Abstract
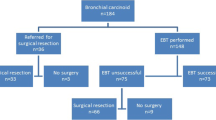
Bronchial carcinoids are slow-growing tumours of the neuroendocrine family. Most of them have a benign course with excellent outcome after complete resection. Due to their location in the primary bronchi, adequate resection with lung preservation requires considerable technical expertise. In this paper we present our surgical experience with endobronchial carcinoids and analyse the factors that predict possibility of lung preservation surgery. Retrospective analysis of a prospectively maintained database of patients operated for endobronchial carcinoids for the period March 2012 to September 2019 was carried out. Demographic factors and peri-operative variables were recorded and analysed. Factors that influence surgical outcome and possibility of lung preservation surgery were analysed. A total of 137 patients underwent surgery for resection of carcinoid tumours, out of which 100 had endobronchial carcinoids whereas 37 had peripheral carcinoids. The surgical procedure in 100 patients with endobronchial carcinoids included 14 left main bronchus sleeve resections, 13 pneumonectomies (7 right sided and 6 left sided), 10 right lower and middle bi-lobectomies, 10 lobectomies (4 left upper, 2 left lower and 4 right upper), and 53 sleeve lobectomies (18 left upper lobe sleeves, 8 left lower lobe sleeves, 20 right upper lobe sleeves, 5 right middle lobe sleeves and 2 right lower lobe sleeve lobectomies). There was no operative mortality. Median tumour size was 3.9 cm (range 5–130 mm). On univariate analysis, longer duration of symptoms was associated with poor surgical outcomes. On multivariate analysis, tumour in the main bronchus, duration of disease < 3 months (p = 0.006), left-sided disease (p = 0.03), and presence of healthy distal lung parenchyma (p < 0.001) were associated with successful lung preservation. Majority of endobronchial carcinoid tumours can be managed with lung-sparing procedures with minimal morbidity and mortality and excellent immediate and short-term outcomes. Early referral and experience of team performing these complex procedures are the key to success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinoid tumours are rare and slow-growing tumours found in the tracheo-bronchial tree and pulmonary parenchyma. WHO has classified them as “well differentiated neuroendocrine tumours” of lung comprising of low-grade (typical carcinoids) and intermediate-grade (atypical carcinoids) tumours [1]. We have used the term carcinoids in this manuscript for ease of understanding as this term is still prevalent in Indian subcontinent. Most of these patients present initially to the general practitioners and are often misdiagnosed as asthma and tuberculosis for many months or even years [1, 2].This is because breathlessness, cough, and hemoptysis, commonly seen in asthma or tuberculosis, are the cardinal symptoms in these young patients with carcinoid tumours also. Fortunately, due to slow growth and minimal risk of distant metastasis, despite initial misdiagnosis and delayed referral, most of these patients have a good prognosis [3]. Surgical resection with negative margins is the treatment of choice. Lung preservation surgery such as sleeve resection should be performed as far as possible [4]. We present herein our experience in the management of these tumours and an analysis of the factors affecting surgical outcome and the possibility of lung preservation surgery.
Material and Methods
This study is a retrospective analysis of prospectively maintained data of operated cases of carcinoid tumour at a tertiary care centre in New Delhi, India, from March 2012 to September 2019. A total of 137 patients underwent surgery for carcinoid tumours, out of which 100 had endobronchial carcinoids whereas 37 had peripheral carcinoids. Only patients having some endobronchial component were included in the study. Demographic data, clinical history, preoperative, intra-operative, and postoperative variables were recorded and analysed. Preoperative evaluation was done with contrast-enhanced computed tomography (CECT) of the chest to assess extent of tumour, extra bronchial component of tumour, and condition of distal lung parenchyma. Pre-operative bronchoscopy was done to know the exact location and extent of tumour. Pre-operative tissue biopsy was obtained in all patients.
