Abstract
The present investigation is an attempt for environmental characterization of two ecologically distinct oxbow lakes using zooplankton taxonomic indices following a comparative approach. These closed and seasonally open oxbow lakes are subjected to eutrophication, impacting their nutrient concentration and eco-hydrological characteristics. The zooplankton are closely linked with environment throughout their life cycle, hence are a potential indicator of eutrophication. The study examined the assemblage pattern of zooplankton community and trophic state of two ecologically distinct oxbow lakes based on eco-hydrological factors and community structure of rotifers and planktonic crustaceans. Comprehensive trophic state index (mTSI), rotifer trophic state indices (mTSIROT) and crustacean based indices (TSICR) were used to assess the degree of eutrophication. The Kruskal–Wallis test confirmed the heterogeneity in eco-hydrological factors between the oxbow lakes. The studied lakes were in transition from high meso-eutrophic to moderately eutrophic state. The mTSI, mTSIROT and mTSICR for Khalsi (seasonally open) and Akaipur (closed) were 54.90 ± 11.71, 56.95 ± 15.64, 59.55 ± 4.54 and 60.26 ± 4.48, 55.79 ± 4.76, 60.00 ± 4.03 respectively. The Canonical Correspondence Analysis (CCA) revealed NO3−N, water temperature and pH positively impacted abundance of eutrophication indicator species Brachionus and Keratella. An overview of worldwide use of rotifer and crustacean based indices in assessment of TSI has also been discussed. The use of these zooplankton indices to evaluate the trophic status of the ecologically distinct lakes is highly recommended for water quality assessment and management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The oxbow lakes are characterized by shallow depth, high sediment nutrient and productivity (Janseen et al. 2014; Tang et al. 2018, 2019). These lakes offer a plethora of ecosystem services for livelihood and nutritional security to the riparian communities (Sarkar et al. 2020; Das et al. 2021). These wetland ecosystems provide safety from natural calamities, serve as habitat for larval rearing, nutrient recycling, water for domestic and irrigation purpose, fishing activities and are considered as “Kidney of ecosystem” due to its efficient sinking capacity to inhale major agricultural runoff (Sarma and Dutta 2012; Meena et al. 2019; Karnatak et al. 2020; Sarkar et al. 2020, 2021a, b).
Oxbow lakes, mainly distributed in eastern and northeastern India are important fishery resources. These lakes are under integrated management practices including fisheries (culture-based fisheries, enclosure culture), agriculture and horticulture (Chaudhuri et al. 2008; Ghosh and Biswas 2015). Despite huge potential, these water bodies are neglected and used irrationally. Anthropogenic pressures resulting from agriculture and industrial developmental activities have accelerated degree of eutrophication of freshwater ecosystem worldwide (Junk et al. 2014). Different types of bioindicators are widely used to assess the aquatic ecosystem health using physical, chemical and biological parameters comprehensively (Oh et al. 2017). Several tools such as macrobenthos based multimetric indices (Meena et al. 2019), phytoplankton indices (Roshith et al. 2018), water quality index (WQI) (Sharma and Bora 2020), biomonitoring and bioassessment (Sanyal et al. 2015), fish assemblage and fisheries (Sandhya et al. 2016, 2019) etc. have been used for wetland health assessment. These have limitations in terms of real time application such as high fluctuations due to climatic and water quality factors. Trophic state is one of the most important characteristics to classify nutritional load of an aquatic ecosystem (Jekatierynczuk-Rudczyk et al. 2014; Dembowska et al. 2015; Ejsmont-Karabin et al. 2016; Wen et al. 2017; Smaoune et al. 2021). Various environmental parameters are used to measure the trophic status of a lake. One of the most widely used trophic state index for classifying lakes is Carlson trophic status index that is based on total phosphorus (TP), Secchi depth (SD) and concentration of Chlorophyll a (Carlson and Havens 2005; Abell et al. 2020).
Chlorophyll a concentration proxies the phytoplankton biomass which signifies the production functions of aquatic ecosystem (Das Sarkar et al. 2021). Easiest one is based on SD but its measures are inclined by both algal and non-algal particulate matters. Zooplankton are one of the important biotic components and a sensitive indicator of water quality, vital for maintaining overall ecosystem productivity and stability of food web (Branco et al. 2002; Kumari et al. 2017; Doukhandji and Arab 2017; Tang et al. 2019; Smaoune et al. 2021).
Although, several reports are available on zooplankton abundance and community structure from water bodies of India (Ganesan and Khan 2008; Sharma 2009, 2011; Sharma and Sharma 2012) and Bangladesh (Biswas and Panigrahi 2015), most are limited to the diversity indices. Only a few studies have attempted to study the relation between species richness and assemblage pattern based on macro-zoobenthos and rotifers to evaluate the wetland health (Kumari et al. 2017; Meena et al. 2019; Ejsmont-Karabin 2012; Dembowska et al. 2015), crustacean (Jekatierynczuk-Rudczyk et al. 2014; Xiong et al. 2016). Rotifer is a tiny, short generation time organism which quickly responds to the environmental changes (Segers 2008) and acts as an effective tool to indicate the ecological status of the lakes and reservoirs (Jiang et al. 2017). Initially taxon-based indicator such as Brachionus:Trichocerca ratio (BT) was used to assess the trophic status of lake, but this has its own limitation of not working in absence of either species (Sládeček 1983). Trophic status of lakes based on zooplankton such as rotifers and crustaceans have been found to be promising to assess the degree of eutrophication in shallow lakes worldwide (Ejsmont-Karabin 2012; Jekatierynczuk-Rudczyk et al. 2014; Dembowska et al. 2015; Ejsmont-Karabin et al. 2016; Smaoune et al. 2021). Rotifer based indicator of trophic status was highly recommended for anthropogenic impacted water bodies (Lodi et al. 2011). Rotifer and crustacean based indices such as TSIROT and TSICR are potential tool for evaluating the degree of eutrophication (Liang et al. 2020a, b).
Therefore, in the present study rotifer trophic state index (TSIROT) and crustacean trophic status index (TSICR) developed by Ejsmont-Karabin (2012) and modified by Ejsmont-Karabin and Karabin (2013) respectively have been used to estimate ecological quality standards of the two different type of shallow open water bodies. The present investigation is an attempt to determine the spatio-temporal patterns and trends of physico-chemical factors by perusal of zooplankton structured taxonomic indices. The findings will reflect spatio-temporal eutrophication in two lower Gangetic Oxbow lakes in relation to zooplankton dynamics.
Materials and Methods
Study Area
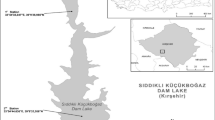
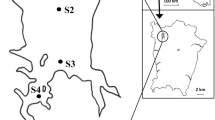
Two Oxbow lakes of West Bengal namely Khalsi (25059′54.02’’N 88038′27’’E) and Akaipur (23005′14.14’’N 88042′56.22’’E) were selected for study (Fig. 1). The study was conducted seasonally covering pre-monsoon (March to May), monsoon (June to August), post-monsoon (September to November) and winter seasons (December to February) during March 2014 to February 2016. Major activities in the catchments of both lakes included intensive agriculture, habitat for birds, cattle, orchards, and human habitation (Sandhya et al. 2016). These lakes are subjected to culture based fisheries management practices involving stocking of both indigenous and exotic carp seed providing livelihood support to the 500 and 300 fishers families in Khalsi and Akaipur respectively. (Meena et al. 2019). Both the lakes are in the lower Gangetic deltaic region and receive runoff from agricultural land, domestic waste, silt and other wastes from jute retting, bathing, washing etc. Both lakes are ecologically distinct. Akaipur has lost its perennial connectivity with the parent river, while Khalsi still maintains a feeble connectivity with the adjacent rivulet facilitating annual flushing. Dominant submerged macrophytes (Hydrilla verticillata (L.f.) Royle, Ceratophyllum demersum L., Vallisneria sp. and floating aquatic vegetations (Eichhornia crassipes Mart., Pistia stratiotes L., Nelumbo nucifera Gaertn., Salvinia molesta D. Mitch., Lemna sp. etc.) have occupied a greater part of Khalsi wetland (40–45%) distinguishing it from Akaipur, where only sporadic occurrence of aquatic plants was noticed (10–15%) during the study period. Floating and submerged macrophytes provide shelter and breeding habitat for many small indigenous fish species in lakes. Standard sampling frequency to effectively monitor biotic and abiotic variables, seasonal variability and inter-annual changes was chosen following Water Framework Directive (WFD) guidelines (European Commission 2000).
