Abstract
Uterine sarcomas are uncommon and aggressive tumors comprising 3–7% of all uterine malignancies. The aim is to evaluate clinical presentation, histopathologic pattern, recurrence pattern, and outcome of patients with uterine sarcomas presenting to a tertiary care cancer center over an 8-year period. A total of 11 cases of uterine sarcoma were diagnosed. The median age of patients at presentation was 51 years (range 30–67 years). Six patients had leiomyosarcoma (54.5%), 4 had endometrial stromal sarcoma (36%), and 1 had adenosarcoma (9%). The main presenting symptoms were abnormal vaginal bleeding, low abdominal pain, and white discharge. Median follow-up was 11 months ranging from 3 to 200 months. Median survivals for leiomyosarcoma, endometrial stromal sarcoma, and adenosarcoma were 6.5, 18, and 56 months. The 3- and 5-year survival by Kaplan–Meier survival analysis of the entire cohort was 30 and 20%. The mitotic index, age, adjuvant therapy (chemotherapy, radiotherapy), and performance of pelvic nodal dissection did not impact survival significantly in the patient with leiomyosarcoma. Stage and histology had the strongest bearing on survival and leiomyosarcoma has the worst survival, whereas adenosarcoma had the best prognosis. Adequately powered prospective studies are required to define the role of radiation therapy and chemotherapy in this rare disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Uterine sarcomas are aggressive and rare tumors accounting for around 1% of female genital tract malignancies and 3 to 7% of uterine cancers [1,2,3]. Their rarity and histopathological diversity have to lead to the lack of consensus on optimal treatment. Histologically, uterine sarcomas are classified as carcinosarcomas, accounting for around 40% of cases, leiomyosarcomas (40%), endometrial stromal sarcomas (10 to 15%), and undifferentiated sarcomas (5 to 10%) [4].
Though carcinosarcomas initially labeled as sarcomas are now classified as the dedifferentiated or metaplastic form of endometrial carcinoma, they are classified as high-grade endometrial cancer [5]. Hence, 20 cases of uterine carcinosarcoma which were treated in the same period have been excluded from the present analysis.
Objectives
The objective of this study is to evaluate clinical presentation, histopathologic pattern, recurrence pattern, and outcome of uterine sarcomas presenting to a tertiary care cancer center over an 8-year period.
Material and Methods
The case records of all histologically proven uterine sarcomas treated at the Basavatarakam Indo American Cancer Hospital, Hyderabad, India, a tertiary cancer care center from June 2008 till January 2015 in the Department of Surgical Oncology were retrieved from a prospectively maintained database. Age, clinical symptoms, signs at presentation and histopathologic diagnosis, recurrence pattern, treatment details, outcomes, and follow-up were analyzed. All the patients underwent routine hematological, biochemical, and imaging tests. Computed tomography and magnetic resonance imaging of the abdomen and pelvis were done. Any additional imaging done was based on the patient’s symptomatology. Stage of disease was determined using the FIGO 2009 classification: stage I—tumor confined to the uterine corpus; stage II—tumor extending to the pelvis; stage III—tumor invades abdominal tissues; and stage IVA—tumor invades bladder and/or rectum and stage IVB distant metastasis [6]. Patients were reviewed every 3 months for the initial 2 years with history, physical examination, and relevant imaging and then 6 months for the next 3 years. All the patients were followed until June 2017. The patients who missed a scheduled follow-up were contacted on the telephone. Numerical data were denoted as mean, median, and frequency appropriately. Mann–Whitney test was utilized for analyzing numerical data and Kruskal–Wallis test for > 2 independent samples. A p value of ≤ 0.05 was considered to be significant. Statistical analysis was done on GraphPad Prism software version 5 for windows and survival analysis by Kaplan–Meier survival estimates using SPSS 20 software.