Surgical Details
All the procedures were performed under general anaesthesia. Single lung ventilation was achieved with a double lumen tube placed under bronchoscopic guidance. Patients were placed in lateral decubitus position with involved side up. Posterolateral thoracotomy was the surgical approach in all patients. Intra-operative bronchoscopy was done in all the patients to reconfirm the site of tumour and also guide in the exact site for bronchotomy. After initial adhesiolysis, the condition of the underlying lung was assessed and decision was made about definitive procedure. If lung parenchyma was completely destroyed, pneumonectomy was performed. Patients with healthy lung parenchyma underwent a procedure depending on the exact location of their tumour. The surgical approach, followed uniformly in all patients, was to go in with a “determination” to do lung preservation, display the tumour site, resect minimum to get macroscopic clear margins, confirm the same by frozen section, and reconstruct, which involved complex manoeuvres at times. Thus, the various procedures performed were as follows: in patients with tumour in the left main bronchus and healthy distal lung parenchyma, left main bronchus sleeve resection was performed. The procedure involved looping of the left main bronchus, bronchotomy, resection of the involved part of main bronchus with macroscopically negative margins, frozen section confirmation of microscopically negative margins, and an end-to-end bronchial anastomosis (Fig. 1). If the tumour involved a lobar bronchus only with clear margin from secondary carina (left side) or main or intermedius bronchus (right side), standard lobectomy was performed (left upper, left lower, and right upper). If the tumour in a lobar bronchus involved secondary carina with or without extension into main bronchus, a sleeve lobectomy was performed. On the left side, this involved resection of the involved lobe with a cuff of secondary carina and main bronchus (with macroscopically clear and frozen negative margins) and re-implantation of the lobar bronchus of the remaining lobe onto the proximal main bronchus (left upper and left lower sleeve lobectomies; Figs. 2 and 3). On the right side, this involved resection of right upper lobe with sleeve of right main bronchus and an end-to-end anastomosis between proximal right main bronchus and distal bronchus intermedius (right upper sleeve lobectomy; Fig. 4), or resection of right middle lobe with sleeve of bronchus intermedius with anastomosis between lower lobar bronchus and bronchus intermedius (right middle sleeve lobectomy; Fig. 5) or resection of right lower lobar bronchus with bronchus intermedius and anastomosis between middle lobe bronchus and proximal bronchus intermedius (right lower sleeve lobectomy; Fig. 6).
Basic principles of minimal circumferential dissection, no cautery near the bronchus, delicate handling with minimal trauma to the cut edges, and a tension-free anastomosis (with 3 0 PDS suture), maintaining correct orientation of the bronchial ends, were followed in all the anastomosis. Standard mediastinal lymphadenectomy was done in all the cases. In case of pneumonectomy, bronchial stump was always reinforced with pericardial fat/intercostal muscle flap. All specimens were sent for histopathological examination. On table, air leak test was done in all cases. Prior to extubating, on table bronchoscopy was done in all patients who underwent sleeve resection to check luminal patency. In case of pneumonectomy, single chest tube was placed and connected to underwater seal bottle. In all other cases, two chest tubes were placed and connected to Thopaz ™ digital negative suction device. All patients were extubated on the table. Patients were monitored overnight in recovery room and shifted to the ward the next morning. Chest physiotherapy was done under physiotherapist’s supervision to maintain good lung expansion. Chest drains were removed when there was no air leak and the drainage was non-purulent /non-haemorrhagic and less than 2 ml/kg in 24 h. Patients were discharged from hospital after drain removal. Hospital stay, duration of chest tube, duration of postoperative air leak, wound infection, and other complications during hospital stay were analysed. Occurrence of any postoperative complication and/or hospital stay > 7 days was considered poor surgical outcome. All patients were followed up in outpatient clinic after discharge with chest X-ray to check for status of lung expansion and any other complication. Follow-up of our patients was done every 6th month for the first 2 years and then yearly for up to 5 years. All patients had a check bronchoscopy at 6 months and annually for 2 years thereafter. In case of any suspicious lesion at anastomotic site at bronchoscopy, a biopsy was performed to rule out tumour recurrence.
Statistical Methods
Statistical testing was conducted with the statistical package for the social science system version SPSS 17.0. Continuous variables were presented as mean ± SD or median (IQR). Categorical variables were expressed as frequencies and percentages. The comparison of normally distributed continuous variables between the groups was performed using Student’s t test. Nominal categorical data between the groups were compared using Chi-squared test or Fisher’s exact test as appropriate. Non-normal distribution continuous variables were compared using Mann-Whitney U test. Multivariate analysis was done using linear regression model. For all statistical tests, a p value less than 0.05 was taken to indicate a significant difference.
