Abstract
Purpose
We intended to assess the clinicopathological features and treatment outcome in patients of uterine sarcoma.
Method
A retrospective review of medical records of patients of uterine sarcoma (2002–2007) was conducted. Overall survival (OS) was analyzed by Kaplan–Meier method.
Results
Forty-two patients met the study criterion [15 carcinosarcoma, 12 endometrial stromal sarcoma, 11 leiomyosarcoma, 3 undifferentiated endometrial sarcoma (UES), and 1 mixed sarcoma]. Median age and performance status were 52 years and ECOG 0, respectively. All patients underwent primary surgery out of which 66.7 % was total abdominal hysterectomy and bilateral salpingo-oophorectomy. FIGO (2009) stage was I, II, III, IV and unknown in 66.7, 7.1, 14.3, 9.5, and 2.4 % of the patients. Eight patients were kept on follow-up only. Adjuvant radiation, chemoradiation, and chemotherapy were offered in 8, 9, and 3 patients, respectively. Pelvic radiation: 46 Gray/23 fractions/4.5 weeks and vincristine, adriamycin, cyclophosphamide (VAC) regimen were most commonly used. Overall clinical complete response (CR), stable disease (SD), and progressive disease (PD) were, respectively, 59.5, 2.4, and 26.2 % (response not evaluable in 12 %). In the evaluable patients (N = 33), median OS was noted to be 7.67 months (mean 30.19 months). 1- and 2-year actuarial survival were 45.45 and 36.36 %. Stratified by histology, median survival in patients with carcinosarcoma, endometrial stromal sarcoma, leiomyosarcoma, and UES were, respectively, 6.57, 18.7, 6.8, and 9.38 months. On univariate analysis, response to therapy (p = 0.0003), disease stage (p = 0.00001), tumor size (p = 0.02), and performance status (p = 0.03) were significant predictors of OS. Disease stage (p = 0.005) and response to therapy (p = 0.01) retained significance on multivariate analysis.
Conclusions
Median OS of only 6.57, 6.8, and 9.38 months, respectively, in patients with carcinosarcoma, leiomyosarcoma, and UES in our series reflect the aggressive clinical course and poor prognosis of these rare neoplasms, which mandate intensive multimodality therapy. Even in low-grade endometrial stromal sarcoma, median survival of 18.7 months in our series is far from satisfying. However, small series, poor treatment compliance and socio-economic constraints in the Indian scenario are limiting factors in the result analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uterine sarcomas constitute 3–9 % of malignant uterine tumors and <1 % of all female genital tract malignancies [1, 2]. The incidence of uterine sarcoma has been estimated to vary between 0.5 and 3.3 cases per 100,000 females per year [3]. Uterine sarcomas exhibit aggressive biology with propensity to local recurrence and distant dissemination [4–6]. Compared with their epithelial counterparts, uterine sarcomas are much less common and portend worse prognosis [7]. The staging of uterine sarcoma is based on the International Federation of Gynaecology and Obstetrics staging system for endometrial cancer. As per the recent staging system (2009), carcinosarcoma is staged similarly as endometrial carcinoma, whereas there is a separate staging system for leiomyosarcoma, endometrial stromal sarcoma, and adenosarcoma. According to WHO classification, uterine sarcomas are classified into four main histological subtypes in order of decreasing incidence: carcinosarcoma, leiomyosarcoma, endometrial stromal sarcoma, and other sarcomas [8]. Carcinosarcoma shows kinship to poorly differentiated endometrial carcinoma and hence recently is being staged and treated differently from other uterine sarcomas. The different histological subtypes have varied clinical course and response to therapy. The aim of this study was to evaluate retrospectively the clinicopathological features and treatment outcome in patients of uterine sarcoma attending Regional Cancer Centre, Postgraduate Institute of Medical Education and Research, Chandigarh, India from 2002 to 2007. To the best of our knowledge, this is the second retrospective analysis of uterine sarcoma from the Indian subcontinent.
Materials and methods
Medical records were reviewed and data collected on all uterine sarcomas over a 6-year period (2002–2007) from the departmental archives. Histopathology was reviewed by two pathologists with expertise in gynecological pathology. Though all patients with a diagnosis of uterine sarcoma were included in the demographic assessment, only those with adequate clinical data on treatment and follow-up were included in the survival analysis. Overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow-up. OS was analyzed by Kaplan–Meier method. Log Rank test and Cox proportional hazard regression model were used, respectively, for univariate and multivariate analysis of factors predictive of OS. p value < 0.05 was considered to be significant. SPSS statistical software version 12 was used for the analysis.
