Abstract
Legacy mercury (Hg) exists in Upper St. Lawrence River wetland hydric soils and is impacted by a new water level management plan (established in 2017) implemented to restore biodiversity and reduce the monotypic nature of riparian wetlands, currently dominated by Typha spp.. The distribution of Hg within the various components of a riparian wetland provides insight into potential impacts of water level fluctuations. Hydric soil represents 83% of the wetland Hg burden while wetland plant biomass contributed 17%, mostly due to organic detritus (13%). Although Typha roots had a bioconcentration factor of 1.2 (relative to hydric soils) and had the highest total Hg among living tissues (25 ± 9.3 ng/g dry wt.), detritus had the highest overall Hg content (110 ± 53 ng/g dry wt.). While root tissue Hg correlated significantly with soil Hg (p = 0.045), it was determined here that Typha spp. has limited use as a biomonitor in wetlands with low levels of Hg contamination, as in this ecosystem. Hg contained within the organic detritus contributed more to the overall Hg burden in these monotypic Typha wetlands than any other tissue or biomass component analyzed. Consequently, shifts in the plant community that are expected to result from a new water level management plan may alter Hg storage within these wetlands and affect its mobility in this ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The threat of environmental mercury (Hg) contamination persists due to the ubiquity of Hg in the biosphere and the compounding effects of other threats, such as climate change (Chen et al. 2018). In northern New York State (US), Hg is a legacy pollutant mostly originating from atmospheric deposition (Driscoll et al. 2007), the majority of which is from coal and oil combustion (Pirrone et al., 2010). Due to recent regulations on atmospheric pollution, Hg deposition to the Great Lakes basin has decreased over the last few decades (Olsen et al. 2020). However, despite the decrease in deposition rates, legacy Hg remains in Upper St. Lawrence River (USLR) wetland soils and is at risk of mobilization, in light of recent changes to water level management protocols in this large river (Brahmstedt et al. 2019).
Riparian wetlands in the USLR are primarily monotypic Typha spp. stands. Stable water levels, regulated due to the presence of a downstream hydroelectric dam, allowed for Typha spp. to dominate riparian wetlands and decrease overall wetland vegetation biodiversity due to the lack of disturbance to the shoreline between the years 1958 and 2017 (Farrell et al. 2010; Wilcox et al. 2008). At present, the Typha stands are still largely intact, but the goal of the new water level management plan is to increase biodiversity within wetland vegetation communities and the overall river by allowing more natural water fluctuations to thus remove or decrease Typha abundance.
Bioaccumulation of Hg has been observed in Typha spp. (Anning and Akoto 2018; Bonanno and Cirelli 2017; Lominchar et al. 2015; Gomes et al. 2014). Willis et al. (2010) observed that Typha spp. accumulated Hg more effectively than other plant species, including Eleocharis parvula, Saururus cernuus, Juncus effuses, and Panicum hemitomon, although other studies, such as that done by Afrous et al. (2011), have found more effective Hg accumulation in other plant species, Phragmites australis and Scirpus (bulrush), respectively. Given the ability for Typha spp. to bioaccumulate Hg, an assessment of their biomass Hg content is necessary when estimating the Hg burden of a monotypic cattail wetland. The monotypic nature of Typha marshes on the USLR presents an opportunity to examine the contribution of Typha alone to Hg storage within riparian wetland with legacy atmospheric Hg content; other herbaceous plant biomass in the wetland is likely negligible to overall wetland Hg storage due to out competition. This study seeks to quantify Hg burden in biomass compared to soil, assess the relative capacity of Typha as a Hg biomonitor within an USLR wetland, and to discuss potential implications of soil and biomass total Hg burden under water level management changes.