Results
One hundred patients were included in the study. Demographic variables are given in Table 1. Tumour was originating in main bronchus or secondary carina in 27 patients, and they underwent left main bronchus sleeve resections (14 patients—tumour in left main bronchus with healthy lung parenchyma) or pneumonectomy (13 patients, 7 right and 6 left—all with destroyed lung parenchyma). Ten patients had tumour in bronchus intermedius and underwent right lower and middle bi-lobectomy. Sixty-three patients had tumour in the lobar bronchus, with or without secondary carina or main bronchus involvement, and they underwent lobectomy (4 left upper, 2 left lower, and 4 right upper, i.e. total 10 lobectomy) or sleeve lobectomy (53). These consisted of 18 left upper sleeves, 8 left lower sleeves, 20 right upper lobe sleeves, 5 right middle lobe sleeves, and 2 right lower sleeve lobectomy. Median tumour size was 3.9 cm (range 0.5–13 cm).
Postpathology TNM staging of these tumours revealed 87 patients in stage 1, 9 in stage 2, 4 in stage 3, and none in stage 4. In 84 patients, the histopathology was low grade (typical carcinoid), whereas 16 patients had intermediate grade (atypical carcinoid). Median number of lymph nodes harvested was 16 (range 4–29). Nine (10.71%) out of 84 patients with low grade (typical carcinoids) had positive lymph nodes, and 4 (25%) out of 16 patients with intermediate grade (atypical carcinoids) had positive lymph nodes, taking total number of patients with positive lymph nodes to 13 (13%). All our patients had an R0 resection, confirmed on table by frozen sections of the margins and postoperative histopathology status.
There was no operative mortality. The median hospital stay was 4.5 days (range 3–15 days), and median chest tube removal was at 3.5 days (range 2–11 days). Six patients had prolonged air leak (> 7 days), 2 patients developed bronchopleural fistula, and 2 patients developed wound infection. Median follow-up in our study was 24 months (range 6–78 months). During follow-up, no patient developed recurrence.
On univariate analysis, duration of symptoms > 3 months was associated with poor surgical outcomes (p < 0.001) (Table 2). On multivariate analysis, patients with tumour in the main bronchus, duration of disease < 3 months (p = 0.006), left-sided disease (p = 0.03), and presence of healthy distal lung parenchyma (p < 0.001) were associated with successful lung preservation (Table 3).
Discussion
Carcinoids are slow-growing neoplasms presently classified by WHO under the category of neuroendocrine tumours (NET) [1]. Differentiation between low grade (typical) and intermediate grade (atypical carcinoids) is by the number of mitoses per 10 high power fields (hpf) and the presence or absence of necrosis. An arbitrary value of 2 or less mitosis per 10 hpf along with no necrosis has been chosen as the cut-off for diagnosing typical carcinoid, and it correlates well with prognosis [5]. The Ki-67 proliferation index of low grade (typical carcinoids) is less than 2%, while that of intermediate grade (atypical carcinoids) may go up to 20%. There is a lot distribution overlap of Ki-67 expression between these two subsets, and it has not been reported to help differentiate effectively between these two subtypes of well-differentiated pulmonary carcinoids. Walts et al. highlighted the Ki-67 index as being significantly different in typical and atypical carcinoids, but with considerable overlap. They concluded that the addition of the Ki-67 index to histological diagnosis does not provide significant prognostic value [6]. Our histopathology department started reporting this index in lung carcinoids only from 2016; hence, we have not included it in our analysis.
The classical triad of presentation of bronchopulmonary carcinoids is cough, haemoptysis, and pneumonia [1, 7]. Similar pattern was observed in our cases also.
A CECT chest was done in all the patients, and it is an invaluable tool for diagnosis as well as surgical planning. To achieve diagnosis, bronchoscopy is mandatory; however, controversy still exists regarding the risks of a biopsy. It seems to be more of a myth that biopsy of a carcinoid can lead to uncontrollable bleeding. Recent studies by Cardillo et al. [8] have shown that biopsy can be safely performed with minimal morbidity. A pre-operative tissue diagnosis is important as it helps differentiate between a typical and an atypical carcinoid. Atypical carcinoids need workup for metastatic disease and warrant more aggressive surgical resection. All 100 patients in our series had a histological diagnosis before surgery.
