Abstract
Background
Transdermal drug delivery systems (TDDS) pose special risks considering a large amount of drug they contain and their modified release properties. We sought to characterize TDDS exposures reported to the National Poison Data System (NPDS).
Methods
NPDS was searched for all human exposures to a TDDS from 1/1/2006 to 12/31/2015. Only single-substance TDDS exposures followed to a known medical outcome were included for final analysis. Specific data analyzed was date, sex, age, TDDS product, exposure reason, route of exposure, medical outcome, management site, level of health care facility care, clinical effects, and interventions.
Results
Over that 10-year period, 6746 adults and 1917 pediatric exposures were identified. Exposures declined over the study period. The most common exposure reason in adults was intentional abuse (n = 1622) compared to unintentional-general (n =1 070) in pediatric cases. TDDS ingestion was reported in 4519 adults and 2825 pediatric cases. Fentanyl was the most common substance encountered in adult (n = 4656) and pediatric cases (n = 474). No or minor effect were the most common medical outcomes in both groups. In fentanyl cases, moderate or major outcomes were seen in 54 % (n = 1062) of adult and 26 % (n = 54) of pediatric cases. Naloxone was given in 1080 cases. Ninety-seven deaths (91 adults, 6 pediatrics) were reported, all involving ingestion of the TDDS. Fentanyl was associated with 80 adult and 5 pediatric deaths.
Conclusion
Overall, single-substance TDDS exposures decreased over the duration of this study and typically resulted in no or mild effects. However, exposures involving fentanyl resulted in higher rates of major or moderate medical outcomes and were associated with multiple deaths.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The skin is one of the largest organ systems, and its main function is to act as a barrier and interface to the surrounding environment. There has long been an interest in using the large surface area of the skin to deliver drugs, whether topical or systemic. Early history documents the use of various ointments, liniments, creams, and even primitive patches as far back as 3000 BC [1]. However, it was not until 1979 that transdermal scopolamine became the first commercially available patch or transdermal drug delivery system (TDDS) [2]. Soon after, transdermal clonidine patches became available in 1984 and there since has been a steady increase in the number of available TDDS [3]. As of 2018, there are over 20 drugs commercially available as a TDDS [4]. They are marketed to treat a variety of conditions including chronic pain, attention deficit hyperactivity disorder, Parkinson’s disease, and coronary artery disease [4].
With the increasing number of TDDS available, there is concern regarding their potential for toxicity. Local toxicity, primarily skin irritation, from TDDS use is a common and well-described phenomenon [5]. More concerning is the potential for systemic toxicity due to the large amount of drug a TDDS may contain. For example, per package inserts a 100 mcg/h fentanyl TDDS containing 10 mg of fentanyl while a 0.3 mg/day clonidine TDDS has 7.5 mg of clonidine [6, 7]. The risk of toxicity and adverse outcomes are further increased if the TDDS is tampered with or used improperly. Case of ingesting, chewing, and smoking transdermal delivery systems have resulted in significant morbidity and mortality [8,9,10,11]. Equally concerning is that large amounts of drugs may remain present in TDDS even when they are correctly used for the prescribed duration. If inappropriately disposed of these, “spent” TDDS may pose a risk for unintentional toxicity, especially in pediatric patients, or for intentional abuse [12, 13].
While there have been several case reports and series describing toxicity from specific TDDS, such as scopolamine, lidocaine, clonidine, and fentanyl TDDS, there is a paucity of literature examining wider trends and outcomes of TDDS toxicity [9, 14,15,16]. We sought to examine TDDS exposures reported to the National Poison Data System (NPDS) to better characterize the nature of the exposures and the outcomes associated with them.