Results
Patient demographics (Table 1)
Forty-two patients met the study criterion. Median age at diagnosis was noted to be 52 years (range 21–78 years). Stratified by histology, median age in patients with carcinosarcoma, leiomyosarcoma, endometrial stromal sarcoma, and UES were, respectively, 60, 50, 37, and 56 years showing a younger age predilection for patients with endometrial stromal sarcoma. Most of the patients (69 %) had performance status of ECOG 0 at first visit to the Radiotherapy department. 45.2 % of the patients were premenopausal, 2.4 % perimenopausal, and 52.4 % postmenopausal. All were parous. Comorbidities included hypertension (11.9 %), diabetes (9.5 %), and goiter (2.4 %). Symptomatology (median duration 4.5 months) comprised bleeding per-vaginum (88.1 %), pain abdomen (45.2 %), and discharge per-vaginum (28.6 %).
Surgical policy
All patients underwent primary surgery out of which 66.7 % was total abdominal hysterectomy and bilateral salpingo-oophorectomy. Pelvic lymph node dissection was carried out in only 4.8 % of the patients (all carcinosarcoma). 57 % of the patients were operated in the institute while the rest were referred after surgery from outside.
Histopathology (Table 2)
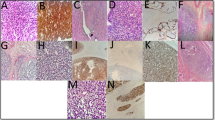
On gross histopathology, 21.4 % of the tumors were located in fundus, 19 % in body, 2.4 % in isthmus, 33.3 % filling the whole uterine cavity, and details were not available in 23.8 %. Tumor size was <5 cm, 5–10 cm, >10 cm in 10, 13, and 6 patients, respectively. On microscopy, histological subtype was as follows: carcinosarcoma/mixed malignant mullerian tumor (MMMT)-15, leiomyosarcoma (LMS)-11, low-grade endometrial stromal sarcoma (LG-ESS)-12, undifferentiated endometrial sarcoma (UES)/high-grade endometrial stromal sarcoma (HG-ESS)-3, and mixed sarcoma (LMS with ESS)-1 (Fig. 1). Myoinvasion, serosal infiltration, adnexal extension, and pelvic-paraaortic lymph nodal involvement were observed in 66.6, 7.1, 16.6, and 2.4 %, respectively. Peritoneal cytology came out to be positive in 7.1 % of the patients. Overall FIGO (2009) stage was I, II, III, IV and unknown in 66.7, 7.1, 14.3, 9.5, and 2.4 % of the patients (Fig. 2).
Composite image panel of uterine sarcoma showing: a leiomyosarcoma with diffuse cytological atypia and mitoses, b carcinosarcoma (MMMT)—a combination of endometriod carcinoma with homologous sarcoma, c low-grade endometrial stromal sarcoma showing classical histological features, and d undifferentiated endometrial sarcoma: hematoxylin and eosin stain; a, c ×200, b ×100, d ×400
Adjuvant therapy
A total of 37 patients were referred for adjuvant therapy. The remaining five patients had presented to our department with advanced and metastatic disease and were treated on merit. Eight patients (19 %) of early stage low-grade ESS were kept on follow-up only. Adjuvant radiation, chemoradiation, and chemotherapy were used in 8 (19 %), 9 (21.4 %), and 3 (7.1 %) patients, respectively (Fig. 3). Nine patients (21.4 %) dropped out following departmental registration. Adjuvant radiation was delivered to whole pelvis, the usual schedule being 46 Gray/23 fractions/4.5 weeks by four-field box technique with 6 MV photons. Radiation interruption was noted in 9.5 % patients. Acute radiation accompaniments were mild-grade1/2 (mostly diarrhea) and severe-grade 3/4 (diarrhea) in 23.8 and 4.8 % of the patients, respectively. Late radiation accompaniments were mild-grade1/2 (telangiectasia and bleeding PR) in 4.8 % of the patients. Chemotherapy regimens used included VAC (Inj vincristine-1.5 mg/m2 IV D1 with top dose of 2 mg; Inj adriamycin-50 mg/m2 IV D1; Inj cyclophosphamide-1 gm/m2 IV D1 q 3 weeks) in 23.8 % patients and Cisplatin-based regimens (CAP) (Inj cyclophosphamide-600 mg/m2 IV D1; Inj adriamycin-50 mg/m2 IV D1; Inj cisplatin-60 mg/m2 IV D1q 3 weeks) in 4.8 % patients (all carcinosarcoma). Chemotherapy interruption was noted in 7.1 % of the patients. Acute chemotherapy accompaniments were mostly mild-grade 1/2 and severe-grade 3/4 (leucopenia) in 26.2 and 2.4 % of the patients, respectively. In adjuvant chemoradiation protocols, radiation was used upfront in three patients (7.1 %) and sequenced in between chemotherapy cycles in six patients (14.3 %). 3 patients of ESS with estrogen and progesterone receptor (ER, PR) bipositivity received adjuvant endocrine therapy with letrozole (2.5 mg OD till 5 years).
