Abstract
Locally advanced breast cancer (LABC) patients require an accurate staging of the disease to rule out distant metastases. Various imaging investigations are used to stage LABC patients. The present study is a prospective comparison of conventional imaging (CI) with fusion positron-emission tomography and computed tomography (PET-CT) scans in the staging of LABC patients. Seventy-three consecutive LABC patients presenting to the breast cancer clinic of the tertiary care cancer institute were included in the study. All patients underwent contrast-enhanced computed tomography, Tv99m bone scintigraphy, and fusion PET-CT. Histology of the metastatic site was confirmed wherever possible. The disparity between the two imaging findings was compared. Doubtful lesions were observed clinically for at least 2 years to confirm their nature. PET-CT detected a higher number of lymph nodes in the axilla, internal mammary, and supraclavicular region as compared to CI. PET-CT upstaged 36.98% and downstaged 5.4% of the patients respectively leading to a change in the management in 30.13% of the patients. Sensitivity, specificity, positive predictive value, and negative predictive value of CI and PET-CT were 71.87%, 87.80%, 82.14%, and 80%, and 90.90%, 90%, 88.23%, and 92.30% respectively. PET-CT was more accurate in staging the LABC patients as compared to CI. PET-CT is more accurate then contrast-enhanced CT and bone scintigraphy for staging locally advanced breast carcinoma patients. It can replace multiple organ–directed imaging in staging breast cancer. It can provide accurate staging of the disease so that patients can be prognosticated and can be directed to the most appropriate treatment plans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In India, locally advanced breast cancer (LABC) is prevalent, and it is among the leading causes of death in cancer patients. The incidence is higher as compared to the western world where early breast cancer is more common than advanced breast cancer. Under current practices, appropriate staging requires multiple organ–based investigations like contrast-enhanced computed tomography (CECT), roentgenograms, Tc99m bone scintigraphy, ultrasonogram (USG), and other investigations based upon the requirement of an individual patient. This may require multiple visits to the hospital. PET exploits the glycolytic activity of the malignant cells and can scan the whole body in a single setting. It can be fused with CECT (PET-CT) to increase its accuracy. Tumors with higher activity are more avid on PET scans and show higher standard uptake value (SUV). The present study is a prospective comparison of CECT and Tc 99 bone scintigraphy with the fusion PET-CT for staging patients with LABC.
Material and Methods
Sequential LABC patients presenting to a breast cancer clinic at a tertiary cancer care hospital from 2015 to 2016 were evaluated for inclusion in the study. Patients with uncontrolled diabetes mellitus, pregnancy, and a history of previous malignancy were excluded from the study. After institute ethical committee approval, 73 consenting patients were subjected to CI, i.e., CECT of chest, abdomen, and pelvis; Tc99m bone scintigraphy; and 18 Flouro-deoxyglucose PET-CT scan. Images were reviewed by two experienced physicians, and the presence of metastasis at various sites on imaging was decided based on classical findings. Rates of detection of metastasis between two modalities were compared. Patients showing a disparity in between the two modalities underwent biopsy and histopathological examination of the metastatic site wherever feasible. Doubtful lesions on PET-CT or CI were followed up for 2 years clinically to confirm their nature.
Results
Seventy-three LABC cases were included in the study. The mean tumor size was 6.71 cm (2–15 cm). Axillary lymph nodes were palpable in 86.3% of patients. Clinically ipsilateral N1, N2, and N3 nodes were present in 63.01%, 17.80%, and 1.36% of patients respectively. Histopathology was infiltrating ductal carcinoma in 95.8%, DCIS in 4.10% of patients.