Surgery remains the mainstay of therapy to treat carcinoid tumours [1]. Prognosis and long-term functional outcomes depend on the ability to achieve a microscopic negative margin [9], and at the same time preserving lung parenchyma, as much as possible, in every single case. Each lobe is precious and all efforts should be made to save every lobe, even if it means doing complex resection and reconstruction. Pneumonectomy in these young patients has long-term consequences and should be avoided as far as possible [10, 11]. However, when the entire lung parenchyma is already destroyed, pneumonectomy is unavoidable. In our series, this was the main reason for pneumonectomy. Patients with tumour in main bronchus with complete obstruction had collapse of the distal lung parenchyma. Delay in referral leads to destruction of the collapsed parenchyma and they required pneumonectomy. As we analysed our data, we found this group of patients to have worse outcome (as defined above). All these patients could have had lung preservation had they come to us in time, before lung parenchyma was destroyed. In all other cases, lung-preserving procedures were performed. A key lesson we learnt early in our experience was to not get influenced by the size of endobronchial tumour as seen in CT scan and at bronchoscopy. The extent of resection is determined not by the size of luminal component of the tumour but by the size of the stalk of origin from bronchial wall. Many times, tumours which look very imposing at bronchoscopy due to huge luminal component had very small stalk of origin and, hence, required limited bronchial wall resection.
Especially noteworthy are 14 patients who underwent left main bronchus sleeve resection and 46 patients who underwent left upper/left lower or right upper sleeve lobectomy, many of whom had visited several centres and had been offered pneumonectomy by some in view of the tumour location. With effort towards lung preservation as a rule rather than exception, we could save significant lung parenchyma which often required complex lung-preserving resection and reconstruction (Figs. 1, 2, 3, and 4). Similarly, 7 patients who underwent right middle and right lower sleeve lobectomy would have undergone right middle and lower bilobectomy, but had one precious lobe saved due to complex resection and reconstruction (Figs. 5 and 6). The role of experience of the surgical team together with strict adherence to basic tissue handling and oncological principles cannot be overemphasised in achieving lung preservation with good outcomes. We ensured macroscopic clear margins on either side of resected specimen and confirmed it by frozen section of the margins in all cases. Such a standardised approach, when ruthlessly applied in each and every case, can give high rate of lung preservation, R0 resection, and excellent operative and short-term outcomes.
There are several reports of complex sleeve resections being performed by thoracoscopy/VATS. Most reports suggest feasibility and good short-term outcomes, but long-term outcomes need to be verified [12,13,14,15,16,17,18]. Due to technical challenges in performing minimally invasive sleeve resection, the learning curve is very steep [19]. One must also realise that these reports are mainly coming from selected very high-volume centres and the results may not be replicated uniformly. Robotic-assisted sleeve resection seems to offer technical advantage over conventional VATS [20,21,22,23]; however, this remains controversial. In our study all the sleeve resections were performed by open approach. Since majority of our patients were young, the anticipated difference in morbidity between minimally invasive and open technique was minimal [24] and hence we decided for open approach in all patients. We strongly feel that such complex resections are a technical challenge even in open approach and that minimally invasive approaches (video-assisted thoracic surgery with/without robotic assistance) are even more demanding. Moreover, with tuberculosis and other inflammatory pathologies being endemic in our country, sticky calcified lymph nodes around the vessels and the bronchi would make matters even worse and a mishap may lead to inadvertently more lung resection than planned. In our opinion, in a country like ours and particularly for carcinoid tumours wherein most patients are young, the difference in morbidity is really minimal and a clean and relatively easier procedure which ensures lung preservation should be chosen, which in our series was the conventional posterolateral thoracotomy.