Results
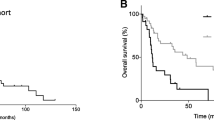
A total of 11 cases of uterine sarcoma were diagnosed. The median age of patients at presentation was 51 years (range 30–67 years). Six had leiomyosarcoma (54.5%), 4 had endometrial stromal sarcoma (36%), and 1 had adenosarcoma (9%). The main presenting symptoms were abnormal vaginal bleeding, low abdominal pain, and white discharge (Table 1). All patients underwent surgery following evaluation. Median follow-up was 11 months ranging from 3 to 200 months. Median follow-up of patients with leiomyosarcoma was 6 months (range 3–65 months), of endometrial stromal sarcoma 17 months (range 4–200 months), and adenosarcoma 55 months. Median survivals of patients with leiomyosarcoma, endometrial stromal sarcoma, and adenosarcoma were 6.5, 18, and 56 months and of the entire cohort was 11 months. The 3- and 5-year survival by Kaplan–Meier survival analysis of the entire cohort was 30 and 20% (Fig. 1).
All patients underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy. Pelvic nodal dissection was done in 5 patients, posterior exenteration in 1, and total pelvic exenteration in one (Table 2). All the patients who were at high risk of recurrence of recurrence were advised adjuvant treatment but many patients defaulted. Two patients received adjuvant radiotherapy and one adjuvant chemoradiation. Adjuvant radiation therapy given was 50 G in 25 fractions along with intravaginal brachytherapy (15 Gy in 3 fractions) application. One patient received adjuvant chemotherapy with ifosfamide and doxorubicin. Tumor extending to pelvis (extrauterine disease), tumor invades adjacent organs, nodal disease, and recurrent disease were the factors considered for advising adjuvant therapy.
Leiomyosarcoma
A total of 6 patients were managed, 2 stage 1A and 4 IB. All patients died of disease. The median survival and disease-free survival of patients with IA stage were 34 and 26.5 months. The median survival of patients with IB stage was 6.5 months with disease-free survival being 2.5 months. The pattern of recurrences, adjuvant treatment, and treatment for recurrence are given in Table 3. Two cases of stage IA leiomyosarcomas were managed. The first case underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH BSO) elsewhere; pre-surgery biopsy was a smooth muscle tumor of uncertain malignant potential. Final histopathology was reviewed here, proven to be high-grade tumor leiomyosarcoma of the uterus. She received adjuvant radiotherapy at our institute. She had lung and retroperitoneal disease at 52 months after surgery and died of disseminated disease at 65 months. The second case had a preoperative endometrial biopsy suggestive of adenocarcinoma, underwent TAH and BSO with bilateral pelvic nodal clearance; final paraffin histopathology was leiomyosarcoma of the uterus. She was planned for adjuvant chemotherapy but developed brain metastasis within a month and died 3-month post-surgery.
Four cases of stage IB leiomyosarcomas of the uterus were managed. In the first case, the preoperative endometrial biopsy was uterine leiomyosarcoma. She underwent TAH and BSO with bilateral pelvic nodal clearance and received adjuvant radiotherapy. She had vault recurrence in 2 months after surgery during the course of radiotherapy. She completed the radiotherapy course and died after 5 months post-surgery. The second case presented with large pelvic mass thought as of adnexal origin; hence, no preoperative biopsy was done. She underwent TAH + BSO; intraoperatively, there was a large mass in relation to the uterus and adnexa with uterus and adnexa were not separately identifiable. Frozen section analysis confirmed the mass to be leiomyosarcoma of the uterus. Operating surgeon did not do nodal dissection based on his preference. The final histopathology corroborated frozen findings. She developed liver metastasis 2 months post-surgery and was advised adjuvant chemotherapy but refused and died from the disseminated disease at 8 months.
The third case had a pre-surgery biopsy of smooth muscle tumor of uncertain malignant potential; underwent TAH and BSO; received 4 cycles of adjuvant ifosfamide and adriamycin; had local regional pelvis, abdominal, and lungs; and died of disease at 3 months from surgery. The fourth case had a preoperative biopsy and intraoperative frozen section both reported as spindle cell neoplasm of the uterus. Therefore, only hysterectomy and bilateral salpingo-oophorectomy were done. The final histology report was high-grade leiomyosarcoma. She developed massive pleural effusion and ascites in 13 months after surgery and died a month later.