Water Sample Collection
Seasonal water quality parameters such as water temperature, transparency (SD), pH, dissolved oxygen (DO), electrical conductivity (EC), total alkalinity (Alk), total hardness (Hard), major dissolved nutrients such as nitrate (NO3-N) and phosphate (PO4-P) and Chlorophyll a (Chl a) concentrations were analysed from 3 different fixed sites in each lake. To analyze the physicochemical quality, major nutrients and Chl a concentration, 1000 ml water was collected in plastic bottles with double stoppers from each sampling station. Water temperature, pH, conductivity was measured using a multi-parameter pcstestr 35 (Eutech) in situ; water transparency was measured by using Secchi-disc. Dissolved oxygen was measured following modified Winkler’s method (Strickland and Parsons 1972). The dissolved inorganic nutrients (NO3-N and PO4-P), total alkalinity and total hardness were analysed in laboratory following standard methods (APHA 2005). For chlorophyll a measurement standard spectrophotometric method (HACH Spectrophotometer, DR 2800, Germany) was used (APHA 2005).
Plankton Data Collection and Analysis
The sampling of zooplankton was done following Sharma and Sharma (2012). Taxonomic identification to the lowest possible taxon was done using standard keys for rotifers (Koste 1978; Battish 1992; Segers 1995; Nogrady & Segers 2002) and cladocerans and copepods (Edmondson 1959; Victor and Fernando 1979; Sehgal 1983; Benzie 2005; Dussart & Defaye 2001). Verification and confirmation of taxonomic nomenclature was done following International Commission on Zoological Nomenclature for rotifers (http://iczn.org/lan/rotifer), Cladocerans (Kotov et al. 2013) and Copepods (Walter and Boxshall 2015). Abundance of zooplankton was expressed as ind/l. Aliquot of 5 ml sample out of 50 ml sample (50 ml sample concentrated from 5 l of lake water) were taken randomly after mixing, and used for counting in a Sedgewick-Rafter chamber under an inverted microscope (Zeiss-Winkel).
Biovolumes of zooplankton were assessed following equations based on geometrical formulae best fitted for each body shape (Ruttner-Kolisko 1977; McCauley 1984). Thirty organisms of each selected species of same taxon were selected randomly. Length, width and height were measured and best fitted geometrical shape formulae were applied. Biovolume of each taxon was converted to fresh weight assuming a specific density of (1 × 10–6). The fresh weight (FW) was later converted to dry weight (DW) (Schindler and Noven 1971; Bottrell et al. 1976). Biomass of ostracods was calculated following Lehette and Hernández-León (2009). Biovolume of Copepod nauplii were assumed 0.400 μg as suggested by Hawkins and Evans (1979). Zooplankton biomass was calculated by multiplying wet weight of individual zooplankton with abundance. Phytoplankton biomass was assessed based on chlorophyll a estimation following Vörös and Padisak (1991).
To determine the ecological indices, number of zooplankton taxa present in each season was included in calculation excluding copepod nauplii and unidentified zooplankton. The commonly used index for biological system was Shannon–Wiener diversity index (H') to characterize species diversity of zooplankton community. Shannon–Wiener diversity index was used both for comparing two distinct water bodies on temporal and spatial scale and to evaluate the health of water bodies. Index value greater than 3 indicates clean water while values in the range of 1–3 indicate moderate pollution (Mason 1966).
Species diversity and homogeneity were calculated using the Shannon–Wiener diversity index H' (Shannon and Weaver 1949), Margalef's richness index (Margalef 1958) and Evenness index J' (Pielou 1966).
The trophic state index (TSI) was used for qualitative assessment of trophic state of the lakes (Carlson 1977; Adamovich et al. 2016). The wetland trophic status classified on a numerical scale between 0–100 is given in Table 1.
The individual trophic state index (TSI) of Chlorophyll a (Chl a), total phosphorus (TP) and Secchi disk transparency (SD) were calculated as follows.
-
(1)
TSI Chl a = 9.76ln (Chl a) + 30.91
-
(2)
TSI TP = 14.43ln (TP) + 4.15
-
(3)
TSISD = -14.39ln (SD) + 59.91
Rotifer community structure have been used for rotifer trophic status index (TSIROT) by following equations given by (Ejsmont-Karabin 2012).
-
(1)
TSIROT1 = 5.38ln (Nr) + 19.28; where Nr number of rotifer (ind/l)
-
(2)
TSIROT2 = 5.38ln (B) + 64.47; where B biomass of rotifer (mgww/l)
-
(3)
TSIROT3 = 3.85(B/ Nr).−0.318
-
(4)
TSIROT4 = 0.144 TECTA + 54.8; percentage of spineless form (tecta) in abundance of Keratella cochlearis
-
(5)
TSIROT5 = 0.203IHT + 40.0; number of species contributed to the high trophic status indicator group
The mean value of TSI (mTSIROT) obtained from of above five equation is used as an indicator of ecological status of lakes.
Crustacean based indices (TSICR) developed by Ejsmont-Karabin and Karabin (2013) for estimation of Crustacean based trophic status indices have been followed for Trophic index of lakes is given below;
-
(6)
TSICR1 = 25.5 Nr.0.142; where Nr number of crustacean abundance (ind/l)
-
(7)
TSICR2 = 57.6B.0.081; where B total wet cyclopoid biomass (mg/l)
-
(8)
TSICR3 = 40.9CB0.097; percentage of cyclopoid biomass in the total crustacean biomass
-
(9)
TSICR4 = 58.3(CY/CL).0.071; CY/CL: ratio of cyclopoids (CY) to cladoceran biomass (CL)
-
(10)
TSI CR5 = 5.08Ln(CY/CA) + 46.6; ratio of cyclopoids (CY) to calanoid biomass (CA)
The mean value of TSI (mTSICR) obtained from of above five equation is used as an indicator of ecological status of lakes.
Statistical Analysis
Statistical analysis was applied on dataset of environmental and biological factors to know the spatio-temporal variability. The Spearman’s bivariate correlation test was performed to explain the level of significant relationship among the environmental factors. Krushkal- Wallis test at the level of significance (5%) were used for analysis of difference among the physico-chemical water quality parameters between the oxbow lakes using SPSS 16.0. One–way ANOVA test was performed at 1% level of significance to determine the annual significant differences between the lakes using SPSS 16.0. Canonical correspondence analysis (CCA) is well known multivariate method to explain the biological identified assemblage and environmental factors. The CCA analysis was performed in R (R Development Core Team 2018).
Results
Variations among Environmental Factors
During the study period, the physical and chemical water quality parameters were assessed seasonally taking average of two years for each station. Correlogram of environmental variables based on their relationship is depicted (Fig. 2). The size of a circle indicates the strength of the correlation and the colour indicates the direction of correlation (that is, blue = positive, orange to red = negative). Only significant correlations (p < 0.01) are shown in the Fig. 2. The correlation analysis shows high correlation between the variables EC and Alk (p < 0.01, r = 0.85), between EC and Hard (p < 0.01, r = 0.83), Hard and Temp (p < 0.01, r = 0.73). It was also found that PO4 − P was positively correlated with Depth (p < 0.01, r = 0.66), Hard, Alk and EC. On the other hand, N:P were negatively correlated with PO4 − P (p < 0.01, r = -0.72).
The spatio-temporal variations of each physico-chemical parameter are given in the Fig. 3. The physico-chemical parameters measured for each lake reflect a spatial difference in majority of variables except Temp (p = 0.81), Depth (P = 0.085), Chl a (0.61), NO3-N (p = 0.24) and N:P (p = 0.13) as confirmed by Kruskal–Wallis test (p < 0.05).