On univariate analysis, duration of symptoms > 3 months was associated with poor surgical outcomes. On multivariate analysis of cases with main bronchial tumour, presence of left-sided disease, duration of symptoms < 3 months, and healthy distal lung parenchyma were associated with higher chance of lung preservation. Higher possibility of lung preservation on the left side is attributable to greater length of left main bronchus, and hence, achieving negative margins becomes easier. Age of the patient and presence of extra-bronchial component of the tumour did not affect the lung preservation.
Lymph node dissection remains a controversial topic. Atypical carcinoids are known to have lymph node metastasis, and a systematic lymphadenectomy seems mandatory [3, 25]. Cardillo et al. [8] have shown that long-term survival is more dependent upon lymph node status rather than on histology (typical/atypical) of the primary tumour. This means that it would be prudent to do a systematic lymphadenectomy in all carcinoid tumours or at least lymph node sampling, as there exists a possibility of the specimen being reported postoperatively as atypical. We routinely perform a systematic mediastinal lymphadenectomy in all carcinoid tumours. In our series, the lymph node positivity rate was 13%. This reinforces our belief and practice that systematic mediastinal lymphadenectomy should be an integral part of all operations for bronchial carcinoids [8, 26].
Upon analysing the immediate postoperative morbidity, it was found that the most common complication was a prolonged air leak (> 7 days), mostly due to surface leaks from adhesiolysis sites which happened in 6 cases and resolved spontaneously. Broncho-pleural fistulae developed in 2 patients, both after right pneumonectomy for a bronchial tumour with destroyed and infected lung. Both patients were managed with window thoracostomy and they improved dramatically postprocedure. Two patients developed wound infection, which was managed by vacuum-assisted closure and secondary suturing. All other patients had uneventful recovery.
Conclusions
Majority of endobronchial carcinoid tumours can be managed with lung-sparing surgery with minimal morbidity and mortality and excellent functional outcome. Early referral and the experience of the surgical team in complex resection and reconstruction are the key to achieve lung preservation.
References
Gustafsson BI, Kidd M, Chan A, Malfertheiner MV, Modlin IM (2008) Bronchopulmonary neuroendocrine tumours. Cancer [Internet] 113(1):5–21 Available from: http://www.ncbi.nlm.nih.gov/pubmed/18473355. Accessed 5 May 2020
Jakhetiya A, Garg PK, Pandey R, Ramanathan P, Kumar S, Nath D et al (2017) Surgical management of bronchopulmonary carcinoids: a single center experience. South Asian J Cancer [Internet] 6(1):6–10 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28413786. Accessed 5 May 2020
Ferolla P, Daddi N, Urbani M, Semeraro A, Ribacchi R, Giovenali P et al (2009) Tumourlets, multicentric carcinoids, lymph-nodal metastases, and long-term behavior in bronchial carcinoids. J Thorac Oncol [Internet] 4(3):383–387 Available from: http://www.ncbi.nlm.nih.gov/pubmed/19247084. Accessed 5 May 2020
Ducrocq X, Thomas P, Massard G, Barsotti P, Giudicelli R, Fuentes P et al (1998) Operative risk and prognostic factors of typical bronchial carcinoid tumours. Ann Thorac Surg 65(5):1410–1414
Travis WD, Giroux DJ, Chansky K, Crowley J, Asamura H, Brambilla E et al (2008) The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumours in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol [Internet] 3(11):1213–1223 Available from: http://www.ncbi.nlm.nih.gov/pubmed/18978555. Accessed 5 May 2020
Walts AE, Ines D, Marchevsky AM (2012) Limited role of Ki-67 proliferative index in predicting overall short-term survival in patients with typical and atypical pulmonary carcinoid tumors. Mod Pathol 25(9):1258–1264
