Abstract
Introduction
Paracetamol dosing errors can cause acute liver injury, with potentially toxic doses only slightly above the therapeutic range. This study aimed to characterise unintentional paracetamol overdose reported to an Australian poisons centre, including time trends, demographics, types of dosing errors, and outcomes.
Methods
Records regarding paracetamol dosing errors for individuals aged ≥12 years were extracted from the New South Wales Poisons Information Centre database, January 2017 to June 2023. Data from 2021 underwent an in-depth screening of free text case notes to examine: dose, duration, products involved, reasons for ingestion and outcomes including hospitalisation, treatment, liver transplantations and deaths. Where possible, complete outcome data were obtained from medical records of New South Wales hospitalised cases in 2021.
Results
There were 14,380 exposures due to paracetamol dosing errors (predominantly self-administered, median age 43 years, 62.6% female), with an average yearly increase of 2.5% (95% CI 1.6–3.8%; p < 0.0001). The in-depth analysis of exposures recorded during 2021 revealed 1899 exposures (median age 46 years, 63.4% female) with 26.8% requiring hospitalisation. Immediate- and modified-release formulations were highly implicated. Multiple paracetamol-containing products were ingested in approximately 20% of exposures. Hospitalised exposures were associated with paracetamol use for dental pain and ingested higher doses for longer durations. Over half of those hospitalised (52%) were treated with the antidote (N-acetylcysteine), and 6% of exposures developed hepatotoxicity.
Conclusion
Paracetamol dosing errors continue to occur, with relatively high rates of hospitalisation and liver injury. Many hospitalisations involved use for dental pain. Possible preventative measures include ingredient name prominence and increased education on appropriate dosing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Paracetamol dosing errors are a common and increasing problem in Australia, occurring with high frequency in middle-aged women. | |
In 2021 over one-quarter required hospital assessment and 6% developed liver injury. | |
One-fifth of exposures involved use of multiple paracetamol-containing products, highlighting a potential role for labelling changes with active ingredient prominence. |
1 Introduction
Paracetamol (acetaminophen) is a first-line treatment for many painful conditions and is one of the most extensively used medicines worldwide [1]. In various countries, overdoses with paracetamol have either been stagnant or steadily increasing over time [2,3,4,5,6,7]. When taken at therapeutic doses the likelihood of adverse effects is minimal. However, overdoses, whether intentional or accidental, carry a risk of acute liver injury. It is still a leading cause of acute liver failure in many high-income countries [8]. Several countries have introduced restrictions and packaging changes in an effort to reduce this burden; however, these interventions have had variable and limited effectiveness [8].
Many studies focus on intentional overdose, possibly due to its increasing occurrence and greater severity. Fewer studies have explored the prevalence and outcomes of unintentional paracetamol overdose. These studies identify some common at-risk groups including those who are female, middle aged, chronic alcohol consumers and people with dental pain [9,10,11,12,13,14]. When compared to intentional ingestions this group appears to take lower doses but present to hospital later [10, 14].
Many consumers lack the understanding of how to appropriately use paracetamol, with some unaware that it can be toxic when taken in excess [15, 16]. Repeated supratherapeutic ingestions (RSTIs) occur when an individual inadvertently takes paracetamol for therapeutic purposes at a dose or interval that does not align with daily dose recommendations. Despite RSTI cases ingesting lower doses than intentional overdose cases, there is a similar risk of acute liver injury, often due to the delay in presentation until the development of symptoms [17]. The gold-standard treatment for paracetamol poisoning, N-acetylcysteine (NAC), is most effective if commenced within 8 h of overdose [18]. Delays in treatment increase the risk of hepatotoxicity, which when severe may be fatal or require liver transplantation.
In Australia, there has been a steady increase in both the number of overdoses and hospitalisations related to paracetamol, which in turn place a large strain on Australia’s health care system [19, 20]. There are several recent events that could impact rates of paracetamol overdose. The coronavirus disease 2019 (COVID-19) pandemic could have influenced rates of unintentional paracetamol overdose in many ways. Consumer behaviour (including stockpiling) and supply chain disruptions could impact paracetamol availability in the home. In addition, people may use excessive paracetamol for relief of pain and fever associated with COVID-19 infection. Of relevance, Australia had relatively low rates of COVID-19 infection until late 2021 when international borders were re-opened. In addition, combination analgesics containing codeine were up-scheduled (made Prescription Only) in 2018 and modified-release paracetamol was up-scheduled (made Pharmacist Only) in 2020. This has the potential to affect dosing errors with paracetamol as this legislation affected access to paracetamol/codeine combination products and modified-release paracetamol, respectively. Finally, in late 2019, updated guidelines were released for the management of paracetamol poisoning in Australia [21]. This resulted in minor changes to the RSTI referral thresholds, which may affect overdoses reported to poisons information centres (PICs).
Characterisation of the potential impact of these recent events is required to monitor the disease burden of paracetamol poisoning. In addition, improved understanding of the drivers of paracetamol overdose could provide avenues for harm minimisation. In this study we aimed to describe patterns and characteristics of stated or presumed dosing errors with paracetamol reported to Australia’s largest PIC in individuals aged ≥12 years.
Specifically, we aimed to:
-
i.