Palliative therapy
Palliative radiation was delivered to whole pelvis in one patient and painful bony metastasis in another. Palliative chemotherapy with VAC regimen was given in three patients. Palliation attained in all five patients was modest.
Response to therapy and symptom control
Overall clinical CR, SD, and PD were, respectively, 59.5, 2.4, and 26.2 %, whereas details were not available in 11.9 % patients. At completion of therapy, 26 patients were free of symptoms, 3 had no symptomatic improvement, and 9 had progressive symptoms.
Patterns of failure and salvage therapy
Four patients with carcinosarcoma failed-one had local recurrence, two had distant metastases (malignant pleural effusion in two patients, bone metastases in one patient), and one had local recurrence along with distant metastasis (liver secondaries). Four patients with leiomyosarcoma failed-one had local recurrence, one had distant metastases (lung secondaries), and two had local recurrence along with distant metastasis (supraclavicular lymph node and lung metastasis). One patient with endometrial stromal sarcoma had distant metastasis in the lungs. Overall, failures were noted to be local in two patients (4.8 %), distant in four patients (9.6 %), and local as well as distant in three patients (7.2 %). Salvage therapy included pelvic radiation (46 Gy/23#/4.5 weeks) in one patient (carcinosarcoma) and chemotherapy with VAC regimen (6 cycles) in one patient (leiomyosarcoma) leading to SD and PD, respectively.
Follow-up and survival analysis (Tables 3, 4, 5)
In the evaluable patients (N = 33), median OS was noted to be 7.67 months (mean 30.19 months). 1- and 2-year actuarial survival were 45.45 and 36.36 % (Fig. 4). Stratified by histology, median survival in patients with carcinosarcoma, endometrial stromal sarcoma, leiomyosarcoma, and UES were, respectively, 6.57, 18.7, 6.8, and 9.38 months. At last follow-up, 20 patients were disease-free and 13 had evidence of disease. On univariate analysis, response to therapy (p = 0.0003), disease stage (p = 0.0005), tumor size (p = 0.02), and PS (p = 0.03) were significant predictors of OS (Figs. 5, 6). Though median survival was numerically more in low-grade tumors (18.7 months) and ESS histology (18.7 months), the impact of age, histology, and tumor grade upon OS was noted to be statistically nonsignificant. Disease stage (p = 0.005) and response to therapy (p = 0.01) retained significance on multivariate analysis. On univariate analysis of factors predicting overall survival in different histological subtypes, only disease stage is a significant prognosticator of overall survival in carcinosarcoma (p = 0.001) (Table 5).
Discussion
Clinical presentation and diagnosis
Most patients of uterine sarcoma are middle-aged to elderly women (40–60 years) presenting with abnormal uterine bleeding or pelvic mass, which may be confused with leiomyoma [7, 9, 10]. The median age at diagnosis in our study was 52 years and the most common symptom was bleeding per vaginum (88.1 %). Screening for uterine sarcoma is a difficult issue because of the rarity of presentation. Preoperative endometrial sampling has a sensitivity of only 64 % and the diagnosis is usually made at the time of surgery or from the final histopathology report [11]. In our series, preoperative tissue diagnosis of uterine sarcoma was available in only 11.9 % patients.
