspectives. Cancer Ther 8:56–70
Masoud V, Pagès G (2017) Targeted therapies in breast cancer: new challenges to fight against resistance. J Clin Oncol 8(2):120–134
Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E et al (2015) Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol 35(Suppl):S185–S198
Seidel JA, Otsuka A, Kabashima K (2018) Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 8:86
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3(5):541–547
Jain N, Nguyen H, Chambers C, Kang J (2010) Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci U S A 107(4):1524–1528
Krummel MF, Allison JP (1996) CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 183(6):2533–2540
Issazadeh S, Zhang M, Sayegh MH, Khoury SJ (1999) Acquired thymic tolerance: role of CTLA4 in the initiation and maintenance of tolerance in a clinically relevant autoimmune disease model. J Immunol 162(2):761–765
Smyth MJ, Godfrey DI, Trapani JA (2001) A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol [Internet] 2(4):293–299
Zhang XF, Pan K, Weng DS, Chen CL, Wang QJ, Zhao JJ et al (2016) Cytotoxic T lymphocyte antigen-4 expression in esophageal carcinoma: implications for prognosis. Oncotarget 7(18):26670–26679
Yu H, Yang J, Jiao S, Li Y, Zhang W, Wang J (2015) Cytotoxic T lymphocyte antigen 4 expression in human breast cancer: implications for prognosis. Cancer Immunol Immunother 64(7):853–860
Domagala-Kulawik J, Osinska I, Hoser G (2014) Mechanisms of immune response regulation in lung cancer. Transl Lung Cancer Res 3(1):15
Bouwhuis MG, Gast A, Figl A, Eggermont AMM, Hemminki K, Schadendorf D et al (2010) Polymorphisms in the CD28/CTLA4/ICOS genes: role in malignant melanoma susceptibility and prognosis? Cancer Immunol Immunother 59(2):303
Rotte A (2019) Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res 38(1):1–12
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H et al (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192(7):1027–1034
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB et al (2002) Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8(8):793–800
Du S, McCall N, Park K, Guan Q, Fontina P, Ertel A, et al. Blockade of tumor-expressed PD-1 promotes lung cancer growth. Oncoimmunology [Internet]. 2018 Apr 3 [cited 2022 Jul 3];7(4). Available from: https://pubmed.ncbi.nlm.nih.gov/29632720/
He R, Ding W, Viswanatha DS, Chen D, Shi M, Van Dyke D et al (2018) PD-1 expression in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and large B-cell Richter transformation (DLBCL-RT): a characteristic feature of DLBCL-RT and potential surrogate marker for clonal relatedness. Am J Surg Pathol 42(7):843–854
Yao H, Wang H, Li C, Fang JY, Xu J (2018) Cancer cell-intrinsic PD-1 and implications in combinatorial immunotherapy. Front Immunol 9:1774
Akinleye A, Rasool Z (2019) Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol 12(1):1–13
Mao H, Zhang L, Yang Y, Zuo W, Bi Y, Gao W et al (2010) New insights of CTLA-4 into its biological function in breast cancer. Curr Cancer Drug Targets 10(7):728–736
Navarrete-Bernal MGC, Cervantes-Badillo MG, Martínez-Herrera JF, Lara-Torres CO, Gerson-Cwilich R, Zentella-Dehesa A et al (2020) Biological landscape of triple negative breast cancers expressing CTLA-4. Front Oncol. 10:1206
Kim A, Kim JY, Lee SJ (2020) Abstract P5–04–26: Expression of cytotoxic T lymphocyte antigen-4 (CTLA-4) is associated with tumor-infiltrating lymphocytes (TILs) levelin HER2-positive breast cancers. Cancer Res 80(4):P5-04–26
Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR et al (2015) Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 6(7):5449–5464
Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G et al (2006) The B7–H1 (PD-L1)T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 8(3):190
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S et al (2014) PD-L1 expression in triple negative breast cancer. Cancer Immunol Res 2(4):361
Peng Z, Su P, Yang Y, Yao X, Zhang Y, Jin F, Yang B (2020) Identification of CTLA-4 associated with tumor microenvironment and competing interactions in triple negative breast cancer by co-expression network analysis. J Cancer 11(21):6365
Planes-Laine G, Rochigneux P, Bertucci F, Chrétien AS, Viens P, Sabatier R, Gonçalves A (2019) PD-1/PD-L1 targeting in breast cancer: the first clinical evidences are emerging—a literature review. Cancers 11(7):1033
Acknowledgements
We acknowledge the Multi-disciplinary Research Unit (MRU) established by the Department of Health Research, Ministry of Health and Family Welfare, at UCMS and GTB Hospital.
Funding
The funding for this project work has been provided by the Department of Health Research-Indian Council of Medical Research, Government of India, New Delhi, under Multi-disciplinary Research Scheme.
Author information
Authors and Affiliations
Contributions
Contributors: Preeti Diwaker; Tanvi Jha; Priyanka Gogoi; Vinod Kumar Arora; Mohammad Ahmad Ansari; Navneet Kaur
Concepts: Preeti Diwaker, Priyanka Gogoi, Vinod Kumar Arora
Design: Preeti Diwaker, Priyanka Gogoi, Vinod Kumar Arora
Definition of intellectual content: Preeti Diwaker, Priyanka Gogoi, Vinod Kumar Arora
Literature search: Preeti Diwaker, Tanvi Jha
Experimental studies: Preeti Diwaker, Tanvi Jha, Mohammad Ahmad Ansari
Data acquisition: Preeti Diwaker, Tanvi Jha, Navneet Kaur
Data analysis: Preeti Diwaker, Tanvi Jha, Mohammad Ahmad Ansari
Manuscript preparation: Preeti Diwaker, Tanvi Jha
Manuscript editing: Preeti Diwaker, Tanvi Jha, Priyanka Gogoi
Manuscript review: Preeti Diwaker, Priyanka Gogoi, Vinod Kumar Arora, Navneet Kaur
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diwaker, P., Jha, T., Gogoi, P. et al. Expression of Immune Checkpoint Regulator Cytotoxic T Lymphocyte Antigen 4 (CTLA-4) and Programmed Cell Death Protein Ligand 1 (PD-L1) in Invasive Ductal Carcinoma Breast. Indian J Surg Oncol (2024). https://doi.org/10.1007/s13193-024-01977-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13193-024-01977-z