Abstract
Tidal wetlands are important blue carbon reservoirs, but it is unclear how sea-level rise (SLR) may affect carbon cycling and soil microbial communities either by increased inundation frequency or via shifting plant species dominance. We used an in-situ marsh organ experiment to test how SLR-scenarios (0, + 7.5, + 15 cm) and vegetation treatments (Spartina alterniflora, Spartina patens, Phragmites australis, unvegetated controls) altered CO2 fluxes (net ecosystem exchange, ecosystem respiration), soil carbon mineralization rates, potential denitrification rates, and microbial community composition. Increasing inundation frequency with SLR treatments decreased the carbon sink strength and promoted carbon emissions with + 15-cm SLR. However, SLR treatments did not alter soil chemistry, microbial process rates, or bacterial community structure. In contrast, our vegetation treatments affected all carbon flux measurements; S. alterniflora and S. patens had greater CO2 uptake and ecosystem respiration compared to P. australis. Soils associated with Spartina spp. had higher carbon mineralization rates than P. australis or unvegetated controls. Soil bacterial assemblages differed among vegetation treatments but shifted more dramatically over the three-month experiment. As marshes flood more frequently with projected SLR, marsh vegetation composition is predicted to shift towards more flood-tolerant S. alterniflora, which may lead to increased CO2 uptake, though tidal marsh carbon sink strength will likely be offset by increased abundance of unvegetated tidal flats and open water. Our findings suggest that plant species play a central role in ecosystem carbon dynamics in vegetated tidal marshes undergoing rapid SLR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tidal marshes straddle the boundary between terrestrial and marine environments and are increasingly targeted for conservation and restoration efforts to mitigate climate change (Pendleton et al. 2012; Watson et al. 2016; Duggan-Edwards et al. 2020). As blue carbon ecosystems (McLeod et al. 2011), tidal marshes have high carbon sequestration rates because productive graminoids and anoxic soils promote organic matter accumulation (Kirwan and Megonigal 2013). Marshes historically transgressed inland in response to sea level rise (SLR) but accelerated SLR coupled with coastal development limits landward migration and promotes marsh submergence (Enwright et al. 2016; Watson et al. 2017). Increased salinity and extended hydroperiods associated with SLR catalyze replacement of high elevation marsh species with low marsh vegetation and ultimately the vegetation dies off (Warren and Niering 1993; Field et al. 2016). How SLR-induced salinity and inundation interactively alter soil-based and plant-mediated carbon processes remains unclear.
Sea level rise increases tidal inundation frequency and salinity of coastal wetlands, both of which can directly affect rates of soil carbon cycling (Luo et al. 2019; Helton et al. 2019). Increased flooding alone tends to decrease rates of soil carbon mineralization (i.e., microbially mediated transformation of soil organic carbon to CO2); inundation promotes reducing conditions that constrain rates of organic carbon breakdown in marsh soils. Increased tidal flooding though is often coupled with marsh exposure to higher concentrations of marine salts. Elevated sulfate reduction rates associated with marine salt exposure have been linked to increased soil C mineralization rates from wetland soils (Chambers et al. 2011; Weston et al. 2011; Neubauer et al. 2013; Simon et al. 2017), although soil mineralization responses are not consistent across salinization studies (Herbert et al. 2015; Luo et al. 2019).
The salinity and hydrologic gradients that directly affect soil carbon cycling also organize tidal marsh plant communities (Bertness 1991), which may differentially respond to SLR and alter marsh carbon cycling. Plant productivity and biomass allocation of dominant species underpin carbon dynamics, as plants sequester atmospheric CO2 and alter the availability of terminal electron acceptors and organic carbon for microbial metabolism, with soil bacterial communities strongly aligning with vegetation zones (Rietl et al. 2016; Barry et al. 2022). Increased salinity associated with tidal inundation reduces plant productivity by increasing ionic stress, accumulating phytotoxic forms of iron, manganese, and sulphur and inhibiting nutrient uptake (Weston et al. 2011; Tobias and Neubauer 2019). SLR-induced reductions in biomass production can in turn limit rhizospheric oxidation via porous wetland plant tissues and allocation to root exudates, labile carbon compounds deposited by roots that stimulate microbial metabolism (Freeman et al. 2001; Farrar et al. 2003; Sutton-Grier and Megonigal 2011). Though differences in carbon cycling among salt marsh vegetation zones have been documented (Martin and Moseman-Valtierra 2015; Barry et al. 2022), greater resolution on how tidal flooding and vegetation independently and interactively affect salt marsh carbon fluxes is needed to inform modeling efforts.
To disentangle two key drivers of salt marsh carbon cycling, inundation frequency and plant-mediated inputs, we manipulated elevation and plant composition using a marsh organ experiment (sensu Morris 2007) to investigate the effect of projected flooding associated with SLR scenarios ~ 10 and 20 years in the future. We predicted that (i) increased inundation associated with SLR treatments would reduce net ecosystem exchange, as stress-induced reductions in plant productivity and CO2 uptake will be greater than increased ecosystem respiration (soil microbial + plant respiration); (ii) low-elevation, salt-tolerant plants will assimilate more carbon than plants adapted to higher elevations under accelerated SLR scenarios; (iii) manipulating plant species dominance and elevation will alter the soil microbial community.
Methods
Study site- We established our experiment within the primary tidal creek of Barn Island Wildlife Management Area Impoundment 3 (Stonington, Connecticut, USA; 41°20’27.4"N 71°51’56.4"W), as it had a consolidated, level bottom and all three target vegetation species (Spartina alterniflora, S. patens, Phragmites australis) present within < 50 m. Tidal creek salinity during site selection (March 2018) was 3–9 ppt, but increased during the 2018 growing season (16–26 ppt).