Characterise demographics, types and duration of errors, products involved, time of presentation, delays to presentation and reasons for ingestion.
-
ii.
Estimate changes over time for dosing errors (per 100,000 population), including any impact of recent events.
-
iii.
Describe outcomes including hospital admissions, the need for NAC, hepatotoxicity, liver unit referral and deaths.
2 Methods
2.1 Design and Setting
We performed a retrospective observational study of calls for paracetamol exposures to the New South Wales Poisons Information Centre (NSWPIC). Calls to the NSWPIC are made from healthcare professionals and members of the public with approximately 35% of these originating from outside of NSW. The NSWPIC handles approximately half of Australia’s annual 250,000 PIC calls and so it may be assumed that national numbers are approximately double what is reported. Australian PICs do not routinely conduct follow-up calls, and thus lack complete outcome data. To supplement this, where available, outcome data for patients presenting to hospital was documented via the electronic Medical Records (eMR) accessed through NSW hospitals. These records contain all data reported during an individual’s stay in hospital including but not limited to progress notes, blood test results and medications administered.
2.2 Eligibility Criteria, Data Extraction and Cleaning
Data on exposure calls which mention paracetamol (both as a single ingredient or combination product) coded as a ‘therapeutic error’ or ‘intentional: other’ (non-deliberate self-poisonings) were obtained from the NSWPIC database between January 2017 and June 2023. While the focus of this paper is unintentional overdose, exposure calls coded as ‘intentional: other’ were screened for inclusion as this code is sometimes used when the individual had therapeutic intent. These exposures will be classified as dosing errors throughout the paper. For the purposes of this paper only individuals aged ≥12 years were included. Dosing errors in those aged <12 are primarily driven by parent/caregiver factors and dosing is on a weight (mg/kg) basis. In addition, different products and formulations are used for the under 12-year age group. People aged ≥12 years are recommended to receive the same ‘adult’ regimen, and the adult formulations are marketed for ages ≥12 years.
Exposure calls were screened to review for inclusion, with data cleaning and re-coding used to capture data from free text fields. For the entire cohort, products and substances were screened to ensure they contained paracetamol. Both single ingredient and paracetamol-containing combination products were included. Exposures missing coded data for age and sex were screened and input if found to be available in the free text and corresponding age category bands were screened to ensure correct coding. Additionally, product names and substances accidentally coded together and multi-patient exposure calls were separated into distinct exposure call records. The NSWPIC has a coded disposition field, which includes a code ‘hospital refer’ (the patient was advised to attend hospital by the NSWPIC), and ‘in hospital’ (the person was already in hospital at the time of the call). For the purposes of reporting, individuals coded with either of these dispositions will henceforth be referred to as the ‘hospital group’. Any individuals whose management did not involve or require hospital attendance (including those already at or referred to a general practitioner for observation) will henceforth be referred to as the ‘community group’. The NSWPIC does not follow-up all cases to obtain outcome data; however, previous studies have shown that PIC advice is followed by 97.6% of callers [22].
Exposure calls taken during the year 2021 underwent an in-depth screen, with the data extracted into a preformatted spreadsheet. All exposure calls were screened by one author, with additional discussion with a second author if any details were unclear. The year 2021 was selected as it is assumed to provide the most recent full year of data least affected by COVID-19 infection waves and the exaggerated use of paracetamol (note: Australia had low COVID-19 infection rates throughout 2021 due to international border closures whilst the government enforced limits to the purchasing of paracetamol in 2020 amid panic buying). Exposure calls were included if there was an error in the dose or interval at which paracetamol was being taken for therapeutic purposes. Therapeutic dosing is generally considered as 1 g every 4 h for immediate-release formulations or 1.33 g every 6 h for modified-release formulations up to a maximum of 4 g per day. For a full definition see Supplementary Methods. Exposure calls were also included if the patient had abnormal results or symptoms of toxicity despite taking up to a maximum daily therapeutic dose due to these cases being managed as RSTIs according to Australian guidelines [21].
Exposure calls were excluded if the paracetamol ingestion was a deliberate self-poisoning, impulsive or due to opioid misuse. Ingestion of therapeutic doses of expired products or ingestion of products that did not contain paracetamol were also excluded. For a full list of exclusion criteria see Supplementary Methods.
From the 2021 call records, along with basic demographics, data extracted included the dose ingested within a 24-h period, the type and duration of the error, paracetamol products involved and their schedule status according to the Australian Poisons Standard, the reason for the ingestion, peak paracetamol, alanine aminotransferase (ALT) and international normalised ratio (INR) levels, treatment with NAC and outcomes such as liver transplantation or death. In Australia, NAC is given intravenously over 20 h. Double-dose NAC is given in some cases when the paracetamol concentration is very elevated. In addition, some patients receive extended NAC regimens, typically because they have prolonged raised paracetamol concentrations and/or signs of hepatotoxicity at the end of the standard course [21]. Need for NAC and need for extended regimens or double-dose NAC can be seen as proxies for risk and severity of the poisoning event.