Spatio-temporal variations of environmental parameters viz: Depth, Secchi depth (SD), Water temperature (Temp), pH, Electrical conductivity (EC), Dissolved oxygen (DO), Total Alkalinity (Alk), Total Hardness (Hard), Chlorophyll a (Chl a), Phosphate (PO4-P), Nitrate (NO3-N), Nitrate: phosphate (N:P). Note: PRM: Pre-monsoon season; MON: Monsoon season; POM: Post-monsoon season; WIN: Winter season
Spatio-temporal Variation of Diversity and Community Characteristics of Zooplankton
Altogether 68 species of zooplankton were identified in the two oxbow lakes: Khalsi (54 species) and Akaipur (45 species) (Table 2). Out of 68 species listed: Rotifera (47 species), Cladocera (12 species), Copepoda (2 orders), Ostracoda (2 species) and Protozoa (5 species), only 3 species of rotifer (Filinia longispina, B. fulcatus, Keratella cochlear) were recorded throughout the study period. Species richness was higher in Khalsi than Akaipur lake. Relative abundance of B. fulcatus and B. budapestiensis were more than 10% each while B. budapestiensis was absent in Khalsi lake. K. longiseta was recorded in all seasons except premonsoon in Khalsi lake while absent in Akaipur lake. K. quadrata was found abundant in Akaipur lake whereas absent in Khalsi lake. Maximum species (14): Filinia longispina, Brachionus rubens, B. fulcatus, B. forficula, Keratella cochlearis, K. tropica, Bosmina longirostris, Polyarthra vulgaris, Ceriodaphnia cornuta, Moina branchiate, M. micrura, Mesocyclops sp., Phyllodiaptomus sp. and Centropyxis aculeate were commonly found in both the lakes. Polyarthra vulgaris was occasionally recorded in warmer season from premonsoon to post-monsoon and absent in winter (Table 2).
The 21 species/genera viz. Polyarthra dolicoptera, Filinia longiseta, F. opoliensis, T. longiseta, P. quadricornis, B. Calyciflurous, B. caudatus f. austerogenitus, K. longiseta, K. tropica, Mytilina mucronata, M. ventralis, Lecane ungulate, Testudinella patina, Synchaeta sp., A. herricki, Leydigia sp., Cypris sp., Stenocypris sp., Difflugia corona, Vorticella sp. and Colpodia colpodia were recorded only in Khalsi lake with varying abundance and frequency. A total of 10 species /genera viz. K. quadrata, T. cylindrica P. multiappendiculata, Lepadella patella, L. ploenensis L. luna, Alona verrucosa, Notholca sp., Asplanchna brightwelli and Diaphanosoma sp. were observed only in Akaipur lake with varying seasonal abundance and frequency (Table 2). Out of 12 species/taxa of cladocerans Ceriodaphnia cornuta, Moina branchiate and M. micrura were recorded in few numbers during monsoon seasons in Khalsi lake. Mesocyclops sp. and Phyllodiaptomus sp. were recorded throughout the study period but had higher abundance during monsoon and post-monsoon. Number of nauplii decreased in 2015–16 as compared to 2014–15. The result of the various indices such as diversity (Shannon–Wiener H′), richness (Margalef D), evenness (Pielou J′) of both lakes varies significantly (Table 3).
Degree of Eutrophication (TSI)
Trophic status was calculated based on three water parameters- transparency (secchi disk depth), total phosphorus and total chlorophyll a, and two taxa: rotifer and crustaceans. TSI results show that TSI (Chl a) varies significantly from 33.57 (pre-monsoon of 1st year) to 53.48 (premonsoon of 2nd year). The study indicated Khalsi lake is in transition phase from oligotrophic to higher mesotrophic state. In Akaipur lake TSI (Chl a) oscillate from 37.14 (pre-monsoon of 1st year) to 54.4 (monsoon of 1st year), indicating similar condition to Khalsi lake. The value of TSI (SD) varies from 53.03 (post monsoon of 1st year) to 70.51 (pre-monsoon of 2nd year) in Khalsi lake, and 63.09 (post-monsoon of 1st year) to 82.51 (pre-monsoon of 2ndyear) in Akaipur. The total phosphorus based TSI (TSI (TP)) ranged from 52.18 (pre-monsoon of 1st year) to 75.43 (post-monsoon of 2nd year) (Fig. 4a & b). The mTSI varied between the lakes (p > 0.05) ranging from 47.28 ± 10.28 to 62.53 ± 13.14 in Khalsi and 51.00 ± 16.23 to 62.91 ± 15.06 in Akaipur. The mTSI values indicated that both the lakes are becoming moderately eutrophic (Table 4).
Temporal variation of TSI in seasonally opens (Khalsi) and closed (Akaipur) oxbow lakes. Note: 1: 2014–2015; 2: 2015–2016; PRM: Pre-monsoon season; MON: Monsoon season; POM: Post-monsoon season; WIN: Winter season; mTSI ROT: mean trophic status index based on Rotifer; mTSI CR: mean trophic status index based on Crustaceans; TSI (Chl a): trophic status index based on Chlorophyll a; TSI (SD): trophic status index based on secchi depth; TSI (TP): trophic status index based on total phosphorus
The TSIROT values varied from 56.41 ± 2.38 to 62.70 ± 6.70 and 58.73 ± 2.10 to 61.80 ± 6.87 in Khalsi and Akaipur lake, respectively (Fig. 4a & b). The calculated mTSIROT values indicated both the lakes were in transition stage from slightly eutrophic to moderately eutrophic condition. The mROTCR value varied from 49.26 ± 3.44 to 62.32 ± 6.08 and 52.63 ± 5.68 to 67.38 ± 2.38 in Khalsi and Akaipur lake, respectively (Fig. 4a & b). The variation of mROTCR in Akaipur lake indicate shifting from meso-eutrophic to hyper eutrophic condition. Single peak value in mROTCR was observed during monsoon of 2014–2015.
Pearson’s correlations among taxonomic diversity indices (D, J, H`), trophic status indices (mTSIROT and mTSICR) and environmental parameters were analysed. The mTSIROT of both lakes were found to exhibit a significantly positive correlation with PO4-P and Chl a, and significantly negative correlation with N:P and DO concentration. The mTSICR were also significantly negatively correlated with N:P and positively correlated with PO4-P and Chl a d in both lakes. These indices were also significantly and negatively correlated with Temp and DO in Khalsi lake and insignificantly correlated in Akaipur lake. The Shannon Wiener (H`) is significantly positively correlated with DO concentration in both lakes but negatively correlated with PO4-P and Chl a concentration. Pielou’s evenness (J) of both the lakes was significantly and negatively correlated with water depth and positively correlated with EC. The significance of Margalef index (D) in both lakes was highly variable (Table 5). Assessment of ecological status based on trophic staus of the lakes have been widely used worldwide (Table 6).
CCA was carried out to identify the important environmental factors influencing the zooplankton abundance in Khalsi and Akaipur lake. The first axis of the CCA (CCA1) explained 23% of the total variation of the model and 15% of the variability was explained by the second component. The first two component CCA indicated that the environmental parameters including phosphate, Secchi disk depth (transparency), alkalinity, hardness, electrical conductivity, chlorophyll a and water depth are the important parameters influencing the zooplankton abundance in Khalsi lake. On the other hand, nitrate, temperature, pH, N:P and DO are the important parameters influencing the zooplankton abundance in Akaipur lake. CCA also indicated that the assemblage pattern of zooplankton in Akaipur lake was distinct from Khalsi lake (Fig. 5).