Methods
This was an IRB approved cross-sectional study of quantitative data obtained from the NPDS. The NPDS is the data warehouse for the 55 poison control centers in the USA. Each center uploads deidentified data after providing information and exposure management services to callers in near real time. The current time for uploading of all poison center data is 9.5 min [17]. The NPDS represents one of the largest repositories of information regarding US exposures and poison management. The NPDS was queried for all single-substance TDDS product human exposures between January 1, 2006 and December 31, 2015. “Single substance” was defined a priori as cases where only the TDDS product was the reported substance exposure. Exposure formulation “patch” and 330 product codes matching TDDS products were identified. Data abstracted from this query included year of exposure, route of exposure, gender, reason for exposure, age of exposed individual (pediatric was defined as 19 years of age or less (≤ 19 years); adult was defined as 20 years of age or greater (≥ 20 years)), TDDS product of exposure, medical outcomes, recorded “related” clinical effects, and “Performed” or “Recommended and Performed” therapeutic interventions. Medical outcomes were coded as follows: no effect—the patient developed no symptoms; minor effect—the patient exhibited some symptoms as the result of the exposure that were minimally bothersome to the patient but self-limiting without treatment and results in full recovery; moderate effect—the patient exhibited symptoms as a result of the exposure which are more pronounced, prolonged, or more of a systemic nature than minor symptoms which require some form of treatment or recommendation for treatment but do not result in permanent or life-threatening effects; major effect—the patient exhibits symptoms as a result of the exposure which were life-threatening or resulted in significant residual disability or disfigurement. Case narratives were not reviewed for this study. Descriptive statistics including frequencies and cross tabs were completed in IBM Statistics for Mac (v. 25, IBM Corporation, Armonk, NY). Proportions are presented with 95 % confidence intervals (CI).
Results
A total of 15,207 single-substance TDDS exposures were identified. Of these, 6364 cases were excluded from further evaluation (Fig. 1). Further evaluation was completed on the remaining 8663 (1917 pediatrics, 6746 adults, and 36 unknown ages) exposures.
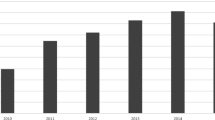
Pediatric TDDS exposures reached a peak in number of exposures in 2007 (240) and declined to a nadir of 144 cases in 2015, while adult exposures reached a peak in 2010 (837) and declined to 477 in 2015 (Fig. 2).
Forty-three percent of pediatric exposures were female (n = 821; CI, 40.6–45) while 56 % (n = 6710; CI, 55.1–57.5) of adult exposures were female.
Ingestion and dermal were the top routes of exposure for all age groups (Table 1). Ingestion represented 67 % (n = 4519; CI, 65.9–68.1) and dermal 42 % (n = 2825; CI, 40.7–43.1) of adult exposures. In pediatric patients age ≤ 5 years, 70 % (n = 768; CI, 67.3–72.8) were ingestions and 35 %(n = 382; CI, 32.1–37.8) were dermal; 6–12 years of age, 59.6 % (n= 240; 95 % CI, 54.6–64.4) were dermal while 44.4 % (n = 179; CI, 39.5–49.4) were ingestions; and 13–19 years of age, 67.4 % were ingestions (n = 277; CI, 62.6–71.9) while 33.8 % (n = 139; CI, 29.3–38.6) were dermal.
More serious outcomes (moderate, major, or death) were more commonly reported in adult encounters (54 %; n = 3630; CI, 52.6–55) than in pediatric encounters (26 %; n = 497; CI, 24–27.9) (Table 2). Among pediatric encounters, rates of serious outcomes increased with age: 15.5 % (n = 170; CI, 13.4–17.8) of ≤ 5 years of age compared to 27.3 % (n = 110; CI, 23–31.9) of 6–12 years old and 52.6 % (n = 216; CI, 47.6–57.5) 13–19 years old.
When a TDDS was reported as being ingested, 41.7 % (n = 845; CI, 40.3–43.2) of the adult cases had a moderate or major outcome compared to 18.5 % (n = 227; CI, 16.3–20.8) of pediatrics cases Among fentanyl exposures, moderate or major outcomes were seen 60 % (n = 2800; CI, 58.7–61.5) of adult fentanyl cases and 43.2 % (n = 205; CI, 38.7–47.8) of pediatric fentanyl cases.
Seventy-eight percent (n = 5253; CI, 76.9–78.9) adult patients were managed in a healthcare facility compared to 53 % (n = 1026; CI, 51.3–55.8) of pediatric patients.
Among pediatric patients rates of management in a healthcare facility increased with age as 46.6 % of age ≤ 5 years (n = 510; CI, 43.6–49.6); 47.9 % (n = 193; CI, 42.9–52.9) of patients age 6–12 years; and 77.9 % (n = 320; CI, 73.5–81.8) of patients age 13–19 years old were managed in a healthcare facility.