It is hypothesized that (a) Hg in Typha angustifolia biomass constitutes a significant (> 5%) fraction of the total Hg contained in the wetland, due to their monotypic presence, and (b) Hg concentrations in Typha organs are positively correlated with total Hg concentrations in the soil from which the plant was harvested. In this study, we use the botanical definitions of each of the three main organs of Typha: the roots, stem (rhizome), and leaves, as well as the root core (Fig. 1).
Typha angustifolia plant with each respective organ that was analyzed for total mercury content: the roots, stem (rhizome), and sessile leaves (not all the leaf structures are depicted), as well as the root core, as described by Lominchar et al. (2015)
Materials & Methods
Site Description
A protected embayment located in Coles Creek State Park in Waddington, New York, USA (44°53′24.7"N 75°08′16.1"W) on fluvial Lake St. Lawrence was chosen as the study site (Fig. 2). The wetland area was 13.2 hectares. The wetland was created in 1958 with flooding caused by a newly created hydropower dam. Wetland vegetation is dominated by a monotypic stand of Typha angustifolia, commonly known as narrowleaf cattail. Typha is a rhizomatous emergent wetland plant. Each plant can produce numerous ramets connected by rhizomes; here we refer to a plant (e.g., Fig. 1) with all intact organs as a ramet. Nine sites were sampled in a 3 × 3 grid format that covered various water interfaces within the wetland, i.e., had greater or less proximity to the water channel or the terrestrial region. Two ramets from each of the three middle sampling locations (#2, 5, and 8) were measured for species identification. Mean leaf width was 1 cm, and the male and female flowers were separated by a mean gap 1.3 cm in length. T. latifolia and the hybrid T. x glauca have also been identified in USLR wetlands (Farrell et al. 2010), but observations during this study indicated that T. angustifolia, the invasive non-native species, was the only species present at the study wetland at Coles Creek State Park.
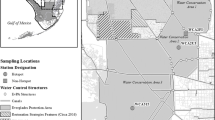
Map of study site on USLR in relationship to the Great Lakes region; a) Eastern end of Great Lakes basin, b) downstream in Lake St. Lawrence on the USLR with the Moses-Saunders power dam indicated within the circle in the upper right, c) aerial imagery of a protected embayment wetland on the St. Lawrence River showing the sampling locations as points. Map was created in ArcGIS Pro 2.7.2 (Esri)
Sampling Description: Samples for Total Hg Analysis
Hydric soil, dry leaf (fallen off the ramet as well as dry leaf still standing from the previous year), fresh green leaves on the ramets, root core, rhizome (stem), and adventitious roots were sampled in June 2018. Senesced leaves still attached to plants were collected in November 2018, representing new growth from the 2018 growing season that was no longer chlorophyllous. Detritus was sampled in May 2019. Hydric soil was sampled within a 1 m radius of the identified sampling location point, using a procedure described elsewhere (Brahmstedt et al. 2019) with alterations including the use of an acrylic dowel. T. angustifolia was sampled within a 1 m radius of the identified sampling location point, using a stainless-steel saw to remove the above-ground organs as well as the below-ground organs intact as one unit. Four ramets (and directly attached rhizomes) were collected within the 1-m radius at each sampling location. Each complete ramet was placed in a food grade polyethylene bag, rinsed in river water to remove loose soil and kept in the food grade bag within a cooler until returned to the lab where samples were refrigerated (4 °C) until processing. Dry leaf material on the ground surface, as well as dry leaves still standing, were also sampled within the 1 m radius, representing last year’s growth. Dry leaf matter was placed in a food grade bag, kept in a cooler until returning to the lab, and placed in a cold room (4 °C) until processing. Detritus was sampled in the field using the same method employed for sampling soil and then placed in a cold room (4 °C) until processing. Senesced leaves collected in November were sampled by removing the leaves at the base of the ramet where green photosynthetic cells ended, and the base turned pale yellow. Four ramets were sampled at each of the 9 sampling locations within the 1 m radius determined for Typha sampling earlier in the season. Senesced leaves were placed in food grade bags, rinsed in river water, placed in a cooler and then in a cold room (4 °C) upon return to the lab.