Concordance between CI and PET-CT imaging in terms of liver, bone, and lung metastases was observed in 56 (76.71%) patients. Stage migration was found in 25(34.24%). PET-CT upstaged the disease in 30 (36.98%) patients as compared to CI. Stage III to stage IV migration occurred in 12 (16.43%) patients due to the identification of distant metastasis. PET-CT detected more lymph nodes at axillary, supraclavicular, and internal-mammary lymph node stations as compared to CI. Upstaging within stage III from IIIA to IIIB or IIIC occurred in 20 (27.39%) patients. PET-CT also detected additional sites of metastases in 4 (5.4%) of patients who were already diagnosed as metastatic by CI. PET-CT downstaged the disease in 4 (5.4%) patients. In these patients, metastasis detected by CI was not shown by PET-CT. Tables 1 and 2 show variations of lymph node detection rates and stage migration respectively.
PET-CT led to a change in the management plan in 22 (30.13%) patients. Histopathological confirmation could be done in 12 patients. In lesions where biopsy is available for conformation, PET-CT was false positive in 3 out of 12 (25%) patients and false negative in 2 out of 12 (16.6%) patients, whereas CI was false negative in 6 out of 12 (50%) patients (Table 3 shows biopsy results and Table 4 compares results of PET-CT and CI).
Discussion
The incidence of metastases increases with the size of the primary lesion and the nodal status of the disease. T3 lesions carry more than 15% chance of metastases [1]. Muller reported incidence of metastases in pN1, pN2, and pN3 disease as 3.8%, 21.7%, and 17.6% respectively [2]. Therefore metastatic workup for locally advanced breast cancer is required. The sensitivity and specificity of CI to detect metastases vary from 31 to 92% and 98 to 100% respectively, whereas for PET-CT, reported rates are 76 to 94% and 86 to 94% respectively [3]. The sensitivity and specificity of PET-CT in a meta-analysis by Isaci et al. are reported as 92.7% and 81.6% respectively [4]. The low specificity of PET is due to nonspecific avidity of 18 FDG during PET scans as inflammatory pathology also shows increased uptake of 18-FDG; however, associated findings may guide the examiner towards the probable etiology of the lesion increasing the specificity. Moreover, the fusion of CT with PET scan increases diagnostic accuracy. Tatsumi reported improved diagnostic ability of PET when combined with CT [5]. Table 5 shows these indices among various studies.
PET-CT is 61–100% sensitive and 75–97% specific for detecting axillary lymph node metastases and is not recommended currently to stage axilla in newly diagnosed breast cancer patients [6,7,8]. CT scans can identify axillary lymph nodes in case of significant enlargement but PET-CT can pick these nodes in a very early stage by their glow. Fink and colleagues have shown a low sensitivity of 20% for axillary lymph node detection by PET; however, they included patients with the early disease [9]. A similar low sensitivity was also shown by Wahl et al. and Barringer et al. with similar T stages of the primary [7, 10]. Danforth et al. studied patients from stages I to IV and showed the sensitivity to detect axillary nodal metastases increased from 43% for stages I and II to 83% in stages III and IV [11]. Kumar et al. have demonstrated that smaller tumors and lower tumor grade are associated with false-negative PET results [12]. In our study, CI detected ipsilateral axillary lymph node enlargement in 94.54%, whereas PET-CT showed avidity in 97.26% of patients. The mean tumor size in our study is 6.71 cm (2–15) and all patients are in stage III. This might be a cause of increased detection of axillary lymph nodes by PET-CT. The incidence of internal-mammary lymph nodal enlargement is reported from 11 to 16% in the literature [13, 14]. Large tumor size and inner quadrant locations are said to be the contributing factors in internal-mammary lymph node metastases. In our study, CI identified internal-mammary lymph nodes in 5.4% of patients and PET-CT detected these nodal enlargements in 27.39% patients.