A canonical correspondence analysis (CCA) of zooplankton species and physicochemical parameters. Sl, Scaridium longicaudum; Pv, Polyarthra vulgaris; Pm, Polyarthra multiappendiculata; Ps, Polyarthra sp.; Fl, Filinia longispina; Ft, Filinia terminalis; Flt, Filinia longiseta; Fo, Filinia opoliensis; Ts, Trichocerca similis; Tl, Trichocerca longiseta; Tc, Trichocerca cylindrical; Ts, Trichocera sp.; Af, Anuraeopsis fissa; Pp, Platyias polycanthus; Pq, Platyias quadricornis; Cg, Cephalodella gibba; Br, Brachionus rubens; Bfu, Brachionus fulcatus; Bfo, Brachionus forficula; Bb, Brachionus budapestiensis; Bc, Brachionus Calyciflurous; Bp, Brachionus patulus; Bq, Brachionus quadridentatus; Bca, Brachionus caudatus f. austerogenitus; Bc, Brachionus caudatus; Bd, Brachionus diversicornis; Ba, Brachionus angularis; Kc, Keratella cochlearis; Kq, Keratella quadrata; Kl, Keratella longiseta; Kt, Keratella tropica; Mm, Mytilina mucronata; Mv, Mytilina ventralis; Ms, Monostyla spp.; Lu, Lecane ungulate; Lb, Lecane bulla; Lp, Lecane ploenensis; Ll, Lecane luna; Lpa, Lepadella patella; Ls, Lepadella sp; Ss, Synchaeta sp.; Ns, Notholca sp.; Ab, Asplanchna brightwelli; Ah, Asplanchna herricki; Asp, Asplanchna sp.; Bl, Bosmina longirostris; Bs, Bosmina sp.; Cc, Ceriodaphnia cornuta; Mb, Moina brachiata; Mmi, Moina micrura; Mma, Moinodaphnia macleayi; Av, Alona verrucosa; As, Alona sp.; Les, Leydigia sp.; Dl, Daphnia lumhottzi; Ds, Daphnia sp.; Cyc, Cyclopoid copepod; Cac, Calanoid copepod; Cs, Cypris sp.; Sts, Stenocypris sp.; Sp, Stylonychia pustulata; Ca, Centropyxis aculeate; Dc, Difflugia corona; Vs, Vorticella sp.; Coc, Colpodia colpoda
Discussion
The ecological state exclusively depends on physico-water quality parameters and is highly variable in shallow lakes which are disconnected from river. Both studied oxbow lakes were significantly different based on electrical conductivity, phosphorus concentration, DO, pH, alkalinity and hardness. Differences in hydrological parameters may be attributed to riverine connectivity of lakes (Amoros and Bornette 2002) and other climatological factors (Sarkar et al. 2020). Seasonal fluctuation of water depth was significantly different in both lakes due to rainfall of the region and latitudinal connectivity to river channel. Water temperature is one of the most influencing affecting chemical and biological process of ecosystem (Cremona and Blank 2021). Fluctuations in temperature were related to regional climatic conditions and air temperature. The pH value in both the lakes was alkaline with limited fluctuation indicating optimum level (BIS 2003) for productivity. The pH is a vital component for biochemical function of aquatic ecosystem (Jena et al. 2013). Slightly higher pH value in Akaipur lake might be due to oscillation of metabolic activity of aquatic organism, and photosynthetic activity (Saha et al. 2021). The coverage of macrophytes in lakes also attributed to diurnal fluctuations in pH value (Rameshkumar et al. 2019). Comparatively lower pH value in Khalsi might be due to decomposition of macrophyte which is in corollary to the study of Tang et al. (2019). Bala and Mukherjee (2010) observed similar finding in Nadia wetland of West Bengal. Dissolved oxygen (DO) is one of the health indicators of aquatic ecosystem. DO of both lakes were well within acceptable range (above 5 mg/L) for ecological wellbeing. DO fluctuations depends on temperature and mixing of nutrient due to influx of runoff from surroundings (Ouhmidou et al. 2015). DO concentration in both the lakes was lowest in monsoon and highest in winter season. Heavy rain during monsoon season brings organic matter such as plant leaves, grasses and other organic matters into the lakes, causing enhanced microbial activity to decompose the vegetation and accelerate the use of oxygen (Mandal et al. 2012).
Chl a is an important component to assess overall algal biomass of lakes and classify their trophic level (Gregor and Mařsalek 2004). Chl a was very high in pre-monsoon (PRM) in both the lakes due to decreased water depth and increased light penetration. Das Sarkar et al. (2021) studied and reported environmental factors influencing Chl a concentration in Gangetic wetlands were water temperature, total alkalinity, electrical conductivity, and pH. The concentration of Chl a in pre-monsoon season was higher due to favourable environmental factors. Annual average Chl a was higher in 2015–16, which might be due to higher influx of organic nutrient in the lakes. Enclosure culture (pen culture) practice was initiated during 2015–16 in both the lakes that might be the source of higher PO4-P leading to eutrophication. Similar observations were made in Baiyangdian lake by Wang et al. (2013). Nutrient concentration (NO3-N and PO4-P) increased from 2014–15 to 2015–16 in both the lakes, with higher deviation in Akaipur. The enhanced organic nutrients particularly, NO3-N and PO4-P due to unscientific pen culture practice in Akaipur as compared to the Khalsi during 2015–2016 is in line with results of Beveridge (1984). The highest concentration of NO3-N during monsoon (June to September) in the present study may also be due to the allochthonous organic input and the decomposition of the aquatic macrophytes and jute retting (Ghosh and Biswas 2018). Enhanced nutrient (NO3-N and PO4-P) concentration during second year of the study, which might be the reason for transition from high mesotrophic to slight eutrophic state. N:P value have decreased significantly in 2015–16 in both the lakes. Tang et al. (2019) suggested N as the only limiting factor in Baiyangdian lake and McCarthy et al. (2013) suggested P as limiting factor in Taihu lake, China for eutrophication. The deviation of the result in our study in contrast to these studies might be due to the fact that supply of both nitrogen and phosphorus from atmospheric deposition, non-point source as agriculture runoff has potentially contributed to increasing nutrients loads in the lakes.
The pattern of zooplankton community structure and abundance are very important for the maintenance of the ecological health of an aquatic ecosystem. Zooplankton are ecological indicators of aquatic environment health (Neto et al. 2014). Rotifers respond quickly to aquatic environmental changes due to their short life cycle and are therefore used as indicators of overall health or condition (Carriack and Schelske 1977). Three common species Filinia longispina, Brachionus fulcatus, Keratella cochlearis of rotifer was recorded throughout the study period. Sharma et al. (1992) noted Filinia longispina is a eurytopic alkaline species. Two commonly occurring species Brachionus fulcatus, Keratella cochlearis of the genus Brachionus and Keratella are considered as cosmopolitan and eurytopic species (Branco et al. 2002; Kumari et al. 2017) due its broader ecological value, thermal tolerance, and geographical distribution (Bennett et al. 2019). Rotifer species Filinialon gispina, Brachionus rubens, B. fulcatus, B. forficula, Keratella cochlearis, K. tropica, Bosmina longirostris and Polyarthra vulgaris were present in both lakes with varying degree of abundance. Keratella cochlearis and K. tropica have been recorded from many freshwater bodies such as ponds to reservoir (Doukhandji and Arab 2017). The seven species of rotifers from both lakes have wide range of pollution tolerance (Sládeček 1983; Sharma et al. 1992; Branco et al. 2002; Kumari et al. 2017). Genus Keratella and Brachionus are considered as indication of eutrophic state of the lake due to their close association with nutrient rich environment (Branco et al. 2002; Kumari et al. 2017; Doukhandji and Arab 2017; Smaoune et al. 2021). Genus Polyarthra was less abundant in both the lakes due to high nutrient availability, as it is less tolerant. Occurrence of Polyarthra is found in oligotrophic, mesotrophic as well as eutrophic lakes (García-Chicote et al. 2018; Obertegger et al. 2008, 2014; Karpowicz et al. 2020). In the present study, in winter (higher PO4-P), Polyarthra were absent, therefore this genus is indicator of oligotrophic to mesotrophic state. Genus Trichocerca was recorded only in post monsoon and winter when nutrient concentration was higher. Castro et al. (2005) also reported this genus from eutrophic environment. Genus Bosmina was relatively abundant throughout the seasons except in monsoon. In monsoon season, generally flow of water is higher compared to other seasons and Cladocerans usually prefer lentic water (littoral zone with macrophytes) (Maia-Barbosa et al. 2008; Jeong et al. 2015), therefore the genus was absent in both lakes in monsoon. Only cladocerans Ceriodaphnia cornuta, Moina branchiate and M. micrura were recorded during monsoon seasons in Khalsi because of their preference towards clear water and macrophyte dominant reasons. Mergeay et al. (2006) have also found similar results and confirmed their association in macrophyte dominated reasons in shallow lakes of southern Kenya. Higher abundance of copepods in monsoon and post-monsoon indicated well mixing of nutrients and favorable temperature. Yin et al. (2018) also reported higher abundance of copepod in mesotrophic lake. Copepod adults have greater preference towards nutrient rich environment than nauplii. Mathews et al. (2018) have reported that favorable temperature range of 25-280C for growth and reproduction of copepod. In this context monsoon and post-monsoon season is most favorable season for higher abundance of copepods. Ostracod communities have close association with aquatic macrophytes (Matsuda et al. 2015) and higher ecological tolerance (Kiss 2007). Centropyxis, Difflugia and Arcella were dominant in Khalsi lake but Centropyxis in Akaipur lake, throughout the study period. All these protozoans have wide range of tolerance to nutrient and are found in oligotrophic to eutrophic conditions (Madoni 2011).