Of the pediatric patients managed at a healthcare facility, 60.5 % (n = 929; CI, 58–62.9) treated/evaluated and released (Table 3). Rates of admission increased with age, as among patients age 13–19 years, 34.7 % (n = 111; CI, 29.5–40.2) were admitted to critical care unit; 12.8 % (n = 41; CI, 9.4–17) were admitted to noncritical care unit; and 6.6 % (n = 21; CI, 4.1–9.9) were admitted to psychiatric care unit.
For adult cases managed at a healthcare facility management, 33.4 % were treated/evaluated and released (n = 1748; CI, 32.1–34.6) with 37.3 % admitted to critical care unit (n = 1956; CI, 36–38.6); 15.7 % admitted to noncritical care unit (n = 821; CI, 14.7–16.7); and 6.3 % admitted to psychiatric care unit (n = 329; CI, 5.6–7).
The 3 most common reasons for exposure in all pediatric patients was unintentional-general (n = 1070; CI, 53.6–58.1); intentional abuse (n = 220; CI, 10.1–13); and adverse reaction drug (n = 189; CI, 8.6–11.3). In the 13–19-year-old age group, intentional abuse (165/411; CI, 35.4–45.1); intentional-suspected suicide (62/411; CI, 11.8–18.9); and adverse reaction drug (55/411; CI, 10.2–17.1) were most common.
The 3 most common reasons for exposure in adult patients was intentional abuse (n = 1622; CI, 23–25.1); intentional-suspected suicide (n = 1332; CI, 18.7–20.6); and adverse reaction drug (n = 977; CI, 13.7–15.3).
>Among adult cases, the vast majority of exposures were to fentanyl (69 %; n = 4656; CI, 67.9–70.1). Tables 4 and 5 list the 5 most common TDDS exposures in adult cases and the corresponding most frequent clinical effects and therapies. In the pediatric population, fentanyl was also the most common TDDS involved in exposures (24.7 %; n = 474; CI,22.8–26.7) but methylphenidate was a close second (21.9 %; n = 419; CI, 20–23.8). The most common TDDS involved in exposures did vary by age group. Table 6 and 7 demonstrates the 3 most common TDDS exposures in the pediatric age subgroups and the most frequent associated clinical effects and therapies.
Naloxone was given in a total of 2807 cases (2551 adults and 256 pediatric cases) and was the most common intervention in adults. Dilute/irrigate/wash, which includes simple dilution with oral liquids and wiping off the skin, was the most common pediatric intervention (n = 421). In cases where a fentanyl TDDS was reported to have been ingested naloxone was documented as being administered in 60 % (n = 2107) of the adult and 52 % (n = 169) of the pediatric cases. In total, decontamination was performed with activated charcoal in 559 cases (490 adult and 69 pediatric cases) and whole bowel irrigation in 244 cases (203 adult and 41 pediatric cases). All of the cases when decontamination was performed were cases where ingestion of the TDDS was reported.
A total of 97 deaths (91 adult and 6 pediatric) were reported over the study period. All deaths involved ingestion of the TDDS. Fentanyl was associated with 80 of the 91 adult deaths while clonidine was involved in 4 adult deaths and buprenorphine TDDS was implicated in 1 adult death. Of the 6 pediatric deaths, 5 cases involved fentanyl and 1 case involved ingestion of a selegiline TDDS. Three of the deaths occurred in children less than 2 years of age.
Discussion
While there continues to be research and development aimed at increasing the use of transdermal drug delivery systems (TDDS) for improved medication compliance and patient comfort, the toxicology of these products has not been well-characterized [18]. In theory, TDDS has the potential to cause significant toxicity due to the relatively large amount of drug they contain and their modified release properties, particularly when used inappropriately, such as being ingested [6, 7]. Furthermore, several of the drugs found in TDDS, such as fentanyl and clonidine, are potentially toxic at microgram doses yet contain milligram amounts of the drug. Thus, it could be reasonably expected that exposures to these agents may cause significant morbidity and even mortality. The best practice management of TDDS exposures, at both the bedside and via poison control centers, is currently not well-established and would vary widely depending on the nature of the exposure. Unintentional exposures may be difficult to detect unless a TDDS exposure is elicited on history or found on physical exam. In such cases, examination of the oral cavity and ensuring the patient’s skin is fully exposed may be critical in detecting the presence of a TDDS. Intentional exposures may be more obvious but have the potential to cause more morbidity and mortality. Based on this study of single-substance NPDS TDDS exposures, most non-fentanyl TDDS exposures can be expected to cause only minimal morbidity, even with reported ingestions of the TDDS. However, cases of possible fentanyl TDDS exposures appear to warrant aggressive care and consideration for decontamination and naloxone therapy.