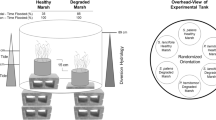
Experimental approach- To test how flooding associated with SLR affects plant and biogeochemical responses, we employed a full-factorial marsh organ experiment, which simulates various elevations at a single location to isolate the effect of hydroperiod (Morris 2007). We tested five vegetation (S. alterniflora, S. patens, P. australis, unvegetated low marsh, unvegetated high marsh) and three SLR scenario (0 cm, control, same elevation and hydroperiod as 2018; +7.5 cm, projected hydroperiod of 2028; and + 15 cm, projected hydroperiod of 2038) treatments, assuming a static marsh surface elevation. Each treatment combination was replicated five-fold; five wooden-framed platforms each contained 15 (5 vegetation x 3 SLR treatments) mesocosms, which were constructed from 15.2-cm diameter PVC pipes cut to varying heights to simulate different flooding scenarios (Fig. 1).
To simulate ecologically relevant SLR scenarios, we first quantified relative elevation differences among vegetation zones using an auto level and stadia rod at 15 randomly stratified locations (three vegetation zones x five random points); S. alterniflora locations averaged 10-cm lower than both S. patens and P. australis. Therefore, we cut PVC pipes for “low marsh” treatments (S. alterniflora, low marsh unvegetated) 10-cm shorter than “high marsh” treatments (S. patens, P. australis, high marsh unvegetated). We also manipulated the lengths of pipes to test three SLR-scenarios (shorter pipes: more frequently flooded). The 0-SLR cm (2018) treatment pipes were the tallest and were installed so that their tops were level with the mean elevation of the surrounding low or high marsh; we reduced the pipe length by 7.5 and 15 cm to simulate projected hydroperiods in 2028 and 2038, respectively, based on the Global Climate Model Maximum SLR estimates for the region (Clough et al. 2015).
The five platforms (each 1.5 × 1 m) were installed within a 10-m stretch of the tidal creek, separated by ~ 1 m (Fig. 1). We randomly oriented platforms (0-360°) to reduce potential bias from shading and randomized the 15 treatment combinations within each platform. Pipes were pounded vertically into tidal creek sediment to target elevations. Vegetation treatments were initiated on 21 May 2018. We excavated ~ 10 × 10 × 10-cm sections of soil (including belowground biomass) from areas dominated (> 50% cover) by the three target species; we visually equalized aboveground biomass and recorded maximum stem height and density of all transplants. Our preliminary analysis of tidal creek sediment suggested poor drainage due to high clay content, so we amended it with commercially purchased sand and Sphagnum peat moss to achieve drainage between tidal cycles. We filled the bottom 20 cm of each pot (15-cm diameter x 30-cm tall, with two, 2-cm wide holes at the bottom) with a 2:2:1 mixture of sand, peat moss, and 5-mm sieved tidal creek sediment; we placed transplants on top and filled in gaps with our soil mixture. Potted plants and unvegetated replicates (completely filled with soil mixture) were placed into the vertical PVC pipes. Two 1.5-cm diameter holes were drilled 10-cm beneath pot bases to ensure drainage; thus, soils were subjected to flooding from below as well as when creek levels overtopped mesocosms.
Field Sampling- To monitor hourly water depth and salinity over the course of a tidal cycle, we installed an In-Situ® Aqua TROLL® 200 (In-Situ, Fort Collins, USA) from 20 July to 1 September 2018 and used barometric pressure readings from the nearest weather station (National Oceanic Atmospheric Administration’s Station NLNC3–8,461,490 in New London, Connecticut) to calibrate water levels. In August 2018, we conducted gas flux sampling campaigns to estimate net ecosystem exchange (using transparent chambers) and ecosystem respiration (using opaque chambers) using a static chamber-based approach (Livingston and Hutchinson 1995) and a Picarro G2201-i cavity ring-down spectrometer (“CRDS”, Santa Clara, California, USA) that measures CO2 and CH4 in real-time (approximately every 3 s).
We strategically sampled gas fluxes on sunny days during peak growing season when low tide occurred around 1200; all measurements were collected between 0900 and 1500. On 6–8 August 2018 we sampled net ecosystem exchange from the 75 mesocosms during 10-minute transparent chamber incubations. On 20–23 August 2018 we estimated ecosystem respiration during 10-minute dark chamber incubations from a subset of mesocosms; we sampled the 0 cm (n = 25) and + 15 cm (n = 25) treatments to examine how our extreme SLR treatments affected ecosystem respiration.
To quantify carbon fluxes, cylindrical chambers (15.2-cm diameter, 50-cm tall) fitted with a vent tube, a Swagelok sample port, a fan to mix chamber air, and an iButton temperature sensor (Maxim Integrated, San Jose, CA, USA) were placed on top of mesocosms, sealed with petroleum jelly and connected to the CRDS via Tygon tubing. During sampling campaigns, we also measured barometric pressure and ambient air temperature using a Kestrel 2500 Weather Meter (Nielsen-Kellerman, Boothwyn, PA, USA), soil temperature with a soil thermometer, and photosynthetically active radiation (PAR) using a Hobo Micro Station H21-USB (Bourne, Massachusetts, USA) placed adjacent to platforms. Gas concentration measurements were corrected for the ideal gas law using air temperature and pressure. Flux rates were calculated based on linear changes in gas concentrations over time; methane accumulation was not linear during our chamber deployments (only 13% of replicates had R2 ≥ 0.85) so were not included analyses. All CO2 fluxes had R2 values > 0.85 and were corrected for chamber volume and surface area.
To characterize soil microbial communities, we collected soil samples 2–5 cm below the surface via an ethanol-sterilized spoon, adjacent to plant roots. These samples were collected on 21 June and again on 1 September 2018. Samples were transferred to sterile Whirl-Pak bags, placed on dry ice during transport to the Connecticut Agricultural Experiment Station, and stored at -80 °C until DNA was extracted.