Endometrial Stromal Sarcoma
Median overall survival in this group was 18 months. A total of 4 patients were managed, 2 in IA, 1 in IVA, and another in IVB. The pattern of recurrences, adjuvant treatment, and treatment for recurrence are given in Table 4. The first case (stage 1A) underwent laparotomy with extra fascial hysterectomy with bilateral salpingo-oophorectomy with bilateral pelvic lymph node clearance. She developed lung metastasis at 10 months. The pros and cons of chemotherapy and hormonal therapy were explained but the patient and family did not prefer any therapy. The patient died after 18 months post-surgery. In the second case (stage 1A), endometrial biopsy along with immunohistochemical studies was suggestive of endometrial stromal sarcoma. Her whole-body PET-CT scan revealed disease localized to the uterus. She underwent extra fascial hysterectomy with bilateral salpingo-oophorectomy with bilateral pelvic lymph node clearance. She was lost to follow up. The third case received radiotherapy for uterine ESS elsewhere. Twelve years later, she presented with central pelvic recurrence invading the rectum and bladder with hydroureteronephrosis. She underwent total pelvic exenteration and died 56 months after surgery due to medical issues. She did not receive any hormonal therapy or adjuvant therapy due to unproven benefit.
The fourth case presented with pain abdomen requiring frequent use of analgesics. She was a diagnosed case of endometrial stromal sarcoma of the uterus. Whole-body PET-CT scan revealed a large pelvic mass in relation to uterus invading the rectosigmoid and displacing the bladder anteriorly. Surgery was planned with a curative intent. Laparotomy was done, and the mass was found arising from the uterus and infiltrating rectosigmoid junction and right distal ureter. Also, there were few deposits in the sigmoid mesentery. As there was no plane of cleavage between the uterus and rectosigmoid junction, a palliative supralevator posterior exenteration (hysterectomy with salpingo-oophorectomy with low anterior resection and defunctioning ileostomy) with right ureteric re-implantation was done. She underwent ileostomy closure 6 weeks later and died 3 months post-surgery.
One case of adenosarcoma was managed. She underwent the subtotal hysterectomy at a periphery hospital and referred here. Final histopathology of the specimen revealed Mullerian adenosarcoma. Exploratory laparotomy and completion of hysterectomy with bilateral salpingo-oophorectomy with bilateral pelvic nodal clearance were done. She had an intraabdominal recurrence in left iliac fossa at 39 months and operated for the same and died at 56 months post primary surgery. Details of DFS and overall survival of patients with uterine sarcomas are given in Table 5.
The patients of leiomyosarcoma with high mitotic index (> 10/HPF) had a median survival of 8 months and those with low mitotic rate (< 5/HPF) 3 months, although not significant (p 0.19). Leiomyosarcoma patients with tumor less than 5 cm (stage 1A) (34 months) had a better survival than those with more than 5 cm (stage 1B) (6.5 months) though it was not statistically significant (p 0.72), similarly patients less than 50 years had survival of 5.5 months, those more than 50 years had 9.5 months although not significant (p 0.88). Survivals of patients with leiomyosarcoma who received chemotherapy and radiotherapy and who did not have any adjuvant therapy did not differ significantly. Also, the performance of pelvic nodal dissection did not confer any survival advantage in the patient with leiomyosarcoma (Table 6). Similar stratification could not be done in patients with ESS and adenosarcoma because of fewer numbers and many of the above parameters were similarly distributed.