Fentanyl was the most commonly encountered TDDS substance in this study and was associated with the most severe outcomes and deaths in adult cases. In pediatric cases, it was the second most commonly encountered substance but was associated with a majority of major outcomes and all deaths. This is consistent with previously published reports [8, 19]. In contrast, the buprenorphine TDDS, which was introduced in 2010, was associated with only 1 adult death, though the number of reported exposures were low. Exposures, including ingestions, of non-opioid TDDS products typically resulted in no effect or mild outcomes and no deaths.
Naloxone was frequently given in this study, again reflecting the impact of fentanyl TDDS, particularly fentanyl TDDS ingestions which accounted for 81 % (n = 2281/2813) of naloxone cases. From the available data, we could not comment on the necessity or efficacy of naloxone. Decontamination with activated charcoal and whole bowel irrigation (WBI) was performed at a relatively low rate, occurring in only 2 % (93/4506) and 1.4 % (17/1229) of adult and pediatric ingestion cases, respectively. The clinical efficacy of decontamination efforts or reason for not performing decontamination could not be ascertained from this study. Ingestion of a TDDS is not specifically mentioned in expert guidelines for the use of activated charcoal or WBI, but intuitively such an exposure could be amenable to decontamination [20]. By their very design, TDDS are modified release products and evidence suggests large amounts of the drug can be liberated when a TDDS is exposed to gastric and intestinal fluids [21]. There is a prior report of the successful use of WBI in TDDS ingestions, but this study suggests it is rarely performed [22]. Further studies into the use of decontamination for reported TDDS ingestions seem warranted.
Results from this study examined outcomes from exposures to currently available TDDS products, but it is important to note that the TDDS market is dynamic. Over the last several decades, there has been an increase in the number of available TDDS products [18]. This study demonstrated that single-substance exposures to TDDS were increasing annually in adults until 2011 but then declined over the next 4 years. By comparison, pediatric exposures peaked in 2007 but have slowly declined since. The reason behind these declines is unclear. It may be due to increasing familiarity with these products resulting in exposures not being reported. It could also be due to increased use of anti-tampering technology such as fentanyl TDDS using matrix technology vs. reservoir technology leading to less misuse. Pediatric exposures may be declining due to campaigns by the Food and Drug Administration promoting proper TDDS disposal. As TDDS technology evolves, new toxicological challenges may present themselves. For instance, micro-needle technology is being developed which uses micro-projections which form micro-channels in the stratum corneum to allow improved drug absorption that could potentially enhance absorption after ingestion [23].
While this study suggests overall TDDS exposures are associated with minimal morbidity and mortality, it does have several limitations. It is a retrospective review of NPDS data which is a compilation of data from the US poison control centers, and thus is subject to reporting bias and unverifiable information in some cases. In addition, there is the possibility that the reported cases may have been miscoded by the poison control center staff resulting in inaccurate documentation of medical outcomes. It is also possible that poison control centers were not contacted for TDDS exposures considered trivial by treating clinicians, or poison control centers may have only been contacted for severely poisoned patients. In addition, there is the possibility of incomplete data collection as individual centers or callers may not have been able to relay all clinically pertinent information as noted in our results where the age was unknown in 36 cases, but fortunately that accounted for < 1% of our total N. Finally, this study is of reported exposures, but the actual exposure or ingestions of these agents were not confirmed.
Conclusion
Both adult and pediatric single-substance TDDS exposures reported to the NPDS decreased over the duration of this study and typically resulted in no or mild clinical effects. However, exposures involving ingestion of the fentanyl TDDS resulted in higher rates of major and moderate medical outcomes and were associated with multiple deaths.