Where available, an individual’s eMR was accessed for patients in NSW presenting to hospitals where the NSWPIC has eMR access. The NSWPIC does not have access to eMR for exposure calls that originated from outside NSW (approximately 35% of the NSWPIC calls), and for some NSW Local Health Districts where access was not granted. As such, follow-up information was not available for all exposures. Available medical records were screened for that particular presentation/admission period. Additional information extracted for this subset of cases was hospital admission and length of stay, time from the last dose until hospital presentation (or from presentation until accidental hospital administration, or from an in-hospital dosing error and contact with the NSWPIC), symptoms developed, detectable paracetamol on presentation, ALT levels on presentation and need for liver unit referral.
For an in-depth explanation of the screening, cleaning and recoding methods see Supplementary Methods. For a flow chart detailing the groups of cohorts analysed see Fig. 1.
Flow chart of cohorts analysed. Data were reported on an event (exposure) basis, rather than on a unique individual basis. Thus, one individual may have had several exposure events contributing to this dataset. Where an exposure event generated several phone calls, that event was only counted once in the analysis. A recall occurs when the NSWPIC receives follow-up calls regarding an exposure already recorded in the system. Overall call numbers count both exposure calls and recalls. NSWPIC New South Wales Poisons Information Centre
2.3 Temporal Trends and Statistical Analysis
To examine any temporal trends, we used weekly counts (consisting of 7-day intervals) and the average daily rate each month to examine time trends in exposure calls.
We also examined weekly trends (exposures by day-of-the-week). We postulated that poorly controlled pain may be more common on weekends when many general practitioners and dentists are closed. This could result in dosing errors with paracetamol.
We calculated yearly population-adjusted exposure rates using population data from the Australian Bureau of Statistics [23]. Since the NSWPIC takes 50% of the nation’s poisoning calls [24], the mid-year population was divided by two. Estimates are expressed as exposures/100,000 population/year. We examined the monthly changes in consultations using Poisson regression and added the logarithm of population as an offset. Results from the Poisson regression, derived from the estimated rate ratios, are presented as yearly changes (in %) accompanied with 95% confidence intervals (CI).
For statistical comparison of groups in 2021 based on hospital referral, we used the proportion test or the Wilcoxon Rank Sum test as appropriate including 95% CIs [25,26,27]. CIs were Bonferroni corrected for multiple comparisons with planned (simultaneous) significance level of 0.05. Statistical analysis was conducted using R statistical software (Version 4.2.1). For more details see Supplementary Methods.
Where there were missing data, this is specified in the tables. Missing data were not extrapolated.
2.4 Ethics
This study was approved by the Sydney Children’s Hospitals Network Human Research Ethics Committee (2021/ETH00165).
3 Results
3.1 Time Trend, Demographics and Exposure Characteristics
Between January 2017 and June 2023 there was a total of 24,648 exposures identified as dosing errors with paracetamol as a recorded substance. After restricting exposure calls to those aged ≥ 12 years, 14,384 exposures were identified; however, four records were excluded due to products which did not contain paracetamol. Therefore, a total of 14,380 paracetamol exposure calls were identified (with a combined total of 15,597 calls, Fig. 1). The majority of exposures involved adults (78.2%, n = 11,239). Where recorded, the median age was 43 years (exact age reported in 47.1% of exposures), and 8996 (62.6%) were female (Table 1). A summary of the patient demographics and details relating to their exposure can be found in Table 1.
There was an average of 42.4 (range 18–76) exposure calls per week (Fig. 2) and 6.1 (range 4.5–8.3) exposure calls per day for dosing errors with paracetamol. The yearly rate of exposures increased from 16.5 per 100,000 population in 2017 to 19.3 in 2022, showing a yearly 2.5% increase (95% CI 1.6–3.8%; p < 0.0001) on average (Fig. 3). Between 2017 and 2023 there was an average of 4.7 (range 4.1–5.0) exposures per 100,000 population per year that were either in hospital or referred to hospital (Fig. 3, note: 2023 rate has been estimated based on data from January to June only). The rate of exposures with a hospital-related disposition showed no increase (95% CI −0.2–3.3% per year; p = 0.07) while the rate of exposures with a community-related disposition increased by 2.9% (95% CI 1.4–4.4%; p < 0.0001) per year. The greatest frequency of exposure calls occurred on Sundays and Thursdays (Supplementary Fig. 1). The majority of all calls (62.6%, n = 9764) came from the community followed by 17.9% (n = 2794) from hospital doctors (Supplementary Table 1).
Weekly count of exposure calls to the NSWPIC about dosing errors with paracetamol, 2017–2023. Exposure calls were calculated as counts per week, consisting of 7-day intervals. The vertical dotted line represents codeine rescheduling to Prescription Only in February 2018. The dot-dash line represents the release of the updated Australian paracetamol poisoning guidelines in December 2019. The dashed line represents the COVID-19 pandemic declaration by the World Health Organisation in March 2020. The double-dot-dash line represents modified-release paracetamol rescheduling to Pharmacist Only in June 2020. The solid line represents the initial stages of international border reopening for Australia in November 2021, which was followed by a surge in COVID-19 cases. COVID-19 coronavirus disease 2019, NSWPIC New South Wales Poisons Information Centre
Exposures per 100,000 population per year split by disposition. Black bars represent exposures that had a hospital-related handling/disposition code. Grey bars represent exposures that had a community-related handling/disposition code. The 2023 rate has been doubled to provide an estimated yearly rate
The most common forms of paracetamol involved included immediate-release (51.8%, consisting of tablets, capsules, liquid and suppository formulations) and modified-release paracetamol (25.6%, consisting of controlled-release tablets only), both of which are single ingredient formulations. This was closely followed by combinations with opioids (primarily consisting of paracetamol and codeine with just 0.7% paracetamol and tramadol) and cold and flu preparations (which often include other ingredients including decongestants and sedating antihistamines). Multiple paracetamol-containing products (which may include more than one brand of the same category) were taken in 2816 (19.6%) exposures with spikes observed in 2019, 2021 and 2022, the greatest of which occurred in 2022 (Supplementary Fig. 2). Most exposures were advised to stay at home and were asymptomatic (Table 1).
