Abstract
Chondroblastoma is a rare benign cartilaginous bone tumor typically seen at the epiphysis of long axial bones. In this regard, there are rare findings about spinal chondroblastomas. We report a 29-year-old man with T1 vertebral chondroblastoma misdiagnosed with a traumatic fracture following an accident. The patient was admitted to our clinic with a chief complaint of axial back pain and kyphosis following posterior spinal fixation. We report his clinical and imaging data before his past operation and at this admission. Our patient underwent a two-stage operation. In the first stage, posterior spinal reconstruction and kyphosis correction was performed. In the second stage, mass resection was performed anteriorly in the T1 vertebral body as much as possible. The results confirmed the chondroblastoma diagnosis histologically. Our patient remained symptom-free with no growth in tumor remnant during 6 months follow-up. Although vertebral chondroblastoma is a sporadic tumor, it should be considered in the differential diagnosis when facing a vertebral infiltrative osteolytic mass, even when mimicking a traumatic fracture after the accident. In addition, histological confirmation is necessary under such conditions. We also reviewed the literature’s clinical presentations, imaging findings, and treatments of 34 case reports with vertebral chondroblastoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chondroblastoma is a rare benign cartilaginous tumor that mainly occurs in long bone epiphysis at the ossification center [1]. The distal end of the femur is the most common site for chondroblastoma. The other preferred sites for this tumor are the proximal end of the humerus, the proximal end of the femur, and the proximal end of the tibia, talus, and innominate bones, in the order of their appearance [2,3,4,5,6]. Chondroblastoma is a rare skeletal tumor that accounts for less than 1% of all primary skeletal tumors. The peak of this lesion is the second and third decades of life, with twice prevalency in men over women [7, 8]. The most common clinical manifestation of chondroblastoma is insidious local pain. Radiological imaging of chondroblastoma typically reveals a well-defined radiolucent lesion with a thin sclerotic rim developed eccentrically at the epiphysis of long bones occasionally extended to the metaphysis. CT scanning may also show internal calcifications giving the lesion a mottled appearance [9,10,11,12]. This tumor appears macroscopically as a cartilaginous lesion with hemorrhagic and cystic patches, the consistency of which varies depending on the calcification degree. Chondroblastoma is characterized by polygonal chondrocyte-like cells and a chondroid matrix with large cells on histological examination [13,14,15,16]. Treatment options for this lesion include local curettage or resection with a margin of normal tissue [11, 17]. The local recurrence rate varies from 24 to 100% [3], depending on the treatment option and the presence of giant cells [11, 12]. Although this pathology is very rare and its most clinical symptom is axial pain, we report a case with thoracic vertebral chondroblastoma discovered incidentally following assessment for proximal junction kyphosis.
Case Presentation
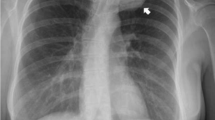
Our case is a 29-year-old man referred to our clinic with a primary complaint of gradually worsening upper back pain and paresthesia in his lower extremities after slipping down the stairs 2 months ago. He mentioned that he had a T1 fracture 6 months ago due to a vehicle accident, which necessitated posterior spinal (C6–C7 and T2–T3) fixation and fusion, as well as laminectomy at another medical institution (Fig. 1).
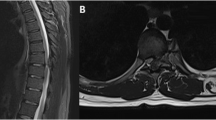
General examination revealed no gross evidence of deformity or atrophy in posterior spinal muscles. The muscle strength examinations were full in all extremities. Deep tendon reflexes were normal and symmetric. Also, he had no specific family history and was taking no medication. In laboratory assessment, no abnormality was found. The neurological examination showed normal findings. An ill-defined radiolucent lesion affecting the body of the T1 vertebra was discovered on lumbar radiography. In addition, a massive lobulated heterogeneous osteolytic tumor affecting the T1 vertebral body with spinal canal invasion was discovered on CT scanning. Magnetic resonance imaging confirmed the T1 fracture appeared hypointense on spin-echo T1-weighted MR image, heterogenous with the prominent thin hyperintensity lobulated rim on FSE T2-weighted MR image, and hypointense on FSE-STIR-weighted image. Furthermore, heterogenous enhancement was observed in contrast with enhanced T1-weighted MR images with fat saturated. This infiltration extended to the spinal canal and narrowed the epidural space without intra-medullary invasion, suggesting bone origin pathologies.