Biomass- On 21 May and 13 August 2018, we quantified stem densities, average stem height, and maximum stem height for each vegetated mesocosm. After 103 days, we ended the experiment on 1 September 2018; pots were extracted from PVC pipes, placed into plastic bags, and transported to the laboratory. Aboveground stems were clipped at the soil surface. The top 10 cm of each pot was cut with a reciprocating saw and then halved vertically, as > 80% of salt marsh belowground biomass occurs in the surface 10 cm (Santini et al. 2019); one half was wet-sieved over a 1-mm screen to capture roots and rhizomes, and the second half was used for soil chemistry analysis. All biomass was dried (65℃ for ≥ 48 h) and weighed.
Soil Chemistry and Microbial Communities- We quantified soil % organic matter, total % carbon and % nitrogen, electrical conductivity (EC), pH, extractable ammonium, carbon mineralization, denitrification potential, N2O/(N2O:N2) product ratio (i.e., N2O yield), and soil microbial community composition using methods similar to Barry et al. (2022) and Ooi et al. (2022) (methodological details available in Supporting Information).
Statistical Analyses- We tested all response variables for normality using Shapiro-Wilk tests, assessed heteroscedasticity by plotting fitted values against residuals, and achieved normality by log transformations when necessary. We conducted t-tests to compare unvegetated and vegetated treatments; since unvegetated treatment soils were our custom soil mixture and vegetated treatment soils were ~ 1:1 custom soil mixture to native soil, we concluded that the unvegetated treatments were not true controls and omitted them from carbon cycling and microbial analyses. We conducted two-way analysis of variance (ANOVA) to test for differences among species, SLR scenarios, and their interaction. For carbon fluxes, we included water depth at the time of measurement to account for potential effects of tidal variation. We ran repeated measures ANOVA for stem measurements taken in May and August (lsmeans package). When categorical factors were significant (α = 0.05), we ran post-hoc Tukey HSD tests. All statistical analyses were run in R version 3.5.1 (R Core Team 2019). Means are presented ± 1 standard error.
We ordinated microbial operational taxonomic units (OTUs; 97% sequence identity) abundance and calculated alpha diversity (phyloseq package). We used non-metric multidimensional scaling (NMDS) on data randomly rarefied to the smallest sample sequence dataset (16,189 sequences). Inter-sample distances were calculated using Bray-Curtis metrics and PERMANOVA was conducted with the Adonis function (vegan package). Sequences generated in this study are available in the NCBI sequence read archive under the accession number PRJNA746321.
Results
Tidal Regime- Mean daily tidal range was 0.64 m (± 0.07 m; 1 Standard Error (SE)), creek salinity ranged from 16 to 26 ppt, and water temperatures varied between 21 and 29 °C. Mesocosms were differentially inundated among SLR and vegetation treatments, with 0-SLR treatments less frequently flooded than + 7.5 and + 15-SLR treatments. Under 0, + 7.5, and + 15 cm-SLR treatments, respectively, low marsh mesocosms were overtopped with tidal waters more frequently (38, 44, and 51%) than high marsh (27, 36, and 43%). Soils within 30-cm deep pots were more frequently submerged than surface soils; low marsh pot bases were submerged 65, 75, and 88% of the time, while those of high marsh treatments were submerged 55, 62, and 72%.
Soil Chemistry- Soil moisture fraction, electrical conductivity, and pH were all greater in vegetated than unvegetated treatments, while organic matter was more abundant in unvegetated mesocosms (Table 1). Measured soil parameters were not different among SLR or species treatments (Supporting Information, Table S1).
Plant Response- Over the course of the experiment we observed differences in plant responses among vegetation treatments, but not among SLR treatments. There were no differences among assigned SLR treatments in stem density or height prior to experiment initiation, but there were differences among species (F2,42 = 83.1, P < 0.001; F2,42 = 152.2, P < 0.001). Similarly, we observed changes in stem density (F2,42 = 61.3, P < 0.001) and stem height (F2,42 = 68.7, P < 0.001) among vegetation treatments from May to August, but not among SLR treatments. Mean S. alterniflora stem density dropped from 33 to 22 stems per mesocosm, but mean height increased from 27 to 39 cm. Mean S. patens stem density increased from 41 to 118 stems, but stem height remained consistent (24 cm). Mean P. australis stem density increased from 3 to 6, but height decreased from 67 to 33 cm; we observed P. australis shoot senescence and new shoot development throughout the growing season. We did not observe differences in above- or belowground biomass among SLR treatments, but aboveground biomass differed among species (F2, 40 = 11.83, P < 0.001); S. alterniflora had greater aboveground biomass (917 ± 85 g m− 2) than S. patens (640 ± 60 g m− 2) and P. australis (459 ± 54 g m− 2; Supporting Information, Table S2).
Net Ecosystem Exchange- We observed differences in net ecosystem exchange among SLR (F2, 35 = 18.65, P < 0.001) and species treatments (F2, 35 = 12.47, P < 0.001), though no interaction (F4,35 = 01.68, P = 0.17) nor an effect of water depth (F1,35 = 0.48, P = 0.49) were observed. Across species, 0-SLR treatments promoted CO2 uptake while + 15-SLR treatments resulted in net CO2 emissions (Fig. 2). Spartina alterniflora had the greatest carbon uptake, whereas P. australis had net carbon emissions. Average PAR during our three-day in-situ field campaign was 1350 ± 14 µmol m− 2 s− 1, though PAR and soil temperature were not correlated with net ecosystem exchange (r = 0.05, 0.02, respectively). Aboveground biomass was positively correlated with net ecosystem exchange (r = 0.29, P < 0.001), though correlative strength differed among species (S. patens: r = 0.41, S. alterniflora: r = 0.11, P. australis: r = 0.00).