Measuring zooplankton species diversity is one of the most important characteristics of aquatic ecosystem to maintain stability as means of coping with any environmental changes. We have compared the value of various indices between two lakes. We have found that lowest diversity indices H′ and D from Akaipur during winter. Diversity has decreased due to unscientific enclosure culture practice and lack of connectivity with main river channel along with abiotic factor, natural predation by copepod and cladoceran, competition for food and increasing eutrophication (Marcus 2004; Perbiche-Neves et al. 2016; Arcifa et al. 2020).
The mTSIROT and mTSICR values were determined annually for both the lakes but spatial as well as temporal difference was not significant. Rotifer community among the zooplankton shows quick response to the environmental changes and is considered as highly suitable for assessing degree of eutrophication (Jekatierynczuk-Rudczyk et al. 2014; Dembowska et al. 2015; Ejsmont-Karabin et al. 2016; Wen et al. 2017; Smaoune et al. 2021). In this study trophic state index was assessed based on rotifer and crustacean groups. The mTSIROT and mTSICR had significantly positive relationship with phosphorus concentration (PO4-P) in both the lakes. Similar finding was reported by Xiong et al. (2016) from two subtropical lakes. Although, there are cumulative effect of many abiotic factors in changing the trophic dynamics of ecosystem PO4-P and Chl a showed significantly positive relationship with eutrophication.
The composition and diversity of zooplankton species with higher trophic state have well explained by combination of environmental parameters with Pearson’s correlations and CCA analysis. García-Chicote et al. (2018) explained the dominance of some of the species in ecological stressful environment. Our result also shows dominance of genus Brachionus influenced by NO3-N rich environment also supported by favorable temp and pH. According to Branco et al. (2002) and Kumari et al. (2017) dominance of genus Brachionus is a considered as good indicator of eutrophication. Present study also reveals the fluctuation in species composition with high level of eutrophication and reported by Obertegger and Manca (2011). The density of rotifers is much more prevalent in both the lakes.
Conclusion
Rotifers proved their strong sensitivity to nutrient load in freshwater ecosystem based on the scientific evidence and fluctuation in species diversity. The present investigation advocates the suitability of rotifers based TSIs, integrating with physico-chemical parameter of the lakes which is first of its kind. The estimated mTSIs value has validated biological indices to suggest an indicator species for determination of water quality and ecosystem health of the lakes. Based on the findings of hydro biological parameters, the degree of eutrophication varies between the lakes. The mTSICR and mTSI based combination of environmental factors have revalidated our finding to assess degree of eutrophication of oxbow lakes. The study highlights the importance riverine connectivity of lakes and short-term impact of enclosure culture on eutrophication of lakes. The present investigation could further help to understand the phenological mechanisms of water quality and rotifer abundance for scientific management of lakes. Nutrient concentration PO4-P, temperature, Chl a, EC, Hard, Alk and SD was found to be most influencing factor influencing trophic state.
Khalsi lake, a partially open wetland has more sinking capacity as compared to Akaipur (closed wetland) having scope for continuation of enclosure culture practices provided impact assessment for long term culture are carried out. Khalsi lake has more fish production potential and essential water quality parameters for open water fisheries emphasizing upon the scientific management and ecosystem-based approach for sustainable fish production. Khalsi lake can be more promising than the Akaipur lake towards biodiversity conservation and enhancement in the present climate change scenario through sustainable management interventions. Akaipur lake requires immediate intervention in terms of restriction of irrational culture practices, management of point source of organic load. Akaipur lake might be succeeding towards super eutrophication if same practices continue for long term without considering scientific management. Present study is first of its kind to validate the physico-chemical and biotic factors with rotifer and crustacean based indices advocating rotifers as excellent indicator organism for ecological assessment, water quality monitoring and assessment of degree of eutrophication for better policy decisions. Thus, addressing the issues related to the eco-hydrological alteration taking place in the closed and semi-closed lakes for ensuring sustainable fisheries enhancement and to carry forward the ecosystem services for wellbeing of the riparian community must be prioritized in the context of degraded ecosystem health, anthropogenic pressures and climate change.
Data Availability
Data will be available based on request.
References
APHA (2005) Standard Methods for the Examination of Water and Wastewater, 21st edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Abell JM, van Dam-Bates P, Özkundakci O, Hamilton DP (2020) Reference and current trophic level index of New Zealand lakes: benchmarks to inform lake management and assessment. New Zealand Journal of Marine and Freshwater Research 54:636–657. https://doi.org/10.1080/00288330.2020.1726974
Adamovich BV, Zhukova TV, Mikheeva TM et al (2016) Long-term variations of the trophic state index in the Narochanskie Lakes and its relation with the major hydroecological parameters. Water Resources 43:809–817. https://doi.org/10.1134/S009780781605002X
Amoros C, Bornette G (2002) (2002) Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshwater Biology 47:761–776
Arcifa MS, de Souza BB, deMorais-Junior CS, Bruno CCC (2020) Functional groups of rotifers and an exotic species in a tropical shallow lake. Scientific Reports 10:14698. https://doi.org/10.1038/s41598-020-71778-1
Bala G, Mukherjee A (2010) Water quality index of some wetland in Nadia district, West Bengal, India. International Journal of Lakes and Rivers 4(1):21–26
Battish SK (1992) Freshwater Zooplankton of India. Oxford and IBH Publishing Co., New Delhi, pp 233
Bennett S, Duarte CM, Marbà N, Wernberg T (2019) Integrating within-species variation in thermal physiology into climate change ecology. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 374(1778):20180550. https://doi.org/10.1098/rstb.2018.0550
Benzie JAH (2005) The genus Daphnia (including Daphniopsis) (Anomopoda: Daphniidae). In: Dumont H. J. (ed.), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 21. Kenobi Productions, Ghent & Backhuys Publishers, Leiden
Beveridge MCM (1984) Cage and pen fish farming. Carrying capacity models and environmental impact. FAO Fisheries Technical Paper 255. FAO, Rome
BIS (2003) Indian standard drinking water specifications IS 10500:1991, Edition 2.2 (2003-2009). Bureau of Indian Standards, New Delhi