Current guidelines recommend PET-CT as an option for staging locally advanced breast cancer. PET-CT is useful to detect occult metastases that are missed by CI. PET thereby accurately stages the patient and helps to choose the appropriate treatment line. The bone, liver, and lung are the common sites of systemic metastases. Ultrasound is the appropriate imaging to detect liver metastases; however, operator dependence and patient body habitus–related factors decrease its sensitivity. CT scans can also detect most liver metastases. In our study, PET-CT detected liver metastases that were missed in CI. The sensitivity PET-CT to detect liver metastases is reported from as low as few cases to 90%. The low sensitivity of PET might be due to background activity of 18 FDG or motion artifacts which might hamper the detection of avidity in the metastatic site. Pulmonary metastases are often missed in conventional chest X-rays. It has a sensitivity of 28% to detect metastases; moreover, early lesions are difficult to detect. CT scans can detect fairly smaller lesions, but they also have difficulty in differentiating certain benign lesions like granuloma or small pleural-based nodules on conventional cross sections. PET-CT produces promising results in the detection of early lesions. However, motion artifacts sometimes pose difficulty in detecting smaller lesions. It can also complement other investigations in differentiating small pulmonary metastases from other benign conditions.
Skeletal metastases are the most common metastatic lesions in breast cancer and can be osteolytic, osteoblastic, or mixed type. Traditionally, bone scintigraphy is performed using Technetium-99m diphosphonates as this tracer is concentrated in bones. In a study by Nakai et al. comparing visualization rates of bone metastases by bone scintigraphy and PET-CT, the rates of detection of bone scintigraphy for osteoblastic, osteolytic, mixed, and invisible lesions are 100%, 70%, 84.2%, and 25% respectively; however, for PET-CT, similar rates were 55.6%, 100%, 94.7%, and 87.5% respectively [15]. Bone scintigraphy detects osteoblastic metastases more accurately, whereas PET-CT provides favorable results in osteolytic and invisible lesions. Tc99m-labeled diphosphonates accumulate in bone in the mineralization phase, and their activity depends upon the new bone formation rate [16, 17]. It shows positive results in reactive bone-forming state, and this can be confounded by conditions like healing fracture [16]. 18-Flouro-deoxyglucose accumulations occur in metabolically active lytic lesions. The reason for increased detection of the lytic lesion by PET might be due to the hypoxic state in these lesions which causes increased uptake of 18-Flouro-deoxyglucose [18]. Potential benefits of PET-CT over bone scintigraphy are the detection of metabolically active bone marrow lesions and absent avidity in benign lesions which often shows diphosphonate uptake in bone scintigraphy. The relative decreased sensitivity of PET to detect the osteoblastic lesions can be overcome by the fusion of CT which can detect these lesions [17]. In the study by Piccardo et al., comparing 18-flouro-deoxyglucose PET scan with CT scan for bone metastases, PET showed a sensitivity and specificity of 91%, whereas CT had a sensitivity and specificity of 77% and 93%. The combined PET-CT sensitivity and specificity improved to 98% and 93% respectively [19].
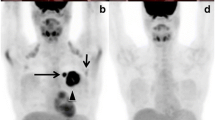
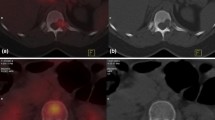
The liver is a common site of visceral metastases in breast cancer. In our study, overall PET-CT detected distant metastases in 35 patients as compared to 28 patients by conventional imaging. CT scan detected liver lesions in 8.2% of patients compared to 17.8% of patients with PET-CT. Figure 1 shows a metastatic liver lesion being picked up by PET-CT and missed by CECT. Liver biopsy was done in four patients with PET-detected lesions which were not shown as metastatic in CT scans. All these lesions were proved to be metastatic by histology. In a few liver metastatic lesions, there was a disparity between two imaging and biopsy was attempted but could not be done due to technical difficulty.
The glowing character of PET scans is somewhat less reliable in the case of lung lesions due to the constant motion of lung fields. In the case of lung lesions, PET scans fused with CT scans can identify metastatic sites. In our study, CT scans identified 10.95% of patients with lung metastases compared to 19.17% of patients on PET fused with CT scans. PET-CT is instrumental in localizing the occult metastatic lesions leading to increments in stage and change in therapeutic management. On the other hand, it can also rule out metastases sites reported by CI imaging and provide accurate staging. PET-CT aids in providing optimal therapy, appropriate prognostication of the patient, and true categorization of patients’ stage for comparing long-term outcomes.