Boxplot of net ecosystem exchange by species and SLR treatments; negative values: carbon uptake, positive values: carbon emission. Box represents first and third quartiles, line represents median values, whiskers extend 1.5 x the interquartile range, and points are outliers. Letters represent differences among species. SLR treatments differed with greater emissions from + 15 cm than 0 cm and + 7.5 cm treatments
Ecosystem Respiration- We observed differences in ecosystem respiration among species (F2, 23 = 8.59, P < 0.01) but not SLR treatments (F1,23 = 0.80, P = 0.38). Neither the interaction between species and SLR treatments (F2,23 = 2.11, P = 0.14) nor the water level during measurement (F1,23 = 2.12, P = 0.16) affected ecosystem respiration. Respiration rates from S. alterniflora were greater than P. australis (Fig. 3a). Aboveground biomass and ecosystem respiration were positively correlated (r = 0.13, P = 0.05), though the strength of correlations differed among species (P. australis: r = 0.35, S. patens: r = 0.25, S. alterniflora: r = 0.23).
Carbon Mineralization and Denitrification- Carbon mineralization rates did not differ among SLR treatments, but we observed differential mineralization among species (F2, 42 = 4.85, P < 0.05), with greater CO2 production from S. patens than P. australis soils (Fig. 3b). We observed a weak but positive correlation between belowground biomass and carbon mineralization across species (r = 0.07, P = 0.05); S. patens had a positive correlation (r = 0.29), but S. alterniflora and P. australis correlations were not significant (r = 0.01 for both species). We also observed a positive correlation between soil electrical conductivity and carbon mineralization (r = 0.10, P < 0.05); when separated among species, S. alterniflora replicates had tighter correlations (r = 0.35) than S. patens (r = 0.16) or P. australis (r = 0.11). We did not find differences in potential denitrification or N2O yield among species or SLR treatments (Supporting Information Tables 2, 3).
Boxplots of (a) in situ ecosystem respiration and (b) laboratory assays of carbon mineralization among species. Box represents first and third quartiles, line represents median values, whiskers extend 1.5 x the interquartile range, and points are outliers. Note the different scales on the y-axes. Letters represent differences among species
Microbial Communities- The number of recovered OTUs from the soil ranged from 589 to 939, with no differences in bacterial richness due to plant species, date, or SLR treatment (Supporting Information, Table 31). Similarly, there were no significant differences in Shannon’s diversity index or the inverse Simpsons index, suggesting no evidence that bacterial diversity was affected by the experimental parameters.
Investigating the composition of the bacterial communities via NMDS clustering showed that the primary factor structuring the microbial communities was the date of sampling (PERMANOVA P > 0.001; Fig. 4a). Thus, to better constrain the effects of plant species and SLR manipulations on the soil bacterial populations, we analyzed the populations on each date separately (Fig. 4b, c). PERMANOVA statistical comparisons showed that in both June and September, plant species were associated with distinct communities (P = 0.036 and 0.049, respectively). In contrast, SLR was not associated with differences in population structure at either date. Taken together, soil microbial observations demonstrate that communities differed in composition, but not the diversity measures we considered, over the course of the experiment.
NMDS plots of microbial community ordinal distances among soil samples (3 SLR scenarios x 3 vegetation zones). (a) Community composition differed between June and September, 2018 sampling campaigns, but there were no differences among SLR treatments in either (b) June, or (c) three months after treatment initiation in September
Discussion
We tested how near-future SLR projections and plant dominance altered tidal marsh carbon cycling and soil microbial communities. We expected carbon-based processes to be more responsive to SLR than species treatments as tidal flooding directly affects plant biomass allocation (Kirwan and Guntespergen 2012) and microbial respiration (Luo et al. 2019). However, we only observed SLR-treatment effects for net ecosystem exchange, whereas ecosystem respiration and carbon mineralization rates were similar among SLR treatments. We found support for our prediction that low marsh S. alterniflora was most resilient to increased flooding, as it consistently had the greatest CO2 uptake rates across our SLR scenarios. Surprisingly, we did not observe differences in soil chemistry (electrical conductivity, pH, soil moisture fraction, % organic matter, total carbon or nitrogen, denitrification) among species nor SLR treatments. While soil microbial communities did not differ among SLR treatments, they varied among vegetation treatments and shifted over the course of the experiment. Overall, our findings suggest that plant-mediated processes rather than near term SLR hydrological modifications alone are critical to tidal marsh carbon cycling and should be incorporated into projections of SLR-induced effects on blue carbon.
Plant Mediated Responses- We observed differences among SLR treatments in net ecosystem exchange, but not ecosystem respiration nor carbon mineralization, suggesting the importance of plant vigor and photosynthetic uptake in moderating carbon dynamics in near future SLR scenarios. SLR treatments of + 7.5 cm reduced the carbon sink strength by 50% and + 15 cm SLR treatments were net carbon sources. Similarly, Kathilankal et al. (2008) found S. alterniflora photosynthetic rates declined under submerged conditions (> 25 cm) due to limited light availability, decreasing net ecosystem exchange by 46%. Mao et al. (2023) found that photosynthetic efficiency was related to the proportion of submerged S. alterniflora leaves, but that fully submerged leaves actively photosynthesize. While our short-term S. alterniflora net ecosystem exchange rates measured during the peak of the growing season were comparable to Cornell et al.’s (2007) estimates, S. patens has a longer photosynthetic period during the growing season resulting in potentially greater annual carbon uptake (Artigas et al. 2015). Thus, phenological differences among species should be considered when evaluating carbon fluxes over longer timescales.