Biswas BC, Panigrahi AK (2015) Ecology and zooplankton diversity of a wetland of jhenidah district, Bangladesh. The International Journal of Innovative Research in Science, Engineering and Technology 1(9):246–249
Bottrell HH, Duncan A, Gliwicz ZM, Grygierek EA, Herzig H, Kurasawa H, Larsson P, Weglenska T (1976) A review of some problems in zooplankton production studies. Norwegian Journal of Zoology 24:419–456
Branco CWC, Roch MIA, Pinto GFS, Gomara GA, Filippo R (2002) Limnological features of ^ Funil Reservoir (R.J., Brazil) and indicator properties of rotifers and cladocerans of the zooplankton community. Lakes Reservoirs: Research and Management 7:87–92. https://doi.org/10.1046/j.1440-169X.2002.00177.x
Carlson RE (1977) A Trophic State Index for Lakes. Limnology and Oceanography 22(2):361–369
Carlson RE, Havens KE (2005) Graphical methods for the interpretation of relationships between trophic state variables. Lakes Reservoirs: Research and Management 21(1):107–118
Carriack JH, Schelske LC (1977) Have we over looked the importance of small phytoplankton in productive waters. Limnology and Oceanography 42:1612–1613
Castro BB, Antunes SC, Pereira R, Soares AMVM, Gonçalves F (2005) Rotifer community structure in three shallow lakes: seasonal fluctuations and explanatory factors. Hydrobiologia 543:221–232
Chaudhuri SR, Mishra M, Salodkar S, Sudarshan M, Thakur AR (2008) Traditional aquaculture practice at east Calcutta Wetland: The safety assessmen. American Journal of Environmental Sciences 4:140–144. https://doi.org/10.3844/ajessp.2008.140.144
Cremona F, Blank K (2021) Haberman J (2021) Effects of environmental stressors and their interactions on zooplankton biomass and abundance in a large eutrophic lake. Hydrobiologia 848:4401–4418. https://doi.org/10.1007/s10750-021-04653-3
Das BK, Roy A, SomS, Chandra G, Kumari S, Sarkar UK, Bhattacharjya BK, Das AK, Pandit, A (2021) Impact of COVID-19 lockdown on small-scale fishers (SSF) engaged in floodplain wetland fisheries: evidences from three states in India. Environmental Science and Pollution Research (2021)https://doi.org/10.1007/s11356-021-16074-9
Das Sarkar D, Sarkar UK, Naskar M, Roy K, Bose AK, Nag SK, … Das BK (2021) Effect of climato-environmental parameters on chlorophyll a concentration in the lower Ganga basin, India. Revista de Biología Tropical 69(1):60-76
Dembowska E, Napiórkowski P, Mieszczankin T, Józefowicz S (2015) Planktonic indices in the evaluation of the ecological status and the trophic state of the longest lake in Poland. Ecological Indicators 56:15–22. https://doi.org/10.1016/j.ecolind.2015.03.019
Doukhandji N, Arab A (2017) The spatio-temporal distribution of Rotifers in a dam in a Saharan climate region (Foum El Gherza, Algeria). Revue d'écologie 72(2):168–176. http://hdl.handle.net/2042/62077.
Dussart B, Defaye D (2001) Introduction to Copepods, 2nd edn. Backhuys Publishers, Leiden
Edmondson WT (1959) Rotifers, p. 420–494. In: Edmondson WT (ed) Fresh-water Biology, 2nd edn. Wiley, New York, p 1248
Ejsmont-Karabin J (2012) The usefulness of zooplankton as lake ecosystem indicators: rotifer trophic index. Pol J Ecol 60:339–350
Ejsmont-Karabin J, Karabin A (2013) The suitability of zooplankton as lake ecosystem indicators: crustacean trophic state index. Polish J Ecology 61:561–573
Ejsmont-Karabin J, Górniak A, Maciej Karpowicz M (2016) Diversity of rotifer communities in lakes of the Suwalki Landscape Park. Limnol. Rev. 16, 4: 207–211https://doi.org/10.1515/limre-2016-0022
European Commission (2000) Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy Off. J Eur Communities 2000
Ganesan L, Khan RA (2008) Studies on the Ecology of Zooplankton in a Oxbow Wetland of West Bengal, India. In Sengupta, M. and R. Dalwani (Eds) Proceedings of Taal 2007: The 12th World Lake Conference, pp 67–73.
García-Chicote J, Armengol X, Rojo C (2018) Zooplankton abundance: a neglected key element in the evaluation of reservoir water quality. Limnologica 69:46–54
Ghosh D, Biswas JK (2015) Impact of jute retting on native fish diversity and aquatic health of roadside transitory water bodies: an assessment in Eastern India. Journal of Ecology Engine 16(4):14–21. https://doi.org/10.12911/22998993/59342
Ghosh D, Biswas JK (2018) Impact of jute retting on physicochemical profile of Chhariganga oxbow lake in Nadia district, West Bengal, India. Archives of Agriculture and Environmental Science 3(1):36–44. https://doi.org/10.26832/24566632.2018.030104
Gregor J, Mařsalek B (2004) Freshwater phytoplankton quantification by chlorophyll a: A comparative study of in vitro, in vivo and in situ methods. Water Research 38:517–522https://doi.org/10.1016/j.watres.2003.10.033
Hawkins BE, Evans MS (1979) Seasonal cycles of zooplankton biomass insoutheastern Lake Michigan. Journal of Great Lakes Research 5:256–263
Janseen ABG, Teurlines S, An S, Janse JH, Paerl HW, Mooij WM (2014) Alternative stable states in large shallow lakes ? Journal of Great Lakes Research 40:813–826
Jekatierynczuk-Rudczyk E, Zieliński P, Grabowska M et al (2014) The trophic status of Suwałki Landscape Park lakes based on selected parameters (NE Poland). Environmental Monitoring and Assessment 186:5101–5121. https://doi.org/10.1007/s10661-014-3763-0
Jena V, Dixit S, Gupta S (2013) Assessment of water quality index of industrial area surface water samples. International Journal of Chemistry Research 5(1):278–283
Jeong H, KotovAA LW, Jeong R, Cheon S (2015) Diversity of freshwater Cladoceran species (Crustacea: Branchiopoda) in South Korea. Journal of Ecology Environment 38(3):361–366
Jiang X, Xie J, Xu Y, Zhong W, Zhu X, Zhu C (2017) Increasing dominance of small zooplankton with toxic cyanobacteria. Freshwater Biology 62(2) https://doi.org/10.1111/fwb.12877
Junk WJ, Piedade MTF, Lourival R, Wittmann F, Kandus P, Lacerda LD, Bozelli RL, Esteves FA, Cunha CN, Maltchik L et al (2014) Brazilian lakes: their definition, delineation, and classification for research, sustainable management, and protection. Aquatic Conservation: Marine and Freshwater Ecosystem 24:5–22
Sandhya KM, Roy A, Hassan MA, Kumari S, Mishal P, Liamthuanluaia.L,KumarV,Aftabuddin M, Bhattacharjya BK, Meena DK, Ali Y, Naskar B (2019) Traditional Fishing Gears, Fish Catch and Species Composition of Selected Floodplain Wetlands of Lower Gangetic Plains, West Bengal, India. Fishery Technology 56:101-109
Karnatak, G, Sarkar, U, Naskar, M et al (2020) Modeling pre-spawning fitness and optimal climate of spotted snakehead Channapunctata (Bloch, 1793) from a Gangetic floodplain wetland of West Bengal, India. International Journal of Biometeorology 64:1889–1898 https://doi.org/10.1007/s00484-020-01976-z
Karpowicz M, Ejsmont-Karabin J, Kozłowska J, Feniova I, Dzialowski AR (2020) Zooplankton Community Responses to Oxygen Stress. Water 12:706 https://doi.org/10.3390/w12030706
Kiss A (2007) Factors affecting spatial a temporal distribution of Ostracoda assemblages in different macrophyte habitats os a shallow lake (Lake Fehér, Hungary). Hydrobiologia 585:89–98
Koste W (1978) Rotatoria. Die RadertiereMitteleuropas. UberordnungMonogononta. Ein Bestimmungswerk, begrundet von Max Voigt. 2. Auflageneubearbeitet von Walter Koste. 2 Bande. Textband: VIII, 673 S., ISBN 3–443–39071–4 II Tafelband: II, 476 S., 234 Taf.