References
Magner LN. A history of medicine. Boca Raton: Taylor & Francis; 2005.
Transdermal scopolamine for motion sickness. Med Lett Drugs Ther. 1981;23(21):89–90.
Weber MA, Drayer JI, McMahon FG, Hamburger R, Shah AR, Kirk LN. Transdermal administration of clonidine for treatment of high BP. Arch Intern Med. 1984;144(6):1211–3.
National Drug Code Directory. Fda.Gov, 2019, https://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm. Accessed Feb 5 2019.
Schmid-Grendelmeier P, Pokorny R, Gasser UE, Richarz U. A comparison of the skin irritation potential of transdermal fentanyl versus transdermal buprenorphine in middle-aged to elderly healthy volunteers. Curr Med Res Opin. 2006;22(3):501–9.
Duragesic (fentanyl transdermal system) package insert, Janssen Pharmaceutical Products, L.P, Titusville, NJ USA 08560;
Catapress-TTS (clonidine) package insert, Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT USA 06877.
Woodall KL, Martin TL, McLellan BA. Oral abuse of fentanyl patches (Duragesic®): seven case reports. J Forensic Sci. 2008;53(1):222–5.
Rapko DA, Rastegar DA. Intentional clonidine patch ingestion by 3 adults in a detoxification unit. Arch Intern Med. 2003;163(3):367–8.
Carson HJ, Knight LD, Dudley MH, Garg U. A fatality involving an unusual route of fentanyl delivery: chewing and aspirating the transdermal patch. Leg Med (Tokyo). 2010;12(3):157–9.
Marquardt KA, Tharratt RS. Inhalation abuse of fentanyl patch. J Toxicol Clin Toxicol. 1994;32(1):75–8.
Marquardt KA, Tharratt RS, Musallam NA. Fentanyl remaining in a transdermal system following three days of continuous use. Ann Pharmacother. 1995;29(10):969–71.
Flannagan LM, Butts JD, Anderson WH. Fentanyl patches left on dead bodies --potential source of drug for abusers. J Forensic Sci. 1996;41(2):320–1.
Nelson L, Schwaner R. Transdermal fentanyl: pharmacology and toxicology. J Med Toxicol. 2009;5(4):230–41.
Zhang XC, Farrell N, Haronian T, Hack J. Postoperative anticholinergic poisoning: concealed complications of a commonly used medication. J Emerg Med. 2017;53(4):520–3.
Shemirani N, Tang D, Friedland DR. Acute auditory and vestibular symptoms associated with heat and transdermal lidocaine. Clin J Pain. 2010;26(1):58–9.
National Poison Data System. https://aapcc.org/data-system. Accessed May 24, 2019.
Watkinson AC, Kearney MC, Quinn HL, Courtenay AJ, Donnelly RF. Future of the transdermal drug delivery market--have we barely touched the surface? Expert Opin Drug Deliv. 2016;13(4):523–32.
Bakovic M, Nestic M, Mayer D. Death by band-aid: fatal misuse of transdermal fentanyl patch. Int J Legal Med. 2015;129(6):1247–52.
Position paper: whole bowel irrigation. J Toxicol Clin Toxicol. 2004;42(6):843–54. Review. Erratum in: J Toxicol Clin Toxicol. 2004;42(7):1000.
Arroyo Plasencia AM, Mowry J, Smith J, Quigley K. In vitro release of fentanyl from transdermal patches in gastric and intestinal fluid. Clin Toxicol (Phila). 2014;52(9):945–7.
Horowitz R, Mazor SS, Aks SE, Leikin JB. Accidental clonidine patch ingestion in a child. Am J Ther. 2005;12(3):272–4.
Moffatt K, Wang Y, Raj Singh TR, Donnelly RF. Microneedles for enhanced transdermal and intraocular drug delivery. Curr Opin Pharmacol. 2017;36:14–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
None.
Sources of Funding
None.
Additional information
Supervising Editor: Supervising Editor: Peter Chai, MD, MMS
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thornton, S.L., Darracq, M.A. Patch Problems? Characteristics of Transdermal Drug Delivery System Exposures Reported to the National Poison Data System. J. Med. Toxicol. 16, 33–40 (2020). https://doi.org/10.1007/s13181-019-00723-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-019-00723-0