Pathology
According to Gynaecology Oncology Group classification, uterine sarcomas are broadly divided into pure (non-epithelial) and mixed (epithelial–non-epithelial) types. Pure uterine sarcomas are further subdivided into the following subtypes: endometrial stromal sarcoma (low grade and high grade), leiomyosarcoma (epitheliod and myxoid), and others. High-grade endometrial stromal sarcoma is recently considered UES. Mixed uterine sarcomas are further subdivided into adenosarcoma (adenoma with sarcoma) and carcinosarcoma/mixed malignant mullerian tumor (carcinoma with sarcoma). Of late carcinosarcoma is being considered metaplastic epithelial tumor and is being staged and treated differently from uterine sarcoma in many centers. Uterine sarcomas can be either homologous, i.e., with native histological features (e.g., leiomyosarcoma with smooth muscle differentiation) or heterologous, i.e., with non-native histological features (e.g., rhabdomyosarcoma with skeletal muscle differentiation, osteosarcoma with osseous differentiation) [7]. In our series, the most common histological subtype was CS (35.7 %) followed by low grade ESS (28.6 %), LMS (26.2 %), UES (7.1 %), and mixed sarcoma (2.4 %).
Clinical course
Mixed malignant mullerian tumor or carcinosarcoma (CS) is the most common type of uterine sarcoma accounting for 43–53 % of these tumors [12, 13]. The most common histological combination is high-grade papillary serous carcinoma with low-grade ESS [7]. The clinical course is akin to that of the epithelial component, which is aggressive with propensity for bulky tumors and early metastasis [14]. CS appears to show more kinship to poorly differentiated endometrial carcinoma. LMS is the second most common uterine sarcoma constituting 33 % of these tumors in SEER database [15]. These tumors have high incidence of distant failure. Metastatic potential is determined by mitoses and cellular atypia. ESS constitute for 6–20 % of all uterine sarcomas [16–18]. The natural history depends upon the grade of the tumor, which is determined by number of mitoses (<10 vs. >10 per 10 hpf) and cellular atypia. Low-grade ESS is characterized by diagnosis in early stage, indolent course, and ER, PR immunopositivity. The 5-year survival is excellent, typically 80–100 % but 37–60 % of patients have late recurrence and 15–25 % eventually die of disease [9, 19]. Recurrences are usually local but late recurrence may involve lung and abdomen. In UES (high-grade ESS), disease tends to be more aggressive and carries poor prognosis, akin to MMMT and LMS [7].
Surgical policy
Surgical management of carcinosarcoma includes total abdominal hysterectomy with bilateral salpingo-oophorectomy, pelvic-paraaortic lymph node dissection, omentectomy, peritoneal washings, and debulking in case of gross extrauterine disease [7, 9, 10]. Comprehensive lymph node dissection is indicated in view of high incidence of lymph node metastasis [20, 21]. Total hysterectomy with removal of gross disease is the surgical treatment of choice in LMS. Ovarian preservation may be attempted in premenopausal lady with early stage LMS [7, 9]. In ESS, primary treatment is total abdominal hysterectomy with bilateral salpingo-oophorectomy [7, 9, 10]. Ovarian ablation is a must as estrogen may act as a trophic agent for these tumors [22]. Lymph node sampling is not the standard of care, as nodal involvement by low-grade ESS is supposed to be rare. In two recently published series, nodal involvement was found in 33–45 % of cases during primary or secondary surgical treatment, and lymphadenectomy has been suggested by the authors as part of the surgical treatment [23, 24]. All patients in our series had undergone primary surgery out of which 66.7 % was total abdominal hysterectomy and bilateral salpingo-oophorectomy. Comprehensive pelvic-paraaortic lymph node sampling/dissection is not routinely carried out in our institute as mirrored by limited use (4.8 %).
Role of radiotherapy
Of all the histological subtypes, carcinosarcoma appears to be the most radiosensitive [7]. Adjuvant radiation to pelvis or whole abdomen with or without vaginal brachytherapy has been used in CS with varied results. Post-operative radiotherapy reduces local recurrence but a survival benefit remains elusive [25, 26]. However, in a case-series of 60 patients of CS comparing surgery followed by observation or adjuvant radiation, there was a benefit in both local recurrence (0 vs. 22 %) as well as 5-year survival (85 vs. 50 %) in favor of adjuvant radiation [27]. In a retrospective analysis of 2,461 women with uterine carcinosarcoma by SEER group, adjuvant radiation led to improved overall and disease specific survival. Five-year OS was 41.5 versus 32.2 % (p < 0.001) in favor of women receiving radiation [28]. The role of adjuvant radiation in LMS is to increase local pelvic control by as much as 50 % [29]. Similarly, post-operative radiation in ESS appears to enhance local control with unclear benefit toward OS [7, 9, 10]. However, two case series demonstrate trend toward improved survival with early stage disease in ESS [30, 31]. In a phase III randomized controlled trial by EORTC group, 224 patients of stages I and II uterine sarcoma (103 LMS, 91 CS, and 28 ESS) were randomized to observation (N = 112) or pelvic radiation-50.4 Gray/28 fractions/5–6 weeks (N = 112) following surgery (TAH and BSO) [32]. Adjuvant radiation led to a decrease in local relapse (14 vs. 24, p = 0.004) without any statistically significant improvement in either overall survival or progression free survival. Increment in locoregional control was discerned in CS but not in LMS. No unexpected toxicity was seen in the radiation arm.