Kotov A, Forró L, Korovchinsky N, Petrusek A (2013) World checklist of freshwater Cladocera species, World Wide Web electronic publication. Available online at http://fada.biodiversity.be/group/show/17. Accessed 20 May 2021
Kumari S, Hassan MA, Lianthuamluaia SKM, Mishal P, Kumar V, Sarkar UK, Jaiswar AK, DeshmukheG SL (2017) Influence of environmental factors on rotifer abundance and biomass in a shallow, tropical oxbow lake, west Bengal. The Bioscan 11(2):129–135
Lehette P, Hernández-León S (2009) Zooplankton biomass estimation from digitized images: a comparison between subtropical and Antarctic organisms. The Association for the Sciences of Limnology and Oceanography 7:304–308
Liang D, Wang Q, Wei N, Tang C, Sun X, Yang Y (2020a) Biological indicators of ecological quality in typical urban river-lake ecosystems: The planktonic rotifer community and its response to environmental factors. Ecological Indicators 112:106–127. https://doi.org/10.1016/j.ecolind.2020.106127
Liang Z, Soranno PA, Wagner T (2020b) The role of phosphorus and nitrogen on chlorophyll a: Evidence from hundreds of lakes. Water Research 185:116236 ISSN 0043–1354 https://doi.org/10.1016/j.watres.2020.116236
Lodi S, VieiraLCG, Velho LFM, BoneckerCC, Carvalho P de, Bini LM (2011) Zooplankton community metrics as indicators of eutrophication in urban lakes. Natureza Conservação 9(1):87–92 https://doi.org/10.4322/natcon.2011.011
Madoni P (2011) Protozoa in wastewater treatment processes: A minireview Italian. Journal of Zoology 78(1):3–11. https://doi.org/10.1080/11250000903373797
Maia-Barbosa PM, Peixoto RS, Guimaraes AS (2008) Zooplankton in littoral waters of a tropical lake: a revisited biodiversity. Brazilian Journal of Biology 68:1069–1078
Mandal S, Debnath M, Ray S, Ghosh PB, Roy M, Ray S (2012) Dynamic modelling of dissolved oxygen in the creeks of Sagar island, Hooghly-Matla estuarine system, West Bengal, India. Applied Mathematical Modelling 36(12):5952–5963. https://doi.org/10.1016/j.apm.2011.10.013
Marcus N (2004) An overview of the impacts of eutrophication and chemical pollutants on copepods of the coastal zone. Zoology Studies 43:211–217
Margalef R (1958) Temporal succession and spatial heterogeneity in phytoplankton. In: Buzzati-Traverso (ed.), Perspectives in Marine biology. Univ. Calif. Press, Berkeley pp 323–347.
Mason CF (1966) Biology of Freshwater Pollution 3rd Edn., Longman Group Ltd., England 356p.
Mathews L, Faithfull CL, Lenz PH (2018) Nelson CE (2018) The effects of food stoichiometry and temperature on copepods are mediated by ontogeny. Oecologia 188(1):75–84. https://doi.org/10.1007/s00442-018-4183-6
Matsuda JT, Lansac-Tôha FA, Martens K et al (2015) Association of body size and behavior of freshwater ostracods (Crustacea, Ostracoda) with aquatic macrophytes. Aquatic Ecology 49:321–331. https://doi.org/10.1007/s10452-015-9527-2
McCauley E (1984) The estimation of the abundance and biomass of Zooplankton in samples. In: Downing JA, Rigler FH (eds) A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters. Blackwell, Oxford, pp 228–265
McCarthy MJ, Gardner WS, Lehmann MF, Bird DF (2013) Implications of water column 750 ammonium uptake and regeneration for the nitrogen budget in temperate, eutrophic Missisquoi 751 Bay, Lake Champlain (Canada/USA). Hydrobiologia 718(1):173–188. https://doi.org/10.1007/s10750-752013-1614-6
Meena DK, Lianthuamluia L, Mishal P, Swain HS, Naskar BK, Saha S, Sandhya KM, Kumari S, Tayung T, Sarkar UK, Das BK (2019) Assemblage patterns and community structure of macro-zoobenthos and temporal dynamics of eco-physiological indices of two lakes, in lower gangetic plains under varying ecological regimes: A tool for wetland management. Ecological Engineering 130:1–20
Mergeay J, Declerck S, Verschuren D (2006) De Meester L (2006) Daphnia community analysis in shallow Kenyan lakes and ponds using dormant eggs in surface sediments. Freshwater Biology 51:399–411
Neto AJG, Silva LC, Saggio AA, Rocha O (2014) Zooplankton communities as eutrophication bioindicators in tropical reservoirs. Biota Neotropica. https://doi.org/10.1590/1676-06032014001814
Nogrady T, Segers H (eds) (2002) Rotifera 6; The Asplanchnidae, Gastropodidae, Lindiidae, Microcodinidae, Synchaetidae, Trochosphaeridae. In Dumont, H. J. (ed.), Guides to the Identification of the Microinvertebrates of the Continental Waters of the World 18. Backhuys Publishers BV, Dordrecht, The Netherlands
Obertegger U, Flaim G, Sommaruga R (2008) Multifactorial nature of rotifer water layer preferences in an oligotrophic lake. Journal of Plankton Research 30(6):633-643https://doi.org/10.1093/plankt/fbn027
Obertegger U, Flaim G, Fontaneto D (2014) Cryptic diversity within the rotifer Polyarthradolichoptera along an altitudinal gradient. Freshwater Biology 59(11):2413-2427https://doi.org/10.1111/fwb.12447
Obertegger U, Manca M (2011) Response of rotifer functional groups to changing trophic state and crustacean community. Journal of Limnology 70:231–238
Oh HJ, Jeong HG, Nam GS, Oda Y, Dai W, Lee EH, Kong D, Hwang SJ, Chang KH (2017) Comparison of taxon-based and trophi-based response patterns of rotifer community to water quality: applicability of the rotifer functional group as an indicator of water quality. Animal Cells and Systems 21(2):133–140 https://doi.org/10.1080/19768354.2017.1292952 To link to this article: https://doi.org/10.1080(Wu and Wang /19768354.2017.1292952
Ouhmidou M, Chahlaoui A, Kharroubi A, Chahboune M (2015) Study of the physico-chemical and bacteriological quality of the barrage Hassan Addakhil of Errachidia (Morocco). Journal of Materials and Environmental Science 6(6):1663–1671
Perbiche-Neves G, Saito VS, Previattelli D, da Rocha CEF, Nogueira MG (2016) Cyclopoid copepods as bioindicators of eutrophication in reservoirs: Do patterns hold for large spatial extents? Ecological Indicators 70:340–347
Pielou EC (1966) The measurement of diversity in different types of biological collections. Journal of Theoretical Biology 13:131–144
Rameshkumar S, Radhakrishnan K, Aanand S (2019) Rajaram R (2019) Infuence of physicochemical water quality on aquatic macrophyte diversity in seasonal wetlands. Applied Water Science 9:12. https://doi.org/10.1007/s13201-018-0888-2
R Development Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org
Roshith CM, Meena DK, Manna RK, Sahoo AK, Swain HS, Raman RK, Sengupta A, Das BK (2018) Phytoplankton community structure of the Genetic (Hooghly-Matla) estuary: Status and ecological implications in relation to eco-climatic variability. Flora 240:133–143
Ruttner-Kolisko A (1977) Suggestions for biomass calculation of plankton rotifers. Arch Hydrobiol Beih Ergebn Limnol 8:71–76
Saha A, Ramya VL, Jesna PK, SibinaMol S, Panikkar P, Vijaykumar ME, Sarkar UK, Das BK (2021) Evaluation of Spatio-temporal Changes in Surface Water Quality and Their Suitability for Designated Uses, Mettur Reservoir, India. Natural Resources Research 30(2):1367–1394
Sandhya KM, Hassan MA, Kumari S, Mishal P, Lianthuamluaia L, Kumar V, Aftabuddin M, Meena DK (2016) Length-weight relationships of four indigenous freshwater fish species from Khalsi wetland in lower Ganga basin, West Bengal, India. Journal of Applied Ichthyology 32:505–506. https://doi.org/10.1111/jai.13005
Sanyal P, Bhattacharya N, Chakraborty S (2015) Biomonitoring of Four Contrasting Wetlands of Kolkata, West Bengal Based on Zooplankton Ecodynamics and Biotic Indices. Journal of Environmental Protection 6:683–699. https://doi.org/10.4236/jep.2015.67062