Discussion
Uterine sarcomas represent 4–9% of all invasive uterine cancers [1,2,3]. They are aggressive tumors with poor prognosis. A series of 42 patients from North India reported a median overall survival (OS) of only 6.6, 6.8, and 9.4 months, respectively, in patients with carcinosarcoma, leiomyosarcoma, and undifferentiated endometrial sarcoma [7]. Another series of 50 patients with uterine sarcomas from India reported 3-year OS of 42% for the entire group of patients (carcinosarcoma, leiomyosarcoma, endometrial stromal sarcoma) [8]. Stage of the disease, histopathological type, and use of postoperative radiotherapy were found to be significant prognostic factors for survival in this study [8]. In a population-based study from Norway including 419 cases of uterine sarcomas, patients with leiomyosarcoma had a poor prognosis with a 5-year survival of 51% for stage I and 25% for stage II, whereas all patients with spread outside the pelvis had died within 5 years. Patients with endometrial stromal sarcoma had a 5- and 10-year survival of 84% and 77% for stage I, 62% and 49% at stage II, and 40% at stage III. Even for adenosarcoma at stage I, the 5- and 10-year survivals were 76% and 61%, respectively. For all tumor types, the stage of disease was found to be the most important prognostic factor. In leiomyosarcomas confined to the uterus, tumor size, and the mitotic index (MI) were significant prognostic factors, whereas the prognosis of endometrial stromal sarcomas confined to the uterus was related to mitotic index and tumor cell necrosis. In adenosarcomas, tumor cell necrosis was the only significant prognostic factor [2]. In the present series, all the 6 patients with leiomyosarcoma treated, died of disease, with median survival being 34 months in stage 1A, and dropping to 6.5 months in stage 1B although not statistically significant. The mitotic index, age, adjuvant therapy (chemotherapy, radiotherapy), and performance of pelvic nodal dissection did not impact survival significantly in the patient with leiomyosarcoma. All the patients of leiomyosarcoma had recurrences and died of disease.
Surgical Staging
Extrafascial hysterectomy/bilateral salpingo-oophorectomy remains the primary and standard treatment for uterine sarcomas stage I disease. The role of pelvic and as well para-aortic nodal dissection is not clear in uterine sarcomas. The incidences of ovarian and lymph node metastases (both pelvic and para-aortic) in uterine leiomyosarcoma (LMS) are very low and commonly found in patients with the extrauterine disease [9]. Ovarian metastases were seen in 3.9% and positive nodes in 8.1% in a study. None of the factors predicted for metastases to the ovary. Only gross extrauterine disease predicted lymph node metastasis. In addition, all of these cases had clinically suspicious (enlarged) lymph nodes. Lymph node dissection for uterine LMS should be reserved for patients with clinically suspicious nodes. Prophylactic nodal dissection (both pelvic and para-aortic) is not warranted [9]. Another study also corroborating the same findings found that the status of nodal involvement has minimally impacted the management of women with uterine leiomyosarcoma (LMS) and endometrial stromal sarcoma (ESS) [10]. In line with the above studies, the present study also demonstrated that the addition of the pelvic nodal dissection did not confer any survival advantage in patients with leiomyosarcoma.
In low-grade endometrial stromal sarcoma (ESS), the relatively high incidence of extrauterine spread and lymph node metastasis calls for surgical staging. Lymph node metastasis occurs in 10 to 40% of the cases and positive lymph nodes portend poorer survival. Late relapses can occur 10 to 20 years after diagnosis. A study evaluating 848 patients of endometrial stromal sarcoma found that among the low-grade endometrial stromal sarcoma patients, the incidences of extrauterine disease were 25%, and lymph node metastasis 7%. Both the univariable and multivariable analysis demonstrated lymph node metastasis and ovarian preservation were not significant prognostic factors for survival. Therefore, neither lymph node metastasis nor ovarian preservation seems to affect the excellent overall survival of these patients [11]. Another large study of 1010 woman with ESS from the SEER database found that adding lymphadenectomy or adjuvant RT to total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO) did not change survival [12].
In a population-based study of 848 patients with ESS, patients with low-grade ESS stage I–II versus III–IV disease had 5 years survival of 96% vs 81% with overall mean survival time was 188 months, and the 5-year survival rate was 92% for the entire cohort [11]. In contrast, high-grade endometrial stromal sarcoma mean survival time was 59 months, and the 5-year survival rate was 25% [11]. The primary treatment for early-stage endometrial stromal sarcoma is the hysterectomy. Lymph node dissection may offer prognostic information, but it has not been shown to improve survival [11]. In the present study, the median survival across all the stages in the ESS group was 18 months, 18 months in stage 1A falling to 4 months in stage IV B disease.
Survival in patients with high-grade endometrial stromal sarcoma tumors is related to the residual disease at the completion of initial surgery and requires an aggressive cytoreduction. The role of systemic lymphadenectomy and optimal adjuvant therapy remains to be defined [13].