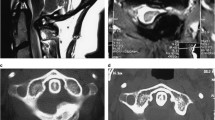
Thus, the patient was prepared for surgical intervention. In the initial operation, previously inserted screws were corrected, and new lateral masses and pedicular screws were applied instead. These operations were performed under fluoroscopy and neuromonitoring guide via the posterior approach. In the second surgical procedure through the anterior approach, the level of the T1 vertebra is determined by C-arm. During the inspection, the whole T1 vertebral body was filled up with the tumor, leading to its relative invasion to surrounding tissue. Corpectomy of the T1 vertebra, including the disc above and below, was performed, followed by placing an expandible cage with a size proportional to the defect. All these procedures were done under neuromonitoring control (Fig. 2). Samples taken during surgery were sent for pathological evaluation. Macrospically, the tissue extracted displayed a rubbery cream pattern. According to histological findings, the sample was a benign tumor with the widespread proliferation of oval cells with well-defined cell boundaries. It also contained monomorphic nuclei with irregular nuclear contours and occasional nuclear grooves. The neoplastic cells were mixed with occasional osteoclast-type multinucleated giant cells. Small areas of immature cartilage formation were also seen, and the findings indicated the correct diagnosis of chondroblastoma (Fig. 3). Retrieved tissue was also sent to a pathologist who specialized in bone tumors to reconfirm the definitive diagnosis of chondroblastoma double. According to pathology confirmation and resection of the lesion, the prognosis is good, and no adjuvant therapy was required. The post-operation imaging showed sagittal balance correction, and the patient affirmed pain relief without any complication. In 6 months, the patient came to our clinic for follow-up, and he expressed neither axial pain nor other complaints with a regular appearance in CT and MRI images (Fig. 4).
Discussion
Although vertebral chondroblastoma is benign in nature, it has a higher tendency to local recurrence than the extra-vertebral ones [3, 7,8,9,10, 18, 19]. In addition, on imaging, vertebral chondroblastoma presents as osteolytic lesions invading the spinal canal with a sclerotic border. On T1-weighted MRI images and T2-weighted MRI images, we observed a hypo-signal mass with varying signal intensities depending on histological parameters (e.g., cellularity and calcification) [20]. These radiological features were compatible with our patient imaging. However, the radiological features of vertebral chondroblastoma may reveal important clues to come into account. When we encountered aggressive tumor is in vertebral body other differential diagnoses (e.g., aneurysmal bone cyst, tuberculosis spondylitis, eosinophilic granuloma, giant cell tumors, chondromyxoid fibroma) should be differentiate from each other by histological assessment [3, 9, 10]. Tathe et al. indicated that intraoperative crash smear cytology is a rapid intraoperative diagnostic tool to confirm chondroblastoma diagnosis. Immunohistochemistry and gene mutation analysis were concerned as a new avenue to differentiate chondroblastoma from other overlap clinicoradiological pathologies [21, 22]. The primary treatment for chondroblastoma is surgery as en bloc resection or curettage, followed by radiosurgery as adjuvant therapy. However, chemotherapy is not performed for the treatment of this tumor [12]. Regarding the controversial role of adjuvant therapy in preventing tumor recurrence, more studies with longer follow-up duration are required to clarify this issue [11, 23, 24].