Role of chemotherapy
In 1980s, doxorubicin was found to elicit limited response in carcinosarcoma [33]. More recent studies have focused on platinum containing regimens. One group at Yale achieved a 92 % 2-year survival in stages I and II CS with chemotherapy combination of cisplatin, adriamycin, and etoposide [34]. In GOG 150 trial, 206 patients with CS were assigned to either whole abdominal radiation or three cycles of chemotherapy with cisplatin, ifosfamide, and mesna (CIM) post surgery. There was a non-significant trend toward both improved local control and survival in the chemotherapy arm [35]. GOG 161 trial compared ifosfamide versus ifosfamide and paclitaxel combination in patients with advanced carcinosarcoma and reported a significant improvement in both response rate and OS in the combination chemotherapy arm [36]. Currently, there are ongoing studies investigating the combination of carboplatin and paclitaxel in both first-line and salvage chemotherapy for CS. Chemotherapy plays a key role in the management of LMS due to its propensity for distant hematogenous metastasis. Though traditionally ifosfamide and adriamycin have been used in LMS, the latest chemotherapy combination exhibiting significant response rate in metastatic LMS is gemcitabine–docetaxel doublet [37–39]. The response rate is 30–40 % in first line setting and 20–30 % in second line setting. Data regarding chemotherapy in ESS is limited. Various chemotherapeutics, e.g., cyclophosphamide, vincristine, adriamycin, actinomycin D, dacarbazine, and methotrexate have demonstrable activity and have been used in various combination in ESS [7, 9].
Endocrine therapy
Low-grade ESS show immunopositivity for estrogen and progesterone receptors. Various endocrine manipulation have been tried in ESS in advanced, metastatic and recurrent setting. These include medroxyprogesterone acetate, megestrole acetate, aromatase inhibitors, and GnRH analogs. Of late hormonal therapy is being tried for the prevention of recurrence in ESS [7, 9].
Departmental protocol
In our department, patients with early stage low-grade ESS are kept on follow-up. Adjuvant radiation to whole pelvis: 46 Gray/23 fractions/4.5 weeks is delivered to patients with CS, LMS, and advanced or high-grade ESS. Adjuvant chemotherapy with VAC regimen is also added to the same cohort. CAP regimen is often preferred in patients with CS. The use of chemotherapy is; however, limited by the poor affordability of our patients. Only 28.5 % of the patients could undergo chemotherapy either alone or in combination with radiation in the adjuvant setting. Adjuvant hormones (letrozole) are given in patients of low-grade ESS with ER, PR immunopositivity. Out of 37 patients referred to our department for adjuvant therapy, 28 patients received treatment as planned and the remaining 9 had dropped out after departmental registration. The high rate of attrition (21.4 %), though not unusual in the fabric of Indian life, is notable. Follow-up schedule includes clinical examination 1 month after completion of therapy, every 3 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Radiological investigations on follow-up are requested only in case of suspicion of disease recurrence. Patients with advanced, metastatic disease are treated on merit: the options being best supportive care, palliative chemotherapy, and palliative radiation to local or metastatic disease.
Treatment outcome in patients of uterine sarcoma in our series has been poor. This may be due to a multitude of reasons: socioeconomic constraints reflected by limited use of chemotherapy (28.5 %), low incidence of comprehensive pelvic-paraaortic lymph node dissection in CS (2/15 patients), relatively heterogeneous treatment strategy, and high rate of attrition (21.4 %). Nevertheless, the present series, the second of its kind from the Indian subcontinent provides a glimpse of management of a rare, aggressive gynecological malignancy in a real world situation in a developing nation.