Greater ecosystem respiration and carbon mineralization rates in Spartina spp. than P. australis may be due to greater biomass or species effects on redox conditions. Roots contribute additional terminal electron acceptors and oxygenate soils (Wolf et al. 2007; Sutton-Grier and Megonigal 2011), with belowground biomass correlating to higher microbial respiration (Barry et al. 2022) and carbon flux in situ (Moseman-Valtierra et al. 2016). Similarly, we detected positive correlations between belowground biomass and carbon mineralization, especially in S. patens-associated soils. However, our estimates of carbon mineralization are likely inflated as we conducted 24-h slurried oxic incubations, which may promote oxidation of accumulated carbon and sulfides. Interestingly, an unpublished companion study used the Tea Bag Index (Keuskamp et al. 2013) to examine soil organic matter decomposition under different species and observed consistently higher rates in P. australis-associated soils, both in this marsh organ experiment and across five marsh complexes, suggesting differential oxygenation or carbon exudate priming among species.
Microbial Community Responses- Plants can exert strong influences on soil microbial assemblages (Rietl et al. 2016; Barreto et al. 2018), but in wetlands this can be confounded by flooding frequency. For instance, Barry et al. (2022) demonstrated that different vegetation zones of tidal wetlands harbor distinct microbial communities, but the study could not differentiate between the abiotic influences related to differences in inundation frequency versus the biotic effects of different plant species. Here we show that plant species have a small but significant influence on the soil microbial community, but those differences were smaller than the temporal changes occurring over the course of sampling. Similarly, a prior mesocosm study demonstrated that soil bacterial communities were far more responsive to salting treatments in comparison to differences between plant species (Donato et al. 2020). Thus, these data suggest that tidal wetland soil microbial populations may be more sensitive to changes in abiotic factors such as salinity and temperature than they are to biotic factors such as plant species or alterations in plant performance as observed under SLR in this study. These data will be important to constraining efforts to predict how coastal wetland soil microbial communities will respond to a variety of climate induced shifts, including increased temperatures and SLR.
SLR and Implications for Blue Carbon- SLR-induced shifts in species dominance have important consequences for tidal marsh carbon dynamics. In coastal New England, SLR facilitates S. alterniflora expansion that reduces less flood and salt-tolerant species such as S. patens and P. australis (Warren and Niering 1993; Doody 2004; Field et al. 2016). Previous studies suggest P. australis sequesters more carbon than native vegetation, due to high plant productivity and more recalcitrant soil carbon (Windham 2001; Caplan et al. 2015; Martin and Moseman-Valtierra 2015). We observed tidal creek salinities during the growing season (July/August: 16–26 ppt) that surpassed the salinity threshold that P. australis can typically tolerate (18 ppt; Chambers et al. 1999), likely due to diminished fresh-water influence after the spring thaw (Yellen et al. 2017). High salinity or stress associated with transplants being severed from their clonal network (Amsberry et al. 2000) may have caused low vigor among P. australis replicates that were often net carbon sources with high ecosystem respiration rates. While our focus was on manipulating near-term SLR scenarios by altering flooding frequency, it is important to note that water quality (e.g., salinity, nitrogen concentration) can affect plant vigor with important implications for carbon cycling (Donato et al. 2020). Phragmites soil also tended to have an abundance of Bacteroidetes, a phylum often associated with carbon breakdown (Fierer et al. 2007; Ai et al. 2015; Elmer et al. 2017). While our experiment did not provide ideal growing conditions for P. australis, we demonstrate that near-future SLR scenarios decrease the carbon sequestration capacity of three dominant plants in North American coastal marshes.
Salt marsh plant-associated bacteria tend to be resilient to environmental changes (Angermeyer et al. 2018), which may explain why we did not see microbial community shifts associated with our SLR treatments. Bacteria are exposed to extreme swings in flooding and anoxia during tidal cycles and thus capable of tolerating a range of environmental conditions. Even in the absence of large taxonomic shifts, the function of microbial communities may be altered by SLR, which were not detected in our 16 S rRNA gene sequence survey. Future studies that better estimate microbial functional profiles, such as transcriptomics, metabolomics, or proteomics, may better identify how microbes are responding to altered carbon cycling in wetland soils by investigating physiological changes rather than taxonomic shifts.
Experimental Caveats- We manipulated elevations to mimic inundation patterns predicted for 2028 and 2038 SLR scenarios, though these treatments were not distinct enough to elicit strong soil chemistry responses. While the tops of our mesocosms were differentially inundated during high tide (27–51% of the time), soils across treatments were saturated at least 55% of the time. In contrast to tidal waters that disperse laterally across a marsh, our vertical mesocosms had limited surface area with a high edge ratio which could increase soil temperature relative to natural marsh soil, especially at low tide when mesocosms are exposed above the water (Fig. 1a). However, our mesocosms had > 2x greater surface area than those used by Langley et al. (2013) and Payne et al. (2021). Further, we did not detect differences in the absolute difference between air and soil temperatures between low and high marsh mesocosms (F1,72 = 1.2, p = 0.276), or among our SLR treatments (F2,72 = 0.51, p = 0.476). This suggests that potential soil warming associated with high edge exposure during low tide did not differentially affect mesocosms of varying height, and that potential soil warming in exposed mesocosms was at least consistent across treatments. Similar to our findings, Abdul-Aziz et al. (2018) did not find water level to have predictive control on greenhouse gas fluxes in salt marshes, though they did observe that fluxes were lower during low than high tides, whereas we only sampled carbon fluxes within three hours of low tide. Further, a longer SLR experiment may have elicited greater response, but executing a multi-year marsh organ experiment during tidal creek freezing events common during New England winters would be extremely challenging, and marsh organ experiments are typically conducted during a single growing season (e.g.: Van Belzen et al. 2017; Kirwan and Guntenspergun 2012, 2015; Langley et al. 2013; Payne et al. 2021).