Alp M, Ucanok K, Dogan R, Kaya S, Cetin G, Unlu M, et al (1987) Surgical treatment of bronchial adenomas: results of 29 cases and review of the literature. Vol. 35, Thoracic and Cardiovascular Surgeon. © Georg Thieme Verlag Stuttgart New York. p. 290–4
Cardillo G, Sera F, Di Martino M, Graziano P, Giunti R, Carbone L et al (2004) Bronchial carcinoid tumours: nodal status and long-term survival after resection. Ann Thorac Surg 77(5):1781–1785
Daddi N, Ferolla P, Urbani M, Semeraro A, Avenia N, Ribacchi R et al (2004) Surgical treatment of neuroendocrine tumours of the lung. Eur J Cardiothorac Surg [Internet] 26(4):813–817 Available from: http://www.ncbi.nlm.nih.gov/pubmed/15450578. Accessed 5 May 2020
Filosso PL, Rena O, Donati G, Casadio C, Ruffini E, Papalia E, Oliaro A, Maggi G (2002) Bronchial carcinoid tumours: surgical management and long-term outcome. J Thorac Cardiovasc Surg 123(2):303–309
Brandt B, Heintz SE, Rose EF, Ehrenhaft JL (1984) Bronchial carcinoid tumours. Ann Thorac Surg 38(1):63–65
Santambrogio L, Cioffi U, De Simone M, Rosso L, Ferrero S, Giunta A (2002) Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest. 121(2):635–636
Mahtabifard A, Fuller CB, McKenna RJ Jr (2008) Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 85(2):S729–S732
He J, Shao W, Cao C, Yan TD, Wang D, Xiong X, Yin W, Xu X, Huang J (2011) Long-term outcome of hybrid surgical approach of video-assisted minithoracotomy sleeve lobectomy for non-small-cell lung cancer. Surg Endosc 25(8):2509–2515
Li Y, Wang J (2013) Video-assisted thoracoscopic surgery sleeve lobectomy with bronchoplasty: an improved operative technique. Eur J Cardiothorac Surg 44(6):1108–1112
Agasthian T (2013) Initial experience with video-assisted thoracoscopic bronchoplasty. Eur J Cardiothorac Surg 44(4):616–623
Chen H, Huang L, Xu G, Zheng B, Zheng W, Zhu Y, Guo Z, Chen C (2016) Modified bronchial anastomosis in video-assisted thoracoscopic sleeve lobectomy: a report of 32 cases. J Thorac Dis 8(8):2233–2240
Soultanis KM, Chen Chao M, Chen J, Wu L, Yang C, Gonzalez-Rivas D, Abu Akar F, Jiang G, Jiang L (2019) Technique and outcomes of 79 consecutive uniportal video-assisted sleeve lobectomies. Eur J Cardiothorac Surg 56(5):876–882
Gonzalez D, de la Torre M, Paradela M, Fernandez R, Delgado M, Garcia J, Fieira E, Mendez L (2011) Video-assisted thoracic surgery lobectomy: 3-year initial experience with 200 cases. Eur J Cardiothorac Surg 40(1):e21–e28
Schmid T, Augustin F, Kainz G, Pratschke J, Bodner J (2011) Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 91(6):1961–1965
Nakamura H, Taniguchi Y, Miwa K, Fujioka S, Matsuoka Y, Kubouchi Y (2013) A successful case of robotic bronchoplastic lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 19(6):478–480
Pan X, Chen Y, Shi J, Zhao H, Chen H (2015) Robotic assisted extended sleeve lobectomy after neoadjuvant chemotherapy. Ann Thorac Surg 100(6):e129–e131
Cerfolio RJ (2016) Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 8(Suppl 2):S223–S226
Flores RM (2020) Commentary: minimally invasive thoracic surgery lobectomy: truth versus hype. J Thorac Cardiovasc Surg 159:295–296
Yaldiz D, Kaya ŞÖ, Ceylan KC (2018) Nodal involvement adversely affects prognosis in pulmonary carcinoid tumours. Turkish J Thorac Cardiovasc Surg [Internet]. [cited 2020 Apr 23];26(3):458–463. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32082778. Accessed 5 May 2020
Pulmonary neuroendocrine (carcinoid) tumours: European Neuroendocrine Tumour Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids - Ann Oncol [Internet]. [cited 2020 Apr 1]. Available from: https://www.annalsofoncology.org/article/S0923-7534(19)31831-9/fulltext. Accessed 5 May 2020
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bishnoi, S., Asaf, B.B., Puri, H.V. et al. Endobronchial Carcinoids: Surgical Outcome in 100 Consecutive Patients and Factors Affecting Lung Preservation. Indian J Surg Oncol 12, 190–198 (2021). https://doi.org/10.1007/s13193-020-01248-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-020-01248-7