Indian perspective
In the only available study on patients of uterine sarcoma from India, Sharma et al. [40] reported the clinical outcome of 50 patients from 2000 to 2008. Median age was noted to be 50 years. Carcinosarcoma was the most common histological type (46 %). Overall FIGO stage was I, II, III, IV and unknown in 27, 7, 12, 2, and 2 patients, respectively. Number of patients undergoing surgery, radiation therapy, and chemotherapy were, respectively, 48, 31, and 16. After a median follow-up of 34 months, 3-year overall survival was noted to be 42 %. Disease stage, histopathological type, and use of post-operative radiation were significant prognosticators of overall survival on univariate analysis.
Conclusion
Median OS of only 6.57, 6.8, and 9.38 months, respectively, in patients with carcinosarcoma, leiomyosarcoma, and UES in our series reflect the aggressive clinical course and poor prognosis of these rare neoplasms, which mandate intensive multimodality therapy. Even in low-grade endometrial stromal sarcoma, median survival of 18.7 months in our series is far from satisfying. However, small series (N = 42), poor treatment compliance, and socio-economic constraints in the Indian scenario are significant deterrents in achieving more meaningful results. Patients of uterine sarcoma should be referred to large academic centers for participation in clinical trials. Future clinical trials should address specific histological subtype rather than clubbing all histological subtypes under the banner of uterine sarcoma.
References
Salazar OM, Bonfiglio TA, Patten SF, Keller BE, Feldstein M, Dunne ME et al (1978) Uterine sarcomas: natural history, treatment and prognosis. Cancer 42:1152–1160
Nordal RR, Thoresen SO (1997) Uterine sarcomas in Norway 1956–1992: incidence, survival and mortality. Eur J Cancer 33:907–911
Harlow BL, Weiss NS, Lofton S (1986) The epidemiology of sarcomas of the uterus. J Natl Cancer Inst 76:399–402
Jonson AL, Bliss RL, Truskinovsky A, Judson P, Argenta P, Carson L et al (2006) Clinical features and outcomes of uterine and ovarian carcinosarcoma. Gynecol Oncol 100:561–564
Berchuck A, Rubin SC, Hoskins WJ, Saigo PE, Pierce VK, Lewis JL Jr (1988) Treatment of uterine leiomyosarcoma. Obstet Gynecol 71:845–850
Berchuck A, Rubin SC, Hoskins WJ, Saigo PE, Pierce VK, Lewis JL Jr (1990) Treatment of endometrial stromal tumors. Gynecol Oncol 36:60–65
Lin JF, Slomovitz BM (2008) Uterine sarcoma 2008. Curr Oncol Rep 10:512–518
Kempson R, Bari W (1970) Uterine sarcomas: classification, diagnosis and prognosis. Hum Pathol 1:331–349
Zagouri F, Dimopoulos AM, Fotiou S, Kouloulias V, Papadimitriou CA (2009) Treatment of early uterine sarcomas: disentangling adjuvant modalities. World J Surg Oncol 7:38
Zagouri F, Linardou H, Dimopoulos AM, Papadimitriou CA (2009) Management of advanced stage uterine sarcomas: a bone of contention. Eur J Gynaecol Oncol 30:483–492
Bansal N, Herzog T, Burke W, Cohen CJ, Wright JD (2008) The utility of preoperative endometrial sampling for the detection of uterine sarcomas. Gynecol Oncol 110:43–48
Kahanpaa KV, Wahlstrom T, Grohn P, Heinonen E, Nieminen U, Widholm O (1986) Sarcomas of the uterus: a clinicopathologic study of 119 patients. Obstet Gynecol 67:417–424
Sherman ME, Devesa SS (2003) Analysis of racial differences in incidence, survival and mortality for malignant tumors of the uterine corpus. Cancer 98:176–186
Sartori E, Bazzurini L, Gadducci A, Landoni F, Lissoni A, Maggino T et al (1997) Carcinosarcoma of the uterus: a clinicopathological multicenter CTF study. Gynecol Oncol 67:70–75
Platz CE, Benda JA (1995) Female genital tract cancer. Cancer 75(1 Suppl):270–294
Diesing D, Cordes T, Finas D, Löning M, Mayer K, Diedrich K et al (2006) Endometrial stromal sarcomas—a retrospective analysis of 11 patients. Anticancer Res 26:655–661
Gadducci A, Sartori E, Landoni F, Zola P, Maggino T, Urgesi A et al (1996) Endometrial stromal sarcoma: analysis of treatment failures and survival. Gynecol Oncol 63:247–253
Oláh KS, Gee H, Blunt S, Dunn JA, Kelly K, Chan KK (1991) Retrospective analysis of 318 cases of uterine sarcoma. Eur J Cancer 27:1095–1099