We tested how SLR scenarios would affect plant-mediated carbon dynamics by establishing our baseline SLR scenario (i.e., 0-SLR) to species-specific elevations that we quantified in the adjacent marsh. We argue that this approach was more ecologically realistic than studies that disregard species distributions along elevation gradients and necessary since we were interested in relatively near-term and small SLR scenarios (+ 7.5, + 15 cm). However, this design resulted in our S. alterniflora treatments being 10 cm lower in the tidal frame than those of S. patens and P. australis, so some may contend that it is challenging to directly compare our species treatments given the different flooding, salinity, and temperature regimes they were exposed to.
While our unvegetated treatments were imperfect controls given their different soil composition than planted replicates, comparisons with vegetated treatments suggest the importance of plant priming on microbial communities. Carbon fluxes were both an order of magnitude higher, carbon mineralization (standardized by soil carbon content) were twice as high, and denitrification rates were > 200X higher in vegetated treatments. Mueller et al. (2016) similarly found that salt marsh vegetation can stimulate soil organic matter decomposition up to 260%. Plants amplify biogeochemical rates by supplying terminal electron acceptors (Wolf et al. 2007), regenerating reduced forms of nitrogen, sulphur, and iron (Sutton-Grier and Megonigal 2011), as well as exuding low molecular weight carbon from roots (Blagodatskaya and Kuzykov 2008).
Conclusions- We manipulated vegetation and elevation to investigate the relative roles of dominant plant species and projected SLR-induced flooding on microbial communities and tidal marsh carbon cycling. We found that plants contributed to carbon cycling primarily through aboveground biomass production that enhanced carbon uptake. Increasing inundation rates associated with our SLR treatments reduced the carbon sink strength by reducing photosynthetic uptake of CO2. Time of sampling emerged as the primary factor influencing microbial populations, highlighting the importance of temporal dynamics and potential species turnovers in sediment bacterial communities. Surprisingly, despite the effects of SLR-induced flooding on plant performance, the bacterial community composition exhibited considerable resilience, with only minor variations observed among different plant species. Our results suggest that carbon cycling is strongly plant-mediated rather than solely dictated by increased inundation in near-future SLR scenarios.
Data Availability
Bacterial sequences generated in this study are available in the NCBI sequence read archive under the accession number PRJNA746321 (https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA746321). All other data will be made publicly available on the Dryad Digital Repository upon article acceptance.
References
Abdul-Aziz OI, Ishtiaq KS, Tang J, Moseman‐Valtierra S, Kroeger KD, Gonneea ME, Mora J, Morkeski K (2018) Environmental controls, emergent scaling, and predictions of greenhouse gas (GHG) fluxes in coastal salt marshes. J Geophys Research: Biogeosciences 123:2234–2256. https://doi.org/10.1029/2018JG004556
Ai C, Liang G, Sun J, Wang X, He P, Zhou W, He X (2015) Reduced dependence of rhizosphere microbiome on plant-derived carbon in 32-year long-term inorganic and organic fertilized soils. Soil Biol Biochem 80:70–78. https://doi.org/10.1016/j.soilbio.2014.09.028
Amsberry L, Baker MA, Ewanchuk PJ, Bertness MD (2000) Clonal integration and the expansion of Phragmites australis. Ecol Appl 10:1110–1118. https://doi.org/10.1890/1051-0761(2000)010[1110:CIATEO]2.0.CO;2
Angermeyer A, Crosby SC, Huber JA (2018) Salt marsh sediment bacterial communities maintain original population after transplantation across a latitudinal gradient. PeerJ 6:e4735–e4735. https://doi.org/10.7717/peerj.4735
Artigas F, Shin JY, Hobble C, Marti-Donati A, Schäfer KVR, Pechmann I (2015) Long term carbon storage potential and CO2 sink strength of a restored salt marsh in New Jersey. Agric For Meteorol 200:313–321. https://doi.org/10.1016/j.agrformet.2014.09.012
Barreto CR, Morrissey EM, Wykoff DD, Chapman SK (2018) Co-occuring mangrovesand salt marshes differ in microbial communities. Wetlands 38:497–508. https://doi.org/10.1007/s13157-018-0994-9
Barry A, Ooi SK, Helton AM, Steven B, Elphick CS, Lawrence BA (2022) Vegetation zonation predicts soil carbon mineralization and microbial communities in southern New England salt marshes. Estuaries Coasts 45:168–180. https://doi.org/10.1007/s12237-021-00943-0
Bertness MD (1991) Zonation of Spartina patens and Spartina alterniflora in New England salt marsh. Ecology 72:138–148. https://doi.org/10.2307/1938909
Blagodatskaya E, Kuzykov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131. https://doi.org/10.1007/s00374-008-0334-y
Caplan JS, Hager RN, Megonigal JP, Mozdzer TJ (2015) Global change accelerates carbon assimilation by a wetland ecosystem engineer. Environ Res Lett 10(11):115006. https://doi.org/10.1088/1748-9326/10/11/115006
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquat Bot 64:261–273. https://doi.org/10.1016/S0304-3770(99)00055-8
Chambers LG, Reddy KR, Osborne TZ (2011) Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Sci Soc Am J 75:2000–2007. https://doi.org/10.2136/sssaj2011.0026