3.2 Characteristics of 2021 Dosing Errors, Stratified by Need for Hospital Referral
Prior to in-depth screening, there were 2259 exposures recorded for the year 2021, which was reduced to 1899 exposures meeting inclusion criteria after screening. Basic exposure characteristics were similar to that recorded for the entire cohort (Table 1). Of these exposures 1204 (63.4%) were females and the median age was 46 years. Immediate- and modified-release paracetamol were again highly implicated and almost one-quarter (22.0%, n = 418) of exposures involved multiple paracetamol-containing products. Similar to the entire cohort, most exposures were advised to stay at home and were asymptomatic (Table 1); however, nearly one-third (30.5%, n = 580) were referred to or had the initial exposure call originate from the hospital.
The 2021 exposures were subject to in-depth screening, and it was determined that over one-quarter of exposure calls (26.8%, n = 509) required hospital management with paracetamol as the primary presenting problem (the remaining 71 hospital presentations were deemed not primarily due to paracetamol, despite a paracetamol error occurring). We compared characteristics of the community group versus the hospital group, to identify possible drivers of more severe cases. Immediate-release and modified-release were the most common formulations used in the community group whilst immediate-release and combinations with opioids were the two most common formulations for those in the hospital group (Table 2). The use of multiple paracetamol-containing products was consistent across both the community and hospital groups. Products classified as Schedule 2/Unscheduled were primarily involved across both groups; however, Schedule 2/Unscheduled products accounted for a higher proportion within the hospital group (Table 2).
A variety of indications were listed for the use of paracetamol with some individuals citing more than one indication. The majority did not have an indication documented in the PIC call record/medical record (Table 2); however, within each individual group the top 5 indications differed. Where known, the top 5 indications for those in the community group included cold/flu symptoms/COVID-19, migraine/headache, none (medicines given to the wrong patient), dental pain/dental work and unspecified pain. A full list of indications recorded in the community and hospital groups is available in Supplementary Tables 2 and 3, respectively. For the hospital group, the top indications were dental pain/dental work, migraine/headache, unspecified pain, back pain and cold/flu symptoms/COVID-19. Proportions were significantly different across all top indications besides migraine/headache, with the biggest difference for dental pain/dental work (21% of the hospital group vs 4% of the community group) (Table 2). Of the 166 unique exposures who were treating dental pain/dental work, approximately 60.8% used only immediate-release and/or modified-release paracetamol. The remaining 39.2% used formulations of paracetamol in combination with other ingredients such as opioids or anti-inflammatories (± single ingredient paracetamol).
The community group had a greater proportion of once-off errors (Table 2) with the greatest sources of error being an acute incorrect dose (35.3%) or an acute interval error (39.3%). In the hospital group, over half of the exposures were complex RSTIs and as expected a much greater proportion had doses associated with risk of acute liver injury based on the Australian and New Zealand guidelines [21] (Table 2). There were only 5 exposures that had no apparent error but developed symptoms of toxicity, all of which were in the hospital group. Only 11 exposures were reported to be due to an individual receiving a therapeutic dose despite needing a dose reduction for their weight.
The dose of paracetamol and duration of error was significantly higher in the hospital group compared to the community group (Table 2). The median dose per 24-h period for the hospital group was 8.0 g with a median duration of error of 2.0 days. This was more than double the median dose of the community group and double their median duration of error (Table 2).
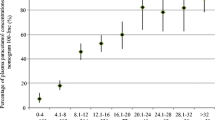
Overall, exposures with an acute dose and/or interval error had lower median doses per 24-h period compared to exposures with a repeated dose and/or interval error or complex RSTIs (Fig. 4). The highest median daily dose was 12.0 g (IQR 9.0–20.0 g) for exposures with a repeated dose and interval error.
3.3 Treatment and Outcomes of Exposures Requiring Hospital Referral/Management, All 2021 Cases
There were 215 (42.2%) exposures that required treatment with NAC of which a small subset required an extended regimen (Table 3). For an explanation of the NAC treatment regimen see Supplementary Methods. It was unknown whether NAC was administered in 194 (38.1%) exposures (as eMR information was only accessible for a subset of cases, see below). Thus, the proportion who received NAC was likely higher. The median recorded peak paracetamol level was 20 mg/L. The median recorded peak ALT level was 51.0 U/L. Approximately one-half of exposures had elevated ALT (>50 U/L), while 26 (8.4%) had a peak ALT >1000 U/L and 2 had an ALT >10,000 U/L. The median recorded peak INR level was 1.1. Only 14 exposures (10.8%) had a peak INR ≥2. No deaths or occurrence of a liver transplantation were recorded for this group.