To this end, we reviewed 34 case reports of vertebral chondroblastoma in the literature (Table 1). In our review, we did not include the patients with a lack of imaging or clinical findings. Overall, 24 men and 10 women with vertebral chondroblastoma aged 11 to 62 years were described. The body and posterior elements of vertebrae were involved in approximately all patients. The affected areas were the thoracic zone in 14 patients, the lumbar zone in 14 patients, the cervical zone in 4 patients, and sacral vertebrae in 2 patients [2,3,4, 6, 7, 10, 13,14,15, 17, 19, 20, 23, 25,26,27,28,29,30]. In our patient, the tumor was discovered by chance during evaluation for proximal junctional kyphosis after undergoing posterior spinal fixation. In another center, his invaded vertebra by tumor was misdiagnosed as a traumatic fracture. Therefore, posterior spinal fixation was performed for him. In literature, symptoms last from 1 month to 2.5 years (with a mean of 9.7 months). In specimens related to 3 patients, ABC was seen [2, 11]. Chondroblastoma accompanied by ABC can be misleading to correct diagnosis. In this regard, two pathologists examined the tissue sample of our patient to confirm the diagnosis.
Furthermore, one of our review studies reported chondroblastoma with pulmonary metastasis [20]. Kunze et al. described 7 patients with metastatic chondroblastoma, but none had vertebral chondroblastoma. They announced that the meantime to diagnose lung metastasis was 8.4 years, and the mean survival time was 12.3 years. They mentioned no difference in pathologic features of metastatic chondroblastoma with the conventional ones [31,32,33]. In our review, we just described one patient with lung metastasis diagnosed by FDG uptake in both lower lobes of the lungs when performing a PET-CT scan to assess its metastatic status [20]. The pointed patient underwent laminectomy of the invaded vertebra and wedge resection of the involved lung, with no tumor growth after 3 years of follow-up. In 13 case reports, both anterior and posterior approaches were used to resect involved vertebral mass lesions and spine stabilization [7, 11, 13, 14, 17, 18, 27, 28]. In the other 16 case reports, the only posterior approach was employed [11, 20, 26]. Aspiration with subtotal resection and surrounding curettage was considered in 3 patients (2 with sacral chondroblastoma and one with lumbar vertebral chondroblastoma), However, one of two patients with sacral chondroblastoma died after 3 months of intervention [18]. In our case described, posterior spinal reconstruction was performed in the first stage. In the 2nd stage, resection of chondroblastoma in the T1 vertebral body was performed as much as possible. If the patient had been referred to our center primarily, an aspiration biopsy to determine the nature of this fracture could have been the better choice. The mean follow-up duration in studies was about 3 years. The recurrence duration ranged from 1.5 to 10 months according to four reports [11, 14, 17]. In these studies, vertebral chondroblastoma originated from the first lumbar vertebrae in 2 patients and the fourth lumbar vertebrae in the other 2 patients. During a 6-month follow-up, our patient was neurologically intact, and no growth in the remnant tumor was observed in MRI images. In this regard, there is no consensus on the duration of these patients’ follow-up, considering their tumor remnant.
Conclusion
Although vertebral chondroblastoma is a rare phenomenon, it should be considered when facing an osteolytic vertebral lesion. This case warned us when facing a young patient with a vertebral fracture following an accident not just relate it to the trauma. It is mandatory to assess its images in more detail to not miss pathologic reasons. Despite benign nature of vertebral chondroblastoma, more studies with longer follow-ups are required to determine its behavior.