In a study by Eubank et al. compared to CI, PET-CT upstaged and downstaged the disease in 43% and 24% of patients respectively, and helped in changing the management of 32% of patients [20]. Yap et al. showed a change in therapeutic management in approximately 60% of patients [21]. In another prospective study of 60 patients by Fuster et al., PET-CT changed the initial staging in 42% patients [22].
PET-CT scan compared to CI changed the management in 30% of patients in our study. In 5.4% of patients, the intent of treatment changed from palliative to curative. In 12 (16.43%) patients, PET-CT changed the stage from III to IV. These patients were planned for neoadjuvant chemotherapy based upon CI image findings, but as distant metastases were identified in these patients, the intent of treatment was changed. Although it is costly, PET-CT has the potential to be replaced as a single investigation in staging locally advanced breast cancer patients. It saves precious time spent by multiple hospital visits for conventional investigations.
The strength of the current study lies in the fact that a diagnosis of all doubtful lesions was made in favor of or against metastases by a reasonable follow-up and secondly in the homogenous population of LABC patients, whereas most studies in the past have a varied patient population. A major limitation of this study is that we were not able to provide histologic proof to all metastatic sites due to ethical and logistic reasons.
Conclusion
PET-CT is more accurate then contrast-enhanced CT and bone scintigraphy for staging locally advanced breast carcinoma patients. It can replace multiple organ–directed imaging in staging breast cancer. It can provide accurate staging of the disease so that patients can be prognosticated and can be directed to the most appropriate treatment plans.
References
Gerber B, Seitz E, Müller H, Krause A, Reimer T, Kundt G, Friese K (2003) Perioperative screening for metastatic disease is not indicated in patients with primary breast cancer and no clinical signs of tumor spread. Breast Cancer Res Treat 82:29–37
Müller D, Kohler G, Ohlinger R (2008) Staging procedures in primary breast cancer. Anticancer Res 28:2397–2400
Mahner S, Schirrmacher S, Brenner W, Jenicke L, Habermann CR, Avril N, Dose-Schwarz J (2008) Comparison between positron emission tomography using 2-[fluorine-18] fluoro-2-deoxy-D-glucose, conventional imaging, and computed tomography for staging of breast cancer. Ann Oncol 19(7):1249–1254
Isasi CR, Moadel RM, Blaufox MD (2005) A meta-analysis of FDG-PET for the evaluation of breast cancer recurrence and metastases. Breast Cancer Res Treat 90:105–112
Tatsumi M, Cohade C, Mourtzikos KA et al (2006) Initial experience with FDG-PET/CT in the evaluation of breast cancer. Eur J Nucl Med Mol Imaging 33(3):254–262
Rosen EL, Eubank WB, Mankoff DA (2007) FDG PET, PET/CT, and breast cancer imaging. Radiographics. 27(Suppl 1):S215–S229
Wahl RL, Siegel BA, Coleman RE, Gatsonis CG, PET Study Group (2004) Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the Staging Breast Cancer with PET Study Group. J Clin Oncol 22(2):277–285
Fehr MK, Hornung R, Varga Z et al (2004) Axillary staging using positron emission tomography in breast cancer patients qualifying for sentinel lymph node biopsy. Breast J 10(2):89–93
Fink D, Steinert HC et al (2004) Axillary staging using positron emission tomography in breast cancer patients qualifying for sentinel lymph node biopsy. Breast J 10:89–93
Barranger E, Grahek D, Antoine M et al (2003) Evaluation of fluorodeoxyglucose positron emission tomography in the detection of axillary lymph node metastases in patients with early-stage breast cancer. Ann Surg Oncol 10(6):622–627