3.4 Further Detail on 2021 Cases Where Complete Outcome Information was Obtained
The NSWPIC had access to complete medical records for a subset of hospitals in the state of NSW only (comprising 12 out of 15 Local Health Districts and 39 out of 64 hospitals). In 2021 in NSW, there were 193 exposures reported to the NSWPIC that were managed in hospital. Of these exposures 73 had an eMR that was not accessible due to either missing patient identifying details in the PIC database, or lack of access to the hospital files at that Local Health District. Therefore 120 exposures were included that allowed for complete follow-up data (Table 4). The median age was 41.5 years and 59 (49.2%) were female. Immediate-release paracetamol, combinations with opioids and modified-release paracetamol were highly implicated (Table 4) with multiple paracetamol-containing products used in 27 (22.5%) exposures.
The median time between the last dose of paracetamol and presentation to hospital was 3.0 h (IQR 1.4–7.1, maximum 70 h, reported in 75.8% of exposures). In 12 (10.0%) exposures, either before the paracetamol dosing error was identified by clinicians or before the patient had been medically cleared, an additional therapeutic dose of paracetamol was given to the patient in hospital (Table 4). There were 2 (1.7%) exposures who had been given the dosing error whilst in hospital (iatrogenic errors).
A total of 62 (51.7%) exposures received NAC with five (4.2%) receiving double-dose NAC and 11 (9.2%) receiving an extended regimen. Symptoms of toxicity were reported for over half of the exposures (Table 4).
The majority of exposures had a detectable paracetamol concentration on presentation (Table 4). Almost half had an abnormal ALT (> 50 U/L), while 7 exposures (5.9% of those with complete outcome data) had an ALT > 1000 U/L. There were no deaths or liver unit transfers in the 120 exposures with complete outcome information and there were 8 patients admitted to the intensive care unit (ICU). The majority (57.5%) were admitted to hospital for paracetamol overdose; however, the overall median length of stay was short, at 12.9 h (Table 4).
4 Discussion
This study of over 14,000 cases underscores the ongoing burden of paracetamol dosing errors in Australia, despite efforts taken to minimise overdose [28, 29]. To our knowledge, this is the largest cohort of paracetamol dosing errors in the literature. There appears to have been a gradual increase in frequency of exposures over the study period. Middle-aged women made up the majority of exposures reported to the NSWPIC. Immediate-release forms of paracetamol were most implicated, which is consistent with their broad availability. One-fifth of exposures involved multiple paracetamol-containing products, suggesting confusion regarding total daily dose allowances for paracetamol. When compared to the community group, people in the hospital group had taken larger doses for longer periods. This is consistent with the dose-dependent nature of paracetamol hepatotoxicity and risk assessment. Dental pain was associated with the hospital group, highlighting the need for better pain management for dental conditions and consumer education. For exposures where medical outcome data were available, we found that hospital admission and minor ALT rises were common. Significant hepatotoxicity was rarer (occurring in approximately 6% of exposures). The Therapeutic Goods Administration (TGA, Australia’s drug regulator) attempts to monitor the safety of medicines and medical devices through the Database of Adverse Event Notifications (DAEN). However, as reporting is not mandatory, suspected adverse events are extremely underreported with only 155 cases mentioning paracetamol-containing products reported on the DAEN during 2023 [30]. Therefore, PIC records form an important source of data to supplement spontaneous adverse drug reaction reporting schemes as they collect many more cases and greater detail.
We documented a gradual increase of exposures over the study period. There was no clear day-of-the-week trend observed; Sunday and Thursday had the greatest number of exposure calls. This does not support the proposition that lack of weekend access to health care (e.g., dentists or medical practitioners) drives these events in those with poorly managed pain. We were also interested in the potential impact of codeine rescheduling and COVID-19 on the frequency of dosing errors. The reduction of the use of multiple paracetamol-containing products in 2018 and 2020 appeared to coincide with the rescheduling of codeine to Prescription Only and modified-release paracetamol to Pharmacist Only, respectively [28, 31]. It is possible that restricting access to these products reduced the risk of multiple paracetamol-containing product dosing errors. However, these scheduling changes did not appear to cause a drop in the daily rate or weekly count of overall exposure calls during those years. The subsequent rise in multiple paracetamol-containing product errors through to 2022 could be driven by the COVID-19 pandemic (international borders re-opened in late 2021), which resulted in paracetamol stockpiling and more people using paracetamol to treat COVID-19 symptoms. The release of the updated paracetamol poisoning guidelines did not appear to affect the rate of exposure calls.