Sarkar S, Sarkar UK, Sajina AM, Kumari S, Mishal P (2021a) Status, ecological services and management of aquatic weeds of floodplain wetlands in India: An overview. Lakes Reservoirs: Research and Management. https://doi.org/10.1111/lre.12353
Sarkar UK, Johnson C, Kumari S, Bakshi S, Karnatak G, Ghosh BD, Mishal P, Lianthuamluaia, Das BK (2021b) Population dynamics of Indian river shad Gudusia chapra (Hamilton, 1822) using length frequency analysis for fisheries management in a floodplain wetland of Ganga River Basin, India. Lakes and Reservoirs 26(3) https://doi.org/10.1111/lre.12365
Sarkar UK, Mishal P, Borah S, Karnatak G, Chandra G, Kumari S, Meena DK, Debnath D, SonaYengkokpam S, Das P, DebRoy P, Yadav AK, Md Aftabuddin, Gogoi P, Pandit A, Bhattacharjya BK, Tayung T, Lianthuamluaia L, Das BK (2020) Status, Potential, Prospects, and Issues of Floodplain Wetland Fisheries in India: Synthesis and Review for Sustainable Management. Reviews in Fisheries Science & Aquaculture 29:1, 1-32https://doi.org/10.1080/23308249.2020.1779650
Sarkar UK, Roy K,Naskar M, Karnatak G, Mishal P, Baksi S, Kumari S, Lianthuamluia L, Das BK (2021b) Assessing vulnerability of freshwater minnows in the Gangetic floodplains of India for conservation and management: Anthropogenic or climatic change risk? Climate Risk Management 33(2021c):100325 ISSN 2212-0963https://doi.org/10.1016/j.crm.2021.100325
Sarma D, Dutta A (2012) Ecological study of two riverine lakes of goalpara District of Assam, India. Nature, Environment and Pollution Technology 1(2):297–302
Schindler DW, Noven B (1971) Vertical distribution and seasonal abundance of zooplankton in two shallow lakes of the Experimental Lakes Area, North western Ontario. Journal of the Fisheries Research Board of Canada 28:245–256
Segers H (1995) Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. 6. Rotifera Volume 2: The Lecanidae (Monogononta). SPB Academic Publishing, The Hague
Segers H (2008) Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 595(1):49–59 https://doi.org/10.1007/s10750-007-9003-7
Sehgal KL (1983) Planktonic Copepods of Freshwater Ecosystems. Interprint, New Delhi, p 169
Shannon CE, Weaver W (1949) The Mathematical Theory of Communication. University of Illinois Press, Urbana
Sharma BK, Sharma S, Dunani VK (1992) Freshwater Rotifer from Darbhanga city, Bihar. Records of the Zoological Survey of India 91(3–4):431–448
Sharma BK (2009) Diversity of Rotifers (Rotifera: Eurotatoria) of Loktak lake, north-eastern India. Tropical Ecology 50:277–285
Sharma BK (2011) Zooplankton communities of Deepor Beel (a Ramsar site), Assam (N. E. India): ecology, richness, and abundance. Journal of Tropical Ecology 52(3):293–302
Sharma BK, Sharma S (2012) Diversity of zooplankton in a tropical Oxbow lake of the Brahmaputra river basin, Assam (Northeast India). Opuscula Zoologica 43(2):187–195
Sharma P, Bora PJ (2020) Water Quality Assessment Using Water Quality Index and Principal Component Analysis: A Case Study of Historically Important Lakes of Guwahati City, North-East India. Applied Ecology and Environmental Sciences 8(5):207-217https://doi.org/10.12691/aees-8-5-4
Sládeček V (1983) Rotifers as indicators of water quality. Hydrobiologia 100:169–171
Smaoune G, Bouchelouche D, Taleb A et al (2021) Evaluation of the trophic status in three reservoirs in Algeria (north west) using physicochemical analysis and rotifers structure. Environmental Science and Pollution Research 28:46627–46642. https://doi.org/10.1007/s11356-020-11233-w
Strickland JD, Parson TR (1972) A practical handbook of seawater analysis. Fishery Research Board, Canada, pp 310
Tang C, Yi Y, Yang Z, Zhang S, Liu H (2018) Effects of ecological flows release pattern on water quality and ecological restoration of a large shallow lakes. Journal of Cleaner Production 174:577–590
Tang C, Yi Y, Yang Z, Zhou Y, Zerizghi T, Wang X (2019) Planktonic indicator of trophiuc states for a shallow lake (Baiyangdian lake China). Limnologica 78:125712
Victor R, Fernando CH (1979) The freshwater Ostracods of India. Records of the Zoological Survey of India 74:147–242
Vörös L, Padisak J (1991) Phytoplankton biomass and chlorophyll a in some shallow lakes in central Europe. Hydrobiologia 215:111–119
Walter TC, Boxshall G (2015) Cyclopidae Rafinesque, 1815, In: World of Copepods database. Available online at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=106413. Accessed 06 May 2021
Wang X, Wang Y, Liu L, Shu J, Zhu Y, Zhou J (2013) Phytoplankton and Eutrophication Degree Assessment of Baiyangdian Lake Wetland, China. Scientific World Journal 25:436965 https://doi.org/10.1155/2013/436965.
Wen X, Zhai P, Feng R et al (2017) Comparative analysis of the spatio-temporal dynamics of rotifer community structure based on taxonomic indices and functional groups in two subtropical lakes. Scientific Reports 7:578. https://doi.org/10.1038/s41598-017-00666-y
Xiong W, Li J, Chen Y, Shan B, Wang W, Zhan A (2016) Determinants of community structure of zooplankton in heavily polluted river ecosystems. Scientific Reports 6(1):22043 https://doi.org/10.1038/srep22043
Yin L, Ji Y, Zhang Y, Chong L, Chen L (2018) Rotifer Community Structure and its Response to Environmental Factors in the Backshore Wetland of Expo Garden, Shanghai. Aquaculture Fish 3:90–97. https://doi.org/10.1016/j.aaf.2017.11.001
Acknowledgements
This work was financially supported by ICAR-CIFRI under the Institute project. The Authors are grateful to the Director, ICAR-CIFRI for providing necessary facilities. The authors also thank Dr. Bandana Das Ghosh for her help in preparing the GIS map. We would like to thank Mr. Y. Ali and Mr. Bablu Naskar for their assistance in sampling. The authors also acknowledge the constructive suggestions of anonymous reviewers which helped us in strengthening the manuscript.
Funding
The research was conducted with the funding support of the ICAR-Central Inland Fisheries Research Institute, Kolkata.
Author information
Authors and Affiliations
Contributions
Conceptualization: Uttam Kumar Sarkar, Suman Kumari and Ashok Kumar Jaiswar, Formal analysis and data collection: Lianthuamluaia Lianthuamluaia, Mishal Puthiyottil, Sandhya Kavitha Mandhir, Data collection: Archan Kanti Das, Darmendra Kumar Meena,, Data Support: Md. AbulHassan, Methodology: Suman Kumari and Mishal Pudyottil, Original draft preparation: Suman Kumari, Lianthuamluaia Lianthuamluaia, Review and editing: Gunjan Karnatak, Uttam Kumar Sarkar, Mishal Puthiyottil, Funding acquisition: Anil Prakash Sharma and Basanta Kumar Das, All the au-thors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The submitted manuscript is not submitted in any other journal.
Consent to Participate
Not applicable.
Consent for Publication
The approval for submitting the manuscript was received from the ICAR-Central Inland Fisheries Research Institute.
Competing Interests
The authors declare no competing interests.
Disclaimer
The statements made herein are solely the responsibility of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, S., Lianthuamluaia, L., Sarkar, U.K. et al. Environmental Characterization of Two Ecologically Distinct Gangetic Oxbow Lakes using Zooplankton Taxonomic Indices Through Comparative Approach for Wetland Monitoring. Wetlands 43, 14 (2023). https://doi.org/10.1007/s13157-023-01658-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-023-01658-7