Clough J, Polaczyk A, Propato M (2015) Application of SLAMM to Coastal Connecticut. Prepared by Warren Pinnacle Consulting, Inc. Accessed April 2, 2019: http://warrenpinnacle.com/prof/SLAMM/LISS/NEIWPCC_Final_CT_Report_Amended.pdf
R Core Team (2019) R: A language and environment for statistical computing. Accessed 1st April 2019
Cornell JA, Craft CB, Megonigal JP (2007) Ecosystem gas exchange across a created salt marsh chronosequence. Wetlands 27:240–250. https://doi.org/10.1672/0277-5212(2007)27[240:EGEAAC]2.0.CO;2
Donato M, Johnson O, Steven B, Lawrence BA (2020) Nitrogen enrichment stimulates wetland plant responses whereas salt amendments alter sediment microbial communities and biogeochemical responses. PLoS ONE 15:e0235225. https://doi.org/10.1371/journal.pone.0235225
Doody JP (2004) Coastal squeeze” - an historical perspective. J Coastal Conserv 10:129. https://doi.org/10.1652/14000350(2004)010[0129:CSAHP]2.0.CO;2
Duggan-Edwards MF, Pagés JF, Jenkins SR, Bouma TJ, Skov MW (2020) External conditions drive optimal planting configurations for salt marsh restoration. J Appl Ecol 57(3):619–629. https://doi.org/10.1111/1365-2664.13550
Elmer WH, Thiel P, Steven B (2017) Response of sediment bacterial communities to sudden vegetation dieback in a coastal wetland. Phytobiomes 11:5–13. https://doi.org/10.1094/PBIOMES-09-16-0006-R
Enwright NM, Griffith KT, Osland MJ (2016) Barriers to and opportunities for landward migration of coastal wetlands with sea-level rise. Front Ecol Environ 16(6):307–316. https://doi.org/10.1002/fee.1282
Farrar J, Hawes M, Jones D, Lindow S (2003) How roots control the flux of carbon to the rhizosphere. Ecology 84(4):827–837. https://doi.org/10.1890/0012-9658(2003)084[0827:HRCTFO]2.0.CO;2
Field CR, Gjerdrum C, Elphick CS (2016) Forest resistance to sea-level rise prevents landward migration of tidal marsh. Biol Conserv 201:363–369
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88(6):1354–1364. https://doi.org/10.1890/05-1839
Freeman C, Ostle N, Kang H (2001) An enzymatic latch on a global carbon store. Nature 409:149–150. https://doi.org/10.1038/35051650
Helton AM, Ardón M, Bernhardt ES (2019) Hydrologic context alters greenhouse gas feedbacks of coastal wetland salinization. Ecosystems 22:1108–1125. https://doi.org/10.1007/s10021-018-0325-2
Herbert E, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardón M, Hopfensperger KN, Lamers L, Gell P (2015) A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6:1–43. https://doi.org/10.1890/ES14-00534.1
Kathilankal JC, Mozdzer TJ, Fuentes JD, D’Odorico P, McGlathery KJ, Zieman JC (2008) Tidal influences on carbon assimilation by a salt marsh. Environ Res Lett 3:044010. https://doi.org/10.1088/1748-9326/3/4/044010
Keuskamp JA, Dingemans BJ, Lehtinen T, Sarneel JM, Hefting MM (2013) Tea bag index: a novel approach to collect uniform decomposition data across ecosystems. Methods Ecol Evol 4:1070–1075. https://doi.org/10.1111/2041-210X.12097
Kirwan ML, Guntenspergen GL (2012) Feedbacks between inundation, root production, and shoot growth in a rapidly submerging brackish marsh. J Ecol 100:764–770. https://doi.org/10.1111/j.1365-2745.2012.01957.x
Kirwan ML, Guntenspergen GR (2015) Response of plant productivity to experimental flooding in a stable and a submerging marsh. Ecosystems, 18, pp.903–913. https://doi.org/10.1007/s10021-015-9870-0
Kirwan ML, Megonigal JP (2013) Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504:53–60. https://doi.org/10.1038/nature12856
Langley AJ, Mozdzer TJ, Shepard KA, Hagerty SB, Patrick Megonigal J (2013) Tidal marsh plant responses to elevated CO 2, nitrogen fertilization, and sea level rise. Glob Change Biol 19(5):1495–1503. https://doi.org/10.1111/gcb.12147
Livingston GP, Hutchinson GL (1995) Enclosure-based measurement of trace-gas exchange: applications and sources of error. In: Matson PA, Harriss RC (eds) Biogenic Trace gases: measuring Emissions from Soil and Water. Blackwell Science, Cambridge, MA, pp 14–51
Luo M, Huang JF, Zhu WF, Tong C, Xie C, An S, Xie D, Davis JA (2019) Impacts of increasing salinity and inundation on rates and pathways of organic carbon mineralization in tidal wetlands: a review. Hydrobiologia 827:31–49. https://doi.org/10.1007/s10750-017-3416-8
Mao L, Mishra DR, Hawman PA, Narron CR, O’Connell JL, Cotten DL (2023) Photosynthetic Performance of Tidally Flooded Spartina alterniflora Salt Marshes. Journal of Geophysical Research: Biogeosciences, p.e2022JG007161
Martin RM, Moseman-Valtierra S (2015) Greenhouse gas fluxes vary between Phragmites australis and native vegetation zones in coastal wetlands across a salinity gradient. Wetlands 35:1021–1031. https://doi.org/10.1007/s13157-015-0690-y
McLeod E, Chumura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560. https://doi.org/10.1890/110004
Morris J (2007) Estimating net primary production of salt marsh macrophytes. In: Fahey T, Knapp A (eds) Principles and standards for measuring primary production. Oxford University Press
Moseman-Valtierra S, Abdul-Aziz OI, Tang J, Ishtiaq KS, Morkeski K, Mora J, Quinn RK, Martin RM, Egan K, Brannon EQ, Carey J, Kroegger KD (2016) Carbon dioxide fluxes reflect plant zonation and belowground biomass in a coastal marsh. Ecosphere 7:e01560. https://doi.org/10.1002/ecs2.1560
Mueller P, Jensen K, Megonigal JP (2016) Plants mediate soil organic matter decomposition in response to sea level rise. Glob Change Biol 22:404–414. https://doi.org/10.1111/gcb.13082