Sampling Description: Samples for Biomass Estimation
Fresh green leaf and dry leaf biomass was collected in August 2018, and below-ground biomass was collected in November 2018. To estimate cattail biomass per unit area, three 1 m2 quadrants were sampled. All living shoots from within the quadrat area were counted, cut, and put into food grade bags. The same was repeated for dry leaf since some dead plants were still standing, with the addition of collecting all dry material on the ground surface resulting from last year’s cattail stand. To measure detritus biomass, defined as organic matter > 2 mm, and below-ground organ biomass, a 7-cm dia. PVC corer was used to collect 4 cores from within each of the 3 quadrat locations. Cores were placed in food grade bags and kept in a cooler until returned to the lab where they were subsequently placed in a cold room until further processing.
Sample Processing
Hydric soil samples were homogenized and processed using the methods described by Brahmstedt et al. (2019). The organs of each ramet were separated cleanly. The fresh green leaves were removed from the ramet by cutting with a stainless-steel scalpel at the base where the leaf transitioned from green to a pale yellow, i.e., where the photosynthesizing cells and above-ground organs ended. The green leaves were rinsed with tap water, sliced into roughly 5 cm pieces, and rinsed with deionized water three times. The dry leaves were also rinsed with tap water, sliced into ~ 5 cm pieces using a stainless-steel scalpel, and rinsed three times with deionized water. To remove the roots from the stem and root mass, the entire mass was rinsed with tap water to remove loose soil. Clean forceps were then used to remove individual adventitious roots, which were rinsed in deionized water and simultaneously brushed gently with an acid-cleaned toothbrush to remove soil from the root surface. Only intact roots were removed and cleaned for further processing. The rhizome was cleaned by rinsing with tap water to remove any soil and remaining debris, including old decomposing root masses. A clean scalpel was used to remove any remaining adventitious roots from the stem surface. An acid-cleaned toothbrush was used to brush soil from the stem surface while simultaneously rinsing in deionized water. The stem was removed from the root core using a clean scalpel and an additional clean slice was made at the end of the stem to ensure no soil remained on or within the stem material. The root core was cleaned last with similar techniques, by rinsing with tap water as needed, using a scalpel to remove fibrous roots and slicing off any soiled broken ends so only the central transition area of the plant remained. The root core was rinsed with deionized water before the next step of processing. The detritus was separated from the soil core by rinsing with tap water on a 2 mm sieve until the water running from the sieve was clear, with the exception of some detritus particles getting broken off into the rinse water. The detritus was then rinsed three times with deionized water. Senesced leaves were cleaned and processed with the same protocol used for the fresh green leaf collected in June, with the exception of using a clean scalpel to make a new clean cut at the base of the leaves to remove any possible contamination from soil. Organ materials from each site were then shredded using a stainless-steel grater and a stainless-steel (Cuisinart Grind Central; Stamford, CT) coffee grinder to obtain biomass resembling consistency of saw dust, thick pesto, ginger root paste, or combination (below ground organs contain greater sugar content and water content, making them both fibrous and starchy). Each sample was sub-sampled and dried at 60 °C until a constant dry weight was obtained and then recorded to calculate percent moisture. Wet samples were frozen (-20 °C) until analysis.
For the determination of biomass dry weight, biomass samples were washed with tap water and separated into respective biomass materials (detritus, root, stem, and root core). Determination of what constitutes as each organ was consistent with that in detailed in the clean protocol outlined above. Each plot biomass material was dried at 60 °C, weighed and averaged to estimate dry material mass per m2. Soil Hg per unit volume was estimated with the same methods as Brahmstedt et al. (2019).