There are over 600 paracetamol products currently listed under the Australian Register of Therapeutic Goods. The majority of exposures involved easily accessible products; however, even those which require a prescription (combinations with opioids with or without other ingredients) resulted in dosing errors. The presence of various formulations including those marketed to target a specific condition (e.g., cold/flu preparations) can increase confusion about appropriate dosing with paracetamol. This is evidenced by multiple paracetamol-containing products being implicated in approximately one-fifth of exposures in this study. The recent decision by the TGA to reduce paracetamol pack sizes in 2025 in an effort to reduce harm from intentional overdose [32] is unlikely to affect the frequency of accidental dosing errors. However, reducing pack sizes may reduce accidental overdose size and severity. Another potential strategy is active ingredient prominence [33], which involves increasing the visibility of the active ingredient on the packaging of a medication to allow for clearer identification. This may reduce the rate of dosing errors involving multiple paracetamol-containing products [33], although labelling changes in Canada did not reduce hospital admissions for accidental paracetamol overdose [6]. Similarly, a simulated scenario with consumers suggested warning labels may not reduce the likelihood of ingesting excessive doses [34]. Increased consumer education may also be warranted to improve knowledge of the maximum daily dose of paracetamol [34]; however, studies showing the impact of education on preventing poisonings are scarce.
Some dosing errors likely result from a lack of efficacy of paracetamol, as individuals could take increased doses or shorten the dosing interval due to persistent pain. A recent systematic review found paracetamol to have moderate to high quality evidence of effectiveness in only 4 out of 44 painful conditions [35]. Paracetamol was shown to be effective for dental procedures; however, the evidence was of poor quality and no data were reported for dental pain [35]. We found dental pain to be the top indication for the hospital group that took a median dose almost double that of a normal daily therapeutic dose. Of all the exposures using paracetamol for dental pain, almost two-thirds used paracetamol alone, which also suggests it is not highly effective for dental pain especially as a sole treatment. Others have also found patients with dental pain are at an increased risk of accidental paracetamol overdose [12]. Just over half of the Australian population have private health insurance [36] meaning a significant proportion of people pay out-of-pocket for dental services. This, in combination with rising costs, may result in delayed dental visits [37] and greater reliance on analgesics such as paracetamol; thus, increasing the potential for dosing errors.
Exposures in the community group were mostly acute dosing errors, which are unlikely to develop severe outcomes. In contrast, most exposures in the hospitalised group consisted of complex repeated supratherapeutic dosing errors. Approximately half of this group received treatment with NAC. The median peak ALT level and hepatotoxicity (6%) were lower than most other studies (6–62%) [9,10,11, 13, 14, 38]. However, previous studies primarily included admitted patients or toxicology unit referrals and are not directly comparable. Only 60% of our exposure group with complete outcome data had a detectable paracetamol level on presentation. Similar values have been reported in other studies [11] and this is likely due to delayed hospital presentations allowing for paracetamol to be metabolised. Typically, in these cases signs of hepatotoxicity prompt the need to seek help and subsequent presentation to hospital.
There are several limitations to this study. Firstly, these results do not capture all dosing errors with paracetamol occurring in NSW or Australia. This dataset comprises only exposures with calls made to the NSWPIC by members of the public or clinicians. There are detailed clinical guidelines for the management of paracetamol overdose in Australia, which may make clinicians less likely to call PICs for advice. Additionally, some hospitals have inpatient toxicology units and do not call PICs for advice. The NSWPIC takes approximately 50% of the nation’s PIC calls, and thus the data for Australia would likely be roughly double those reported here [39]. Due to this being a retrospective observational study, the data rely on the accuracy of an individual’s recall of dose, duration, and products. In addition, documentation from calls to the NSWPIC sometimes had missing data (e.g., exact age). Due to the volume of exposure calls, it was only feasible to conduct in-depth screening for a 1-year subset of the data for this study. Thus, data quality is best for the 2021 data. The vast majority of exposures in this study lack complete outcome data, as Australian PICs are not resourced to conduct follow up for all cases. For patients advised to present to hospital, there is no way of confirming they followed the NSWPIC advice unless the NSWPIC received a subsequent call from the hospital. Similarly, for patients in the emergency department, rates of admission, treatment with acetylcysteine, ICU admission, and hepatotoxicity are impossible to ascertain for the whole dataset. We were able to obtain complete outcome data for the subset of 2021 exposure calls for which we had eMR access. However, this was limited to a subset of exposures from one state only, and thus the overall number with follow-up data is small. This means that rare outcomes (liver unit admission, liver transplant, death) from other exposures may have been missed. Finally, the process of data abstraction/modification was done by a single investigator, although any unclear records were then discussed with a second reviewer. However, despite these limitations, the much larger sample size compared to previous studies allows us to provide a far more representative and in-depth assessment of demographics, reasons for errors, products involved and time trends.
5 Conclusion
Dosing errors with paracetamol continue to occur in Australia. Targeted efforts focusing on dosing errors could use ingredient name prominence and consumer education on the potential for toxicity, appropriate dosing and the use of multiple paracetamol-containing products. Forthcoming pack size restrictions aimed at reducing harm from intentional paracetamol overdose may reduce the severity of dosing errors, but this requires evaluation. Better pain management might also reduce dosing errors. This may include use of other analgesics, combinations, and non-pharmaceutical strategies. Dental pain should be of particular focus as it was strongly associated with larger overdoses.
References
Brune K, Renner B, Tiegs G. Acetaminophen/paracetamol: a history of errors, failures and false decisions. Eur J Pain. 2015;19:953–65. https://doi.org/10.1002/ejp.621.
Hopkins AG, Spiller HA, Kistamgari S, et al. Suicide-related over-the-counter analgesic exposures reported to United States poison control centers, 2000–2018. Pharmacoepidemiol Drug Saf. 2020;29:1011–21. https://doi.org/10.1002/pds.4997.