References
Negri S, Wangsiricharoen S, Chang L, Gross J, Levin AS, Morris CD, McCarthy EF, James AW (2021) Clinicopathologic analysis of chondroblastoma in adults: a single-institution case series. Int J Surg Pathol 29(2):120–128. https://doi.org/10.1177/1066896920927794
Kurth AA, Warzecha J, Rittmeister M, Schmitt E, Hovy L (2000) Recurrent chondroblastoma of the upper thoracic spine. A case report and review of the literature. Arch Orthop Trauma Surg 120(9):544–547. https://doi.org/10.1007/s004029900125
Venkatasamy A, Chenard MP, Massard G, Steib JP, Bierry G (2017) Chondroblastoma of the thoracic spine: a rare location. Case report with radiologic-pathologic correlation. Skeletal Radiol 46(3):367–372. https://doi.org/10.1007/s00256-016-2550-0
Vialle R, Feydy A, Rillardon L, Tohme-Noun C, Anract P, Colombat M, De Pinieux G, Drape JL, Guigui P (2005) Chondroblastoma of the lumbar spine. Report of two cases and review of the literature. J Neurosurg Spine 2(5):596–600. https://doi.org/10.3171/spi.2005.2.5.0596
Shakir TM, Li W, Wang H, Niu C, Zhang M (2018) Chondroblastoma Of The Lumbar Vertebra. J Ayub Med Coll Abbottabad 30(4):608–610
Tathe SP, Parate SN, Jaiswal KN, Randale AA (2018) Intraoperative crush smear cytology of vertebral chondroblastoma: A diagnostic challenge. Diagnostic cytopathology 46(1):79–82. https://doi.org/10.1002/dc.23799
Attar A, Ugur HC, Caglar YS, Erdogan A, Ozdemir N (2001) Chondroblastoma of the thoracic vertebra. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 8(1):59–60. https://doi.org/10.1054/jocn.2000.0780
Bloem JL, Mulder JD (1985) Chondroblastoma: A clinical and radiological study of 104 cases. Skeletal Radiology 14(1):1–9. https://doi.org/10.1007/BF00361187
Kim S, Cho K-J, Park Y, Lee J-S, Kwon H-J, Chung H, Kim M-J (2011) Chondroblastoma of the lumbar spine - a case report and review of the literature. Korean J Pathol 45:532. https://doi.org/10.4132/KoreanJPathol.2011.45.5.532
Nishida J, Kato S, Murakami H, Ehara S, Satoh T, Okada K, Shimamura T (2003) Tetraparesis caused by chondroblastoma of the cervical spine: a case report. Spine (Phila Pa 1976) 28(9):E173–E178. https://doi.org/10.1097/01.BRS.0000058731.83977.61
Jia Q, Liu C, Yang J, Ji Y, Wei H, Liu T, Yang X, Yang C, Xiao J (2018) Clinical features, treatments and long-term follow-up outcomes of spinal chondroblastoma: report of 13 clinical cases in a single center. J Neuro-Oncol 140(1):99–106. https://doi.org/10.1007/s11060-018-2935-0
Chen W, DiFrancesco LM (2017) Chondroblastoma: An Update. Arch Pathol Lab Med 141(6):867–871. https://doi.org/10.5858/arpa.2016-0281-RS
Hernández Martínez SJ, Campa Núñez H, Ornelas Cortinas G, Garza Garza R (2011) Chondroblastoma of the fourth lumbar vertebra diagnosed by aspiration biopsy: case report and review of the literature. Acta cytologica 55(5):473–477. https://doi.org/10.1159/000328709
Leung LY, Shu SJ, Chan MK, Chan CH (2001) Chondroblastoma of the lumbar vertebra. Skeletal Radiol 30(12):710–713. https://doi.org/10.1007/s002560100432
Howe JW, Baumgard S, Yochum TR, Sladich MA (1988) Case report 449: Chondroblastoma involving C5 and C6. Skeletal Radiol 17(1):52–55. https://doi.org/10.1007/bf00361456
Ilaslan H, Sundaram M, Unni KK (2003) Vertebral chondroblastoma. Skeletal Radiol 32(2):66–71. https://doi.org/10.1007/s00256-002-0599-4
Chung OM, Yip SF, Ngan KC, Ng WF (2003) Chondroblastoma of the lumbar spine with cauda equina syndrome. Spinal cord 41(6):359–364. https://doi.org/10.1038/sj.sc.3101458
김긍년, 윤도흠 (2005) A case of thoracic vertebral chondroblastoma, treated with 3-D image-guided resection and reconstruction. Journal of Korean Neurosurgical Society (대한신경외과학회지) 37(2):154–156.