Total Mercury Analysis
Total mercury was analyzed using a Milestone Direct Mercury Analyzer (DMA-80, Sorisole, Italy) in the CAARES laboratory at Clarkson University. Replicates of ~ 0.1 g of wet sample were run until either a relative standard deviation (RSD) of < 10% was achieved or until 5 replicates were analyzed. A maximum of 5 replicates was determined as acceptable due to the low concentrations of many samples, making low RSD difficult to achieve. RSD ranged from 0.17—178%. Initially, a sediment standard (3,400 ng Hg/g dry wt) (1944—New York/New Jersey Waterway) from the National Institute of Standards and Technology (Gaithersburg, MD) was used per batch in duplicate (106% recovered; 3602 ± 181.5 ng Hg/g dry wt), but Apple leaf SRM (43.2 ng Hg/g dry wt) from the National Institute of Standards and Technology (Gaithersburg, MD) was used for the remaining analyses in duplicate because concentration was more comparable to those of the samples (93.2% recovered; 40.28 ± 2.82 ng Hg/g dry wt). System blanks and boat blank samples were run to check system background Hg concentrations. For samples with low Hg concentrations, particularly the living cattail tissues other than the roots, the system background Hg was often > 20% of the sample Hg. To correct for this, the system background Hg was subtracted from all sample concentrations using the Hg read in the system blanks.
Data Analysis
All statistical analyses were completed using R Studio (R project; R ver. 1.0.153). A Shapiro–Wilk normality test was used to assess normality among the total Hg concentrations (W = 0.57, p < 0.001). Total Hg concentrations were log (base 10) transformed to normalize the skewed distribution among concentrations prior to completing correlations, an ANOVA, and Tukey test comparing the materials and biomass types.
Bioconcentration factors (Eq. 1) and translocation factors (Eq. 2) were calculated to assess accumulation into Typha organs relative to the soil as well as within the plant, with the assumption that a value > 1.0 indicated bioaccumulation of total Hg, [Hg]T, into the numerator organ (Bonanno and Cirelli 2017). Translocation factors were used to compare Hg accumulation in the roots to upper organs.
Results
Among the living tissue types of T. angustifolia, root biomass had significantly higher [Hg]T dry wt. compared to the rhizome, root core, fresh green leaf, and dry leaf biomass (p < 0.001, Fig. 3). Detritus had significantly higher [Hg]T relative to all other materials analyzed (p < 0.001, Table 1, 110 ± 53 ng/g d.w.). Root biomass had higher average [Hg]T than soil, 25 ± 9.3 ng/g d.w. and 22 ± 11 ng/g d.w. respectively, but not at a statistically significant difference (p = 0.99, Table 1). Dry leaf biomass had significantly higher [Hg]T compared to fresh green leaf biomass (p < 0.001, Table 1), 8.4 ± 1.5 ng/g d.w. and 1.8 ± 0.73 ng/g d.w. respectively. A post-hoc hypothesis explained the higher dry leaf biomass concentrations. It was predicted that senesced leaf collected at the end of the growing season (November), would have a greater Hg burden (per unit dry weight) than that collected earlier in the season due to translocation to the leaf over time. Results indicated that senesced leaf tissue collected in November was significantly higher in [Hg]T (6.4 ± 1.9 ng/g d.w.) than the fresh green leaf collected in June (p < 0.001, Fig. 3).
The BCF for [Hg]T in root relative to that of the soil was 1.2, whereas BCFs comparing soil concentrations to other organ concentrations remained below 1.0 (Table 1). TFs comparing upper organ [Hg]T to root [Hg]T were all below 1.0, indicating a lack of translocation of Hg among organs (Table 1). The greater TF for senesced leaf/root (TF = 0.26) compared to the TF for fresh green leaf/root (TF = 0.073) is consistent with the increase in [Hg]T in senesced leaf tissue compared to the fresh green leaf tissue (Table 1). BCF and TF were not calculated for detritus or dry leaf because they are no longer living tissue and have not been for over a full growing season; the Hg is likely mostly adsorbed, rather than taken up into the tissue via cellular internalization. Root tissue [Hg]T was positively correlated with soil [Hg]T (p = 0.045, Table 1). All other correlations comparing tissue [Hg]T to soil [Hg]T were not statistically significant (p ≥ 0.05).