A single institution retrospective analysis of 38 patients with endometrial stromal sarcoma from south India is recently published. Seventy-four percent were low-grade ESS and rest others were high-grade ESS. At a median follow up of 28 months, the 5-year disease-free survival (DFS) and overall survival for the entire cohort were 62.9 and 89.1%. Complete surgical staging with adjuvant therapy had a 5 years DFS of 90% irrespective of grade, whereas incomplete staging or avoiding adjuvant therapy could decrease DFS [14].
Addition of Adjuvant Radiotherapy
A phase III randomized study (EORTC 55874) of 224 patients of uterine sarcomas, including 103 uterine leiomyosarcomas patients, evaluated the role of adjuvant pelvic radiotherapy (51Gy). No difference was found in either overall or disease-free survival or local control for patients with uterine leiomyosarcomas [15]. SARCGYN phase III randomized study conducted by the French Sarcoma Group compared adjuvant chemotherapy followed by pelvic radiotherapy (RT) versus RT alone in patients with completely resected stages I–III uterine sarcoma, including high-grade endometrial sarcoma, uterine leiomyosarcoma (uLMS), and carcinosarcoma. The addition of chemotherapy to RT improved 3-year disease-free survival (55 vs. 41%) with no improvement in 3- or 5-year overall survival [16].
Addition of Adjuvant Chemotherapy
In a well-planned, retrospective study of 110 patients of uterine sarcomas, 30% were treated with adjuvant gemcitabine/docetaxel and 70% were observed. No significant difference in unadjusted disease-free or overall survival was found between both the groups [17]. A recent meta-analysis [18] and an observational cohort study from the National Cancer database [19], found that adjuvant chemotherapy in early uterine leiomyosarcoma (uLMS) did not improve outcomes in comparison to observation. SARC 005 trial a single-arm study tested adjuvant gemcitabine and docetaxel followed by doxorubicin in women with completely resected, high-grade uterine-confined LMS and found 78% remained progression-free at 2 years, and 57% remained progression-free at 3 years [20].
Administration of adjuvant therapy in patients with the high-risk uterine-confined disease has not been shown to change the outcome in the few trials that have been conducted [21]. There is also no proven advantage of chemotherapy for completely resected advanced stage disease, though it is commonly considered empirically [21].
EORTC 62012, a phase 3 randomized controlled study evaluated single-agent doxorubicin to combination chemotherapy, i.e., doxorubicin and ifosfamide in locally advanced, unresectable, or metastatic high-grade soft tissue sarcoma patients. Four hundred fifty-five patients were randomly allocated to doxorubicin or doxorubicin plus ifosfamide in a 1:1 pattern. There were 103 patients of leiomyosarcoma. The intensified doxorubicin and ifosfamide regimen did not translate in overall survival over single-agent doxorubicin [22]. Only one prospective study investigated the role of chemotherapy following a complete resection for advanced uterine leiomyosarcoma. In a GOG trial, 25 women received adjuvant gemcitabine plus docetaxel for completely resected stages I–IV high-grade uterine leiomyosarcoma. This study, however, did not possess a control arm, and it still remains unclear whether treatment led to an improvement in survival [23]. The present study also found that the addition of adjuvant chemotherapy or radiotherapy did not significantly impact the survival in patients with leiomyosarcoma.
Therefore, in conclusion, adjuvant therapies including radiation therapy, chemotherapy, and combined modalities, have been evaluated. However, heterogeneous patient populations, small sample sizes, and the inclusion of carcinosarcoma, lack of standard imaging techniques for recurrence detection have severely restricted the interpretation of these studies. Till to date, there is no properly conducted randomized or prospective study conducted evaluating adjuvant chemotherapy against an observation arm [21].
Therefore, in the absence of clear benefit of adjuvant chemotherapy or radiotherapy for women with surgical stage I or II LMS, observation remains as the standard option [21].