Neubauer SC, Franklin RB, Berrier DJ (2013) Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10:8171–8183. https://doi.org/10.5194/bg-10-8171-2013
Ooi SK, Barry A, Lawrence BA, Elphick CS, Helton AM (2022) Vegetation zones as indicators of denitrification potential in salt marshes. Ecol Appl 32(6):e2630
Payne AR, Burdick DM, Moore GE, Wigand C (2021) Short-term effects of thin-layer sand placement on salt marsh grasses: a marsh organ field experiment. J Coastal Res 37(4):771–778. https://doi.org/10.2112/JCOASTRES-D-20-00072.1
Pendleton L, Donato DC, Murray BC, Crooks S, Jenkins WA, Sifleet S, Craft C, Fourqurean JW, Kauffman JB, Marbà N, Megonigal P, Pidgeon E, Herr D, Gordon D, Baldera A (2012) Estimating “Blue Carbon” emissions from conversion and degradation of vegetated ecosystems. PLoS ONE 7:–e43542. https://doi.org/10.1371/journal.pone.0043542
Rietl AJ, Overlander ME, Nyman AJ, Jackson CR (2016) Microbial community composition and extracellular enzyme activities associated with Juncus roemerianus and Spartina alterniflora vegetated sediments in Louisiana salt marshes. Microbiol Aquat Syst 71:290–303. https://doi.org/10.1007/s00248-015-0651-2
Santini NS, Lovelock CE, Hua Q, Zawadzki A, Mazumder D, Mercer TR, Muñoz-Rojas M, Hardwick SA, Madala BS, Cornwell W, Thomas T (2019) Natural and regenerated saltmarshes exhibit similar soil and belowground organic carbon stocks, root production and soil respiration. Ecosystems 22:1803–1822. https://doi.org/10.1007/s10021-019-00373-x
Simon MR, Zogg GP, Travis SE (2017) Impacts of sea-level rise on sediment microbial community structure and function in two New England salt marshes, USA. J Soils Sediments 17:2847–2855. https://doi.org/10.1007/s11368-017-1710-8
Sutton-Grier AE, Megonigal JP (2011) Plant species traits regulate methane production in freshwater wetland soils. Soil Biol Biochem 43:413–420
Tobias CR, Neubauer SC (2019) Salt marsh biogeochemistry: An overview. pp. 539–596 in: G. Perillo, E. Wolanski, D. Cahoon, C. Hopkinson (eds). Coastal wetlands: An integrated ecosystem approach. 2nd edition. Elsevier
Van Belzen J, Van De Koppel J, Kirwan ML, Van Der Wal D, Herman PM, Dakos V, Kéfi S, Scheffer M, Guntenspergen GR, Bouma TJ (2017) Vegetation recovery in tidal marshes reveals critical slowing down under increased inundation. Nat Commun 8(1):15811. https://doi.org/10.1038/ncomms15811
Warren RS, Niering WA (1993) Vegetation change on a northeast tidal marsh: interaction of sea-level rise and marsh accretion. Ecology 74:96–103. https://doi.org/10.2307/1939504
Watson EB, Szura K, Wigand C, Raposa KB, Blount K, Cencer M (2016) Sea level rise, drought, and the decline of Spartina patens in New England marshes. Biol Conserv 196:173–181. https://doi.org/10.1016/j.biocon.2016.02.011
Watson EB, Wigand C, Davey EW, Andrews HM, Bishop J, Raposa KB (2017) Wetland loss patterns and inundation-productivity relationships prognosticate widespread salt marsh loss for southern New England. Estuaries and Coasts, 40, 662–681. https://doi.org/10.1007/s12237-016-0069-1
Weston NB, Vile MA, Neubauer SC, Velinsky DJ (2011) Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102:135–151. https://doi.org/10.1007/s10533-010-9427-4
Windham L (2001) Comparison of biomass production and decomposition between Phragmites australis (Common Reed) and Spartina patens (Salt Hay Grass) in brackish tidal marshes of New Jersey. USA Wetlands 21:179–188. https://doi.org/10.1672/0277-5212(2001)021[0179:COBPAD]2.0.CO;2
Wolf AA, Drake BG, Erickson JE, Megonigal JP (2007) An oxygen-mediated positive feedback between elevated carbon dioxide and soil organic matter decomposition in a simulated anaerobic wetland. Glob Change Biol 13:2036–2044. https://doi.org/10.1111/j.1365-2486.2007.01407.x
Yellen B, Woodruff JD, Ralston DK, MacDonald DG, Jones DS (2017) Salt wedge dynamics lead to enhanced sediment trapping within side embayments in high-energy estuaries. J Geophys Research: Oceans 122(3):2226–2242
Acknowledgements
We thank Bowen Dou, Ron Rosza, Roger Wolfe, Alaina Bisson, Kayleigh Granville, Fiona Liu, Kevin Jackson, Olivia Johnson, Jacquelyn LaReau, Anna Puchkoff, Gary Ulatowski, and Samantha Walker for their assistance in project implementation.
Funding
This manuscript is a resulting product from project R/CMB-42-CTNY funded under award LI96172701, U.S. Environmental Protection Agency, on behalf of Connecticut Sea Grant, and in collaboration with NYSG. The statements, findings, conclusions, views and recommendations are those of the authors and do not necessarily reflect the views of any of those organizations. This project was also supported by the Connecticut Institute for Resilience and Climate Adaptation’s (CIRCA) Matching Funds Program.
Author information
Authors and Affiliations
Contributions
Beth Lawrence, Ashley Helton, and Chris Elphick conceived the project. Aidan Barry, Sean Khan Ooi, Ashley Helton, Blaire Steven, Chris Elphick, and Beth Lawrence designed methodology; Aidan Barry and Sean Khan Ooi collected and analyzed the data; Aidan Barry and Beth Lawrence led manuscript writing and all authors revised drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barry, A., Ooi, S.K., Helton, A.M. et al. Carbon Dynamics Vary Among Tidal Marsh Plant Species in a Sea-level Rise Experiment. Wetlands 43, 78 (2023). https://doi.org/10.1007/s13157-023-01717-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-023-01717-z