Of the biomass types, organic detritus was the largest contributor of Hg to total wetland burden (13%) (Table 2), despite only making up 11% of the overall wetland biomass weight, whereas green leaf made up 52% of biomass weight, but only contributed to 1% of the total wetland Hg burden. Collectively, the living biomass (root, stem, core, fresh green leaf) contributed only 1.1% to the overall wetland mercury burden. The fresh green leaf contributed the majority of the living biomass Hg, 1.0%, which can be attributed to its 52% contribution to the overall biomass weight within the wetland, rather than its [Hg]T.
Discussion
T. angustifolia Hg content
The increase in [Hg]T between fresh green leaf tissue collected in June and senesced leaf tissue collected in November, as well as the higher translocation factor for senesced leaf tissue, supports the post-hoc hypothesis that there may be translocation of Hg into the leaf tissue over the course of the growing season. However, if translocation occurred then it is reasonable to expect leaf [Hg]T to correlate with the soil [Hg]T. Since fresh green leaf and senesced leaf do not correlate significantly with Hg content in soil, other factors should be considered to explain the increase in [Hg]T over time. Ambient atmosphere concentrations could influence leaf Hg content more than accumulation from the soil. Total Hg content in leaves was more dependent upon air concentrations than soil Hg content in controlled mesocosm experiments with T. latifolia (Fay and Gustin 2007).
Meng et al. (2012) made similar observations in the aboveground tissues of rice (Oryza sativa L.). Therefore, concentrations of Hg in T. angustifolia in this study site may be influenced by a combination of translocation, wet deposition, and ambient concentrations of Hg in air.
The highest [Hg]T among living organ tissues was found in the adventitious roots (p < 0.001, Fig. 3). Elevated Hg content in roots is consistent with other studies that examined Hg bioaccumulation in Typha species (Afrous et al. 2011; Bonanno and Cirelli 2017; Lominchar et al. 2015; Willis et al. 2010) and metal uptake by other plant species (Beauford et al. 1977; Carranza-Alvarez et al. 2008; Patra and Sharma 2000; Taylor et al. 1983; Weis and Weis 2004). Accumulation of Hg in roots may be related to a mechanism for Typha to keep toxicity isolated in roots and away from critical photosynthesis in leaves. Beauvais-Fluck et al. (2018) observed that inorganic mercury (Hginorg) decreased chlorophyll content, whereas MeHg increased antioxidant production in Elodea nuttallii exposed to Hginorg or MeHg in mesocosm experiments. Krupp et al. (2009) identified phytochelatins in rice (Oryza sativa) and common horehound (Marrubium vulgare) that preferentially bound Hginorg (rather than MeHg) within the roots, and this prevented movement of Hg into adjacent tissues, which suggests phytochelatins immobilize Hg in the roots and prevent transport to the leaf. Mercury may behave similarly to other metals, such as lead, upon uptake in plants. Using an electron microscope, Panich-pat et al. (2005) observed the accumulation of lead (Pb) granules near vacuoles in the parenchyma tissue of a T. angustifolia root exposed to Pb-spiked soil. Due to potential growth and photosynthesis impairments, it is reasonable to expect that T. angustifolia in USLR wetlands would preferentially keep bioaccumulated mercury within the roots, away from photosynthetic tissue. Additionally, metal oxides (likely Fe and Mn) were observed on the outer surface of the roots while cleaning. Although the soil and other surficial debris was removed, the oxides were not. Therefore, the [Hg]T may be in part, or in full, due to the oxides on the surface tissue rather than direct uptake. The oxides could have been removed by washing with a reducing agent and a metal complexing ligand solution. For the purposes of estimating the overall Hg budget of the wetland, the roots were cleaned only with deionized water and gentle brushing to remove mineral and organic detritus that were included in the Hg analysis of hydric soils.