Gedeborg R, Svennblad B, Holm L, et al. Increased availability of paracetamol in Sweden and incidence of paracetamol poisoning: using laboratory data to increase validity of a population-based registry study. Pharmacoepidemiol Drug Saf. 2017;26:518–27. https://doi.org/10.1002/pds.4166.
Friðriksdóttir ÞA, Jónsdóttir F, Snook CP, et al. Paracetamol poisoning: a population-based study from Iceland. Scand J Gastroenterol. 2021;56:832–9. https://doi.org/10.1080/00365521.2021.1921254.
Tong HY, Medrano N, Borobia AM, et al. Hepatotoxicity induced by acute and chronic paracetamol overdose in adults. Where do we stand? Regul Toxicol Pharmacol. 2015;72:370–8. https://doi.org/10.1016/j.yrtph.2015.05.011.
Antoniou T, Guan Q, Martins D, et al. Impact of acetaminophen product labelling changes in Canada on hospital admissions for accidental acetaminophen overdose: a population-based study. Can Med Assoc J. 2022;194:E542–8. https://doi.org/10.1503/cmaj.210842.
Casey D, Geulayov G, Bale E, et al. Paracetamol self-poisoning: Epidemiological study of trends and patient characteristics from the multicentre study of self-harm in England. J Affect Disord. 2020;276:699–706. https://doi.org/10.1016/j.jad.2020.07.091.
Chidiac AS, Buckley NA, Noghrehchi F, et al. Paracetamol (acetaminophen) overdose and hepatotoxicity: mechanism, treatment, prevention measures, and estimates of burden of disease. Expert Opin Drug Metab Toxicol. 2023;19:297–317. https://doi.org/10.1080/17425255.2023.2223959.
Alhelail MA, Hoppe JA, Rhyee SH, et al. Clinical course of repeated supratherapeutic ingestion of acetaminophen. Clin Toxicol. 2011;49:108–12. https://doi.org/10.3109/15563650.2011.554839.
Schiødt FV, Rochling FA, Casey DL, et al. Acetaminophen toxicity in an urban county hospital. N Engl J Med. 1997;337:1112–8. https://doi.org/10.1056/NEJM199710163371602.
Egan H, Isbister GK, Robinson J, et al. Retrospective evaluation of repeated supratherapeutic ingestion (RSTI) of paracetamol. Clin Toxicol. 2019;57:703–11. https://doi.org/10.1080/15563650.2018.1547829.
Vogel J, Heard KJ, Carlson C, et al. Dental pain as a risk factor for accidental acetaminophen overdose: a case-control study. Am J Emerg Med. 2011;29:1125–9. https://doi.org/10.1016/j.ajem.2010.08.006.
Daly FFS, O’Malley GF, Heard K, et al. Prospective evaluation of repeated supratherapeutic acetaminophen (paracetamol) ingestion. Ann Emerg Med. 2004;44:393–8. https://doi.org/10.1016/j.annemergmed.2004.05.005.
Gyamlani GG, Parikh CR. Acetaminophen toxicity: suicidal vs accidental. Crit Care. 2002;6:155. https://doi.org/10.1186/cc1475.
Hornsby LB, Whitley HP, Hester EK, et al. Survey of patient knowledge related to acetaminophen recognition, dosing, and toxicity. J Am Pharm Assoc (2003). 2010;50:485–9. https://doi.org/10.1331/JAPhA.2010.08175.
Wolf MS, King J, Jacobson K, et al. Risk of unintentional overdose with non-prescription acetaminophen products. J Gen Intern Med. 2012;27:1587–93. https://doi.org/10.1007/s11606-012-2096-3.
Saccomano SJ. Acute acetaminophen toxicity in adults. Nurse Pract. 2019;44:42–7. https://doi.org/10.1097/01.NPR.0000586020.15798.c6.
Hodgman MJ, Garrard AR. A review of acetaminophen poisoning. Crit Care Clin. 2012;28:499–516. https://doi.org/10.1016/j.ccc.2012.07.006.
Cairns R, Brown JA, Wylie CE, et al. Paracetamol poisoning-related hospital admissions and deaths in Australia, 2004–2017. Med J Aust. 2019;211:218–23. https://doi.org/10.5694/mja2.50296.
Sood S, Howell J, Sundararajan V, et al. Paracetamol overdose in Victoria remains a significant health-care burden. J Gastroenterol Hepatol. 2013;28:1356–60. https://doi.org/10.1111/jgh.12196.
Chiew AL, Reith D, Pomerleau A, et al. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med J Aust. 2020;212:175–83. https://doi.org/10.5694/mja2.50428.
Huynh A, Cairns R, Brown JA, et al. Health care cost savings from Australian Poisons Information Centre advice for low risk exposure calls: SNAPSHOT 2. Clin Toxicol. 2020;58:752–7. https://doi.org/10.1080/15563650.2019.1686513.
Australian Bureau of Statistics. National, state and territory population. https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population. Accessed 12 May 2024.
Huynh A, Cairns R, Brown JA, et al. Patterns of poisoning exposure at different ages: the 2015 annual report of the Australian Poisons Information Centres. Med J Aust. 2018;209:74–9. https://doi.org/10.5694/mja17.01063.
Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–12. https://doi.org/10.1080/01621459.1927.10502953.
Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–90. https://doi.org/10.1002/(SICI)1097-0258(19980430)17:8%3c873::AID-SIM779%3e3.0.CO;2-I.
Hollander M, Wolfe DA. Nonparametric statistical methods. 1st ed. New York: Wiley; 1973.
Therapeutic Goods Administration. 1.4. Final decision in relation to paracetamol (modified release). 2019. https://www.tga.gov.au/resources/publication/scheduling-decisions-final/notice-final-decision-amend-or-not-amend-current-poisons-standard-august-2019/14-final-decision-relation-paracetamol-modified-release. Accessed 31 August 2022.
NPS MedicineWise. Rates of paracetamol overdose continue to rise in Australia. 2019. https://www.nps.org.au/news/paracetamol-overdoses-rise. Accessed 6 May 2024.
Therapeutic Goods Administration. Database of Adverse Event Notifications (DAEN) - medicines. https://daen.tga.gov.au/medicines-search/. Accessed 9 May 2024.
Therapeutic Goods Administration. Final decision on re-scheduling of codeine: frequently asked questions. 2016. https://www.tga.gov.au/final-decision-re-scheduling-codeine-frequently-asked-questions. Accessed 15 November 2023.
Therapeutic Goods Administration. TGA makes final decision to reduce paracetamol pack sizes. 2023. https://www.tga.gov.au/news/media-releases/tga-makes-final-decision-reduce-paracetamol-pack-sizes. Accessed 15 November 2023.
Therapeutic Goods Administration. Active ingredient prominence. 2013. https://www.tga.gov.au/labelling-and-packaging-practices-summary-some-evidence/active-ingredient-prominence. Accessed 26 October 2023.
Rotella J-A, Wong A, Howell J, et al. High-visibility warning labels on paracetamol-containing products do not prevent supratherapeutic ingestion in a simulated scenario. Clin Toxicol. 2015;53:935–40. https://doi.org/10.3109/15563650.2015.1098657.
Abdel Shaheed C, Ferreira GE, Dmitritchenko A, et al. The efficacy and safety of paracetamol for pain relief: an overview of systematic reviews. Med J Aust. 2021;214:324–31. https://doi.org/10.5694/mja2.50992.
Australian Prudential Regulation Authority. Quarterly private health insurance statistics December 2023. 2024. https://www.apra.gov.au/sites/default/files/2024-02/Quarterly%20Private%20Health%20Insurance%20Statistics%20December%202023_0.pdf. Accessed 5 May 2024.
Australian Institute of Health and Welfare. Oral health and dental care in Australia. https://www.aihw.gov.au/reports/dental-oral-health/oral-health-and-dental-care-in-australia/contents/costs. Accessed 5 May 2024.
Myers RP, Shaheen AAM, Li B, et al. Impact of liver disease, alcohol abuse, and unintentional ingestions on the outcomes of acetaminophen overdose. Clin Gastroenterol Hepatol. 2008;6:918–25. https://doi.org/10.1016/j.cgh.2008.02.053.
Buckley N, Calear A, Cairns R, et al. Independent expert report on the risks of intentional self-poisoning with paracetamol. 2022. https://www.tga.gov.au/sites/default/files/2022-09/paracetamol_report_final.pdf. Accessed 23 September 2022.
Acknowledgements
The authors wish to thank all staff at the New South Wales Poisons Information Centre whose work contributed to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. RC is supported by an NHMRC Investigator Grant (ID: 1196516), NAB is supported by an NHMRC Investigator Grant (ID: 2007726). RC is the recipient of an untied educational grant from Reckitt Benckiser to study over-the-counter medicine poisonings, which includes a PhD stipend for ASC.
Conflicts of interest
ASC is supported by a PhD scholarship funded by Reckitt Benckiser, as part of an untied educational grant awarded to RC. RC has also received conference speaker fees/honoraria from Reckitt Benckiser and The Pharmacy Guild of Australia. These funders had no role in the design, conduct, or interpretation of the study’s findings. NAB is an Editorial Board member of Drug Safety. NAB was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. FN has no conflicts of interest to be declared.
Availability of data and material
The data supporting this study’s findings are not publicly available due to the sensitive nature of the contents. Select data is available on request to the NSW Poisons Information Centre. For access, please email rose.cairns@sydney.edu.au
Ethics approval
This study was approved by the Sydney Children’s Hospitals Network Human Research Ethics Committee (2021/ETH00165).
Consent for participation
Not applicable.
Consent for publication
Not applicable.
Code availability
R code used for the Wilcoxon Rank Sum test is available on request. For access, please email firouzeh.noghrehchi@sydney.edu.au
Author contributions
RC and NAB contributed to the design of the study. Data cleaning and extraction was performed by ASC. ASC and FN performed the data analysis. The first draft of the manuscript was written by ASC and all authors commented on subsequent versions of the manuscript. All authors read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chidiac, A.S., Buckley, N.A., Noghrehchi, F. et al. Paracetamol Dosing Errors in People Aged 12 Years and Over: An Analysis of Over 14,000 Cases Reported to an Australian Poisons Information Centre. Drug Saf (2024). https://doi.org/10.1007/s40264-024-01472-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s40264-024-01472-y