Hormonal Therapy for Uterine Endometrial Stromal Sarcoma
By virtue of estrogen and progesterone receptors in ESS, hormone therapy has shown to be efficacious. Gonadotrophic releasing hormone (GnRH) agonists create a hypoestrogenic state by downregulating GnRH receptors in the anterior pituitary gland. Estrogen deprivation is also achieved by blocking the last step in the biosynthesis of estrogens from androgens by the aromatase enzyme. ESS expresses aromatase enzyme and aromatase inhibitors such as letrozole and anastrozole can be utilized as an adjuvant treatment. Studies have shown that estrogen receptor therapy (ERT) may be detrimental in low-grade endometrial stromal sarcoma patients in early-stage ESS. Retention of normally functioning ovaries, may not affect the recurrence rate significantly following hysterectomy in stage I patients [24]. Letrozole, as well as progestins, could be the first choice of treatment for patients with recurrent or residual low-grade ESS, which are difficult to resect as an effective alternative to radiation and chemotherapy [25]. There is no clarity in the literature regarding the optimal usage of hormonal therapy in completely resected recurrent uterine ESS. Some studies suggest patients with a previous history of low-grade ESS should not be treated with estrogens or tamoxifen. If such history is present, withdrawal of estrogen receptor therapy or tamoxifen is strongly advocated for disease stabilization. Medroxyprogesterone acetate and letrozole are highly effective for a sustained disease control in most such cases [26].
Overall, the prognosis of patients with uterine sarcomas is poor. Due to the rarity of the disease, the studies performed earlier were underpowered and suffer from multiple flaws, included carcinosarcomas, and cannot be taken as benchmark trials to base the decision. The role of adjuvant therapy including single agent or combination chemotherapy, adjuvant radiotherapy or combined radiotherapy and chemotherapy both in early and advanced uterine sarcomas remains unclear at this time. Also, the role of hormonal therapy in uterine endometrial stromal sarcomas remains to be defined. The present study also has a number of limitations. Less number of patients, retrospective studies, lack of pelvic nodal dissection in all patients could contribute to the shortcoming. The need of the day is to conduct adequately powered cooperative trials which can define the role of neoadjuvant and adjuvant therapies.
Abbreviations
- CS:
-
Carcinosarcoma
- ESS:
-
Endometrial stromal sarcoma
- LMS:
-
Leiomyosarcoma
- AS:
-
Adenosarcoma
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
References
Harlow BL, Weiss NS, Lofton S (1986) The epidemiology of sarcomas of the uterus. J Natl Cancer Inst 76:399–402
Abeler VM, Røyne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB (2009) Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 54:355–364
Brooks SE, Zhan M, Cote T, Baquet CR (2004) Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989–1999. Gynecol Oncol 93:204–208
D'Angelo E, Prat J (2010) Uterine sarcomas: a review. Gynecol Oncol 116(1):131–139
Cantrell LA, Blank SV, Duska LR (2015) Uterine carcinosarcoma: a review of the literature. Gynecol Oncol 137(3):581–588
Prat J (2009) FIGO staging for uterine sarcomas. Int J Gynaecol Obstet 104(3):177–178
Biswas A, Patel F, Kumar P, Srinivasan R, Bera A, Sharma SC, Rajwanshi A (2013) Uterine sarcoma-current management and experience from a regional cancer centre in North India. Arch Gynecol Obstet 288(4):873–882
Sharma DN, Rath GK, Kumar S, Kumar L, Bhatla N, Gandhi AK, Hariprasad R (2011) Clinical outcome of patients with uterine sarcomas. J Cancer Res Ther 7(3):270–274
Leitao MM, Sonoda Y, Brennan MF, Barakat RR, Chi DS (2003) Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol 91:209–212
Goff BA, Rice LW, Flelschhacker D, Muntz HG, Falkenberry SS, Nikrui N, Fuller AF Jr (1993) Uterine leiomyosarcoma and endometrial stromal sarcoma: lymph node metastases and sites of recurrence. Gynecol Oncol 50(1):105–109