Gadducci A, Cosio S, Romanini A, Genazzani AR (2008) The management of patients with uterine sarcoma: a debated clinical challenge. Crit Rev Oncol Hematol 65:129–142
Temkin SM, Hellmann M, Lee YC, Abulafia O (2007) Early-stage carcinosarcoma of the uterus: the significance of lymph node count. Int J Gynecol Cancer 17:215–219
Nemani D, Mitra N, Guo M, Lin L (2008) Assessing the effects of lymphadenectomy and radiation therapy in patients with uterine carcinosarcoma: a SEER analysis. Gynecol Oncol 111:82–88
Pink D, Lindner T, Mrozek A, Kretzschmar A, Thuss-Patience PC, Dörken B et al (2006) Harm or benefit of hormonal treatment in metastatic low-grade endometrial stromal sarcoma: single center experience with 10 cases and review of the literature. Gynecol Oncol 101:464–469
Riopel J, Plante M, Renaud MC, Roy M, Têtu B (2005) Lymph node metastases in low-grade endometrial stromal sarcoma. Gynecol Oncol 96:402–406
Reich O, Winter R, Regauer S (2005) Should lymphadenectomy be performed in patients with endometrial stromal sarcoma? Gynecol Oncol 97:982–983
Ferrer F, Sabater S, Farrus B, Guedea F, Rovirosa A, Anglada L et al (1999) Impact of radiotherapy on local control and survival in uterine sarcomas: a retrospective study from the Grup Oncologic Catala-Occita. Int J Radiat Oncol Biol Phys 44:47–52
Hornback NB, Omura G, Major FJ (1986) Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys 12:2127–2130
Gerszten K, Faul C, Kounelis S, Huang Q, Kelley J, Jones MW (1998) The impact of adjuvant radiotherapy on carcinosarcoma of the uterus. Gynecol Oncol 68:8–13
Clayton Smith D, Kenneth Macdonald O, Gaffney DK (2008) The impact of adjuvant radiation therapy on survival in women with uterine carcinosarcoma. Radiother Oncol 88:227–232
Gadducci A, Landoni F, Sartori E (1996) Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecol Oncol 62:25–32
Weitmann H, Knocke TH, Kucera H, Potter R (2001) Radiation therapy in the treatment of endometrial stromal sarcoma. Int J Radiat Oncol Biol Phys 49:739–748
Leath C, Huh W, Hyde J, Cohn DE, Resnick KE, Taylor NP et al (2007) A multi-institutional review of outcomes of endometrial stromal sarcoma. Gynecol Oncol 105:630–634
Reed NS, Mangioni C, Malmstrom H, Scarfone G, Poveda A, Pecorelli S et al (2008) Phase III randomized trial to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stage I and II: an European Organisation for Research and Treatment of cancer Gynaecological Cancer Group Study (protocol 55874). Eur J Cancer 44:808–818
Omura GA, Blessing JA, Major F (1985) A randomized clinical trial of adjuvant adriamycin in uterine sarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 3:1240–1245
Resnik E, Chambers S, Carcangiu M (1995) A phase II study of etoposide, cisplatin and doxorubicin chemotherapy in mixed mullerian tumors (MMT) of the uterus. Gynecol Oncol 56:370–375
Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ et al (2007) A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I–IV carcinosarcoma (CS) of the uterus. Gynecol Oncol 107:177–185
Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, Kilgore LC et al (2007) Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. J Clin Oncol 25:526–531
Fleming G (2008) Gemcitabine/docetaxel—welcome to a new standard. Gynecol Oncol 109:313–315
Hensley ML, Blessing JA, Degeest K, Abulafia O, Rose PG, Homesley HD (2008) Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol 109:323–328
Hensley ML, Blessing JA, Mannel R, Rose PG (2008) Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncology 109:329–334
Sharma DN, Rath GK, Kumar S, Kumar L, Bhatia N, Gandhi AK, Hariprasad R (2011) Clinical outcome of patients with uterine sarcoma. J Cancer Res Ther 7:270–274
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biswas, A., Patel, F., Kumar, P. et al. Uterine sarcoma-current management and experience from a regional cancer centre in North India. Arch Gynecol Obstet 288, 873–882 (2013). https://doi.org/10.1007/s00404-013-2843-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-013-2843-7