Osman W, Zaoui A, Abdelkrim SB, Hamida RB, Ayèche MB (2014) Chondroblastoma of the lower thoracic spine. A Case Report and Review of the Literature. 2(4):78–81
Sohn SH, Koh SA, Kim DG, Park SW, Lee KH, Kim MK, Choi JH, Hyun MS (2009) A case of spine origin chondroblastoma metastasis to lung. Cancer Res Treat 41(4):241–244. https://doi.org/10.4143/crt.2009.41.4.241
Mu H, Jiang Y, Xue L, Hua Y, Lin J, Cai Z (2021) H3.3 K36M mutation as a clinical diagnosis method of suspected chondroblastoma cases. Orthopaedic Surgery 13(2):616–622. https://doi.org/10.1111/os.12878
Konishi E, Nakashima Y, Mano M, Tomita Y, Kubo T, Araki N, Morii E, Yoshikawa H, Haga H, Toguchida J, Ueda T, Osawa M, Hoshi M, Inoue T, Aono M, Yanagisawa A (2017) Chondroblastoma of extra-craniofacial bones: Clinicopathological analyses of 103 cases. Pathol Int 67(10):495–502. https://doi.org/10.1111/pin.12586
Zheng BW, Niu HQ, Wang XB, Li J (2021) Sacral chondroblastoma - a rare location, a rare pathology: A case report and review of literature. World J Clin Cases 9(20):5709–5716. https://doi.org/10.12998/wjcc.v9.i20.5709
Angelini A, Hassani M, Mavrogenis AF, Trovarelli G, Romagnoli C, Berizzi A, Ruggieri P (2017) Chondroblastoma in adult age. European Journal of Orthopaedic Surgery & Traumatology : Orthopedie Traumatologie 27(6):843–849. https://doi.org/10.1007/s00590-017-1996-7
Hoeffel JC, Brasse F, Schmitt M, Plenat F, Vignaud JM, Czorny A, Montaut J, Marchal AL (1987) About one case of vertebral chondroblastoma. Pediatr Radiol 17(5):392–396. https://doi.org/10.1007/BF02396616
Buraczewski J, Lysakowska J, Rudowski W (1957) Chondroblastoma (Codman's tumour) of the thoracic spine. J Bone Joint Surg Br 39-B(4):705–710. https://doi.org/10.1302/0301-620X.39B4.705
Sagoo NS, Southern EP, King AG, Stark MW, McBride LA (2020) Missed radiographic and clinical findings in a case of non-idiopathic scoliosis resulting from chondroblastoma. Spine Deform. https://doi.org/10.1007/s43390-020-00185-3
Shin BJ, Yun T-K, Lim K-C, Lee JC, Kim K-J, Kim D-W, Kim Y-I (2001) Chondroblastoma of the first lumbar vertebra - a case report. J Korean Soc Spine Surg 8. https://doi.org/10.4184/jkss.2001.8.1.90
Akai M, Tateishi A, Machinami R, Iwano K, Asao T (1986) Chondroblastoma of the sacrum. A case report. Acta Orthop Scand 57(4):378–381. https://doi.org/10.3109/17453678608994417
Giri P, Chavan V (2017) Chondroblastoma of thoracic vertebra in young adult causing paraparesis. Romanian Neurosurgery 31. https://doi.org/10.1515/romneu-2017-0054
Baumhoer D, Harder D, Ameline B, Dawson H, Kollar A (2020) Metastasizing chondroblastoma: a rare bone tumor no longer supported by the WHO classification. Skeletal Radiol. https://doi.org/10.1007/s00256-020-03525-6
Edel G, Ueda Y, Nakanishi J, Brinker KH, Roessner A, Blasius S, Vestring T, Müller-Miny H, Erlemann R, Wuisman P (1992) Chondroblastoma of bone. A clinical, radiological, light and immunohistochemical study. Virchows Archiv A, Pathological Anatomy and Histopathology 421(4):355–366. https://doi.org/10.1007/bf01660984
Kunze E, Graewe T, Peitsch E (1987) Histology and biology of metastatic chondroblastoma: Report of a case with a review of the literature. Pathol Res Pract 182(1):113–120. https://doi.org/10.1016/S0344-0338(87)80151-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tabibkhooei, A., Javadnia, P. Chondroblastoma of Thoracic Vertebrae: a Case Report and Review of the Literature. Indian J Surg Oncol 15 (Suppl 1), 22–28 (2024). https://doi.org/10.1007/s13193-022-01659-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-022-01659-8