Danforth DN, Aloj L, Carrasquillo JA et al (2002) The role of 18F-FDG-PET in the local/regional evaluation of women with breast cancer. Breast Cancer Res Treat 75(2):135–146
Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A (2006) Clinicopathologic factors associated with false-negative FDG-PET in primary breast cancer. Breast Cancer Res Treat 98(3):267–274
Zhang YJ, Oh JL, Whitman GJ, Iyengar P, Yu TK, Tereffe W, Woodward WA, Perkins G, Buchholz TA, Strom EA (2010) Clinically apparent internal mammary nodal metastasis in patients with advanced breast cancer: incidence and local control. Int J Radiat Oncol Biol Phys 77(4):1113–1119
Savaridas SL, Spratt JD, Cox J (2015) Incidence and potential significance of internal mammary lymphadenopathy on computed tomography in patients with a diagnosis of primary breast cancer. Breast Cancer (Auckl) 9:59–65
Nakai T, Okuyama C, Kubota T, Yamada K, Ushijima Y, Taniike K, Suzuki T, Nishimura T (2005) Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur J Nucl Med Mol Imaging 32(11):1253–1258
Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I (1998) Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol 16(10):3375–3379
O’Sullivan GJ, Carty FL, Cronin CG (2015) Imaging of bone metastasis: an update. World J Radiol 7(8):202–211
Clavo AC, Brown RS, Wahl RL (1995) Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med 36(9):1625–1632
Piccardo A, Altrinetti V, Bacigalupo L, Puntoni M, Biscaldi E, Gozza A, Cabria M, Iacozzi M, Pasa A, Morbelli S, Villavecchia G, DeCensi A (2012) Detection of metastatic bone lesions in metastatic breast cancer patient fused (18)F-Flouride- PET-MDCT has higher accuracy than MDCT. Preliminary experience. Eur J Radiol 81:2632–2638
Eubank WB, Mankoff D, Bhattacharya M, Gralow J, Linden H, Ellis G, Lindsley S, Austin-Seymour M, Livingston R (2004) Impact of FDG PET on defining the extent of disease and on the treatment of patients with recurrent or metastatic breast cancer. AJR Am J Roentgenol 183(2):479–486
Yap CS, Seltzer MA, Schippers C (2001) Impact of whole-body 18 FDG PET on staging and imaging patients with breast cancer: the referring physicians prospective. J Nucl Med 42:1334–1337
Fuster D, Duch J, Paredes P, Velasco M, Muñoz M, Santamaría G, Fontanillas M, Pons F (2008) Pre-operative staging in large primary breast cancer with 18 Fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedure. J Clin Oncol 26:4746–4751
Piperkova E, Raphael B, Altinyay ME, Castellon I, Libes R, Sandella N, Heiba S, Abdel-Dayem H (2007) Impact of PET/CT in comparison with same day contrast-enhanced CT in breast cancer management. Clin Nucl Med 32(6):429–434
Radan L, Ben-Haim S, Bar-Shalom R et al (2006) The role of FDG-PET/CT in suspected recurrence of breast cancer. Cancer 107:2545–2551
Kamel EM, Wyss MT, Fehr MK, von Schulthess GK, Goerres GW (2003) [18F]-Flourodeoxyglucose positron emission tomography in patients with suspected recurrence of breast cancer. J Cancer Res Clin Oncol 129(3):147–153
Moon DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK (1998) Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med 39(3):431–435
Kim TS, Moon WK, Lee DS, Chung JK, Lee MC, Youn YK, Oh SK, Choe KJ, Noh DY (2001) Fluorodeoxyglucose positron emission tomography for detection of recurrent or metastatic breast cancer. World J Surg 25(7):829–834
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhoriwal, S., Deo, S.V.S., Kumar, R. et al. A Prospective Study Comparing the Role of 18 FDG PET-CT with Contrast-Enhanced Computed Tomography and Tc99m Bone Scan for Staging Locally Advanced Breast Cancer. Indian J Surg Oncol 12, 266–271 (2021). https://doi.org/10.1007/s13193-021-01299-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-021-01299-4