Shah JP, Bryant CS, Kumar S, Ali-Fehmi R, Malone JM Jr, Morris RT (2008) Lymphadenectomy and ovarian preservation in low-grade endometrial stromal sarcoma. Obstet Gynecol 112(5):1102–1108
Barney B, Tward JD, Skidmore T, Gaffney DK (2009) Does radiotherapy or lymphadenectomy improve survival in endometrial stromal sarcoma? Int J Gynecol Cancer 19(7):1232–1238
Leath CA, Huh WK et al (2007) A multi-institutional review of outcomes of endometrial stromal sarcoma. Gynecol Oncol 105(3):630–634
Agarwal R, Rajanbabu et al (2017) Endometrial stromal sarcoma-a retrospective analysis of factors affecting recurrence. Eur J Obstet Gynecol Reprod Biol 216:92–97
Reed NS, Mangioni C, Malmstrom H et al (2008) Phase III randomized study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: a European Organization for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer 44:808–818
Pautier P, Floquet A, Gladieff L, Bompas E, Ray-Coquard I, Piperno-Neumann S, Selle F, Guillemet C, Weber B, Largillier R, Bertucci F, Opinel P, Duffaud F, Reynaud-Bougnoux A, Delcambre C, Isambert N, Kerbrat P, Netter-Pinon G, Pinto N, Duvillard P, Haie-Meder C, Lhomme C, Rey A (2013) A randomized clinical trial of adjuvant chemotherapy with doxorubicin, ifosfamide, and cisplatin followed by radiotherapy versus radiotherapy alone in patients with localized uterine sarcomas (SARCGYN study). A study of the French Sarcoma Group. Ann Oncol 24:1099–1104
Littell RD, Tucker LY, Raine-Bennett T, Palen TE, Zaritsky E, Neugebauer R, Embry-Schubert J, Lentz SE (2017) Adjuvant gemcitabine-docetaxel chemotherapy for stage I uterine leiomyosarcoma: trends and survival outcomes. Gynecol Oncol 147(1):11–17
Bogani G, Fucà G, Maltese G, Ditto A, Martinelli F, Signorelli M, Chiappa V, Scaffa C, sabatucci I, Lecce F, Raspagliesi F, Lorusso D (2016) Efficacy of adjuvant chemotherapy in early stage uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol 143(2):443–447
Seagle BLL, Sobecki-Rausch J, Strohl AE, Shilpi A, Grace A, Shahabi S (2017) Prognosis and treatment of uterine leiomyosarcoma: a National Cancer Database study. Gynecol Oncol 145(1):61–70
Hensley ML, Wathen JK, Maki RG, Araujo DM, Sutton G, Priebat DA, George S, Soslow RA, Baker LH (2013) Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma: results of a phase 2 trial (SARC 005). Cancer 119(8):1555–1561
Friedman CF, Hensley ML (2018) Options for adjuvant therapy for uterine leiomyosarcoma. Curr Treat Options in Oncol 19(2):7
Judson I, Verweij J, Gelderblom H, Hartmann JT, Schöffski P, Blay JY, Kerst JM, Sufliarsky J, Whelan J, Hohenberger P, Krarup-Hansen A, Alcindor T, Marreaud S, Litière S, Hermans C, Fisher C, Hogendoorn PC, dei Tos A, van der Graaf W, European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group (2014) European organisation and treatment of cancer soft tissue and bone sarcoma group. Lancet Oncol 15(4):415–423
Hensley ML, Ishill N et al (2009) Adjuvant gemcitabine plus docetaxel for completely resected stages I-IV high grade uterine leiomyosarcoma: results of a prospective study. Gynecol Oncol 112(3):563–567
Chu MC, Mor G, Lim C, Zheng W, Parkash V, Schwartz PE (2003) Low-grade endometrial stromal sarcomas: hormonal aspects. Gynecol Oncol 90(1):170–176
Yamaguchi M, Erdenebaatar C, Saito F, Motohara T, Miyahara Y, Tashiro H, Katabuchi H (2015) Long-term outcome of aromatase inhibitor therapy with letrozole in patients with advanced low-grade endometrial stromal sarcoma. Int J Gynecol Cancer 25(9):1645–1651
Pink D, Lindner T et al (2006) Harm or benefit of hormonal treatment in metastatic low-grade endometrial stromal sarcoma: single center experience with 10 cases and review of the literature. Gynecol Oncol 101(3):464–469
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nusrath, S., Bafna, S., Rajagopalan, R. et al. Uterine Sarcomas: Experience from a Tertiary Cancer Care Center from India. Indian J Surg Oncol 10, 342–349 (2019). https://doi.org/10.1007/s13193-018-0860-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-018-0860-5