The potential use for T. angustifolia as a biomonitor in this environment is low. Root tissue [Hg]T was the only biomass material that correlated significantly with soil [Hg]T (p = 0.045) (Table 1). However, root tissue does not make an ideal biomonitor from a methodological standpoint. Based on the extensive cleaning and processing required for analyzing root tissue [Hg]T in the methodology used here, it may not be the most efficient means to determine if a wetland is Hg contaminated. From an efficiency standpoint, sampling and analyzing the soil itself would be easier than the root tissue.
The role of cattails in Hg stabilization in wetlands
Many species of wetland plants are useful for the remediation of contaminated sites due to their ability to bioaccumulate contaminants (Ashraf et al. 2019; Song et al. 2018; Weis and Weis 2004). Toxic trace metal contamination, including Hg, has the potential to be treated using phytoremediation due to the ability for trace metals to bioaccumulate in plant tissue (Muthusaravanan et al. 2018; Weis and Weis 2004). Many studies discussed here that have examined Hg in Typha were designed to assess the ability for Typha to facilitate phytoremediation. Therefore, our results are discussed here in context of phytoremediation-focused Typha studies for the purposes of comparing Hg burden and mechanisms for bioaccumulation.
Although a BCF > 1.0 is commonly accepted as an indication of bioaccumulation, there is not convincing evidence for the widespread application of T. angustifolia in Hg phytoremediation efforts for three main reasons: failure to meet current accepted criteria for hyperaccumulation, overall minimal contribution of roots to the overall wetland total Hg burden, and USLR wetlands exemplify conditions under which Typha performs poorly as a means of phytoremediation relative to other studies.
T. angustifolia did not classify as a hyperaccumulator given the criteria for other metals (Baker and Brooks 1989). Concentrations in plant tissue must be ≥ 1000 µg/g (Co, Cu, Cr, Pb, Ni) or 10,000 µg/g (1%) (Mn, Zn), to be considered a hyperaccumulator. Although Hg is not included in the metals listed in their criteria, the difference in scale of accumulation (ng/g vs. µg/g) is enough to conclude that T. angustifolia from this wetland did not hyperaccumulate Hg. However, higher [Hg]T in the root tissue relative to above-ground organ tissue is characteristic of species used for phytostabilization (Mahar et al. 2016), suggesting the potential for Hg stabilization within USLR riparian wetlands.
The entire root biomass of Typha within the studied wetland made up only 0.12% of the overall Hg burden within the wetland (Table 2). Although the roots had the highest [Hg]T, and a BCF of 1.2, they made up only 0.43% of the total biomass weight. Higher root [Hg]T may fit with the description of a species capable of phytostabilization (Mahar et al. 2016), but in context of the rest of the wetland Hg burden, the living intact roots do not stabilize a significant amount of Hg, at a given time during the growing season.
Studies elsewhere have observed greater phytoremediation potential in Typha, which may be due to environmental differences, such as pollution sources, soil chemistry, and the evolutionary history of the Typha populations in the USLR. Bonanno and Cirrelli (2017) observed a BCF of 5.35 ± 0.28 in T. angustifolia sampled in the spring, and 6.40 ± 0.13 in the fall. Lominchar et al. (2015) observed BCFs in T. domingensis ranging from 121 – 3168. The difference in BCF here may be due to the Hg in USLR wetlands being deposited from the atmosphere, which created a lower base [Hg]T in the hydric soils of this wetland as compared to studies with point-source contamination from mining or wastewater (Bonanno and Cirrelli 2017; Lominchar et al. 2015). However, findings from Lominchar et al. (2015) indicate that the BCF and amount of Hg bioaccumulated relative to the total Hg in the soil is largely determined by the fraction of total Hg that is soluble. BCF values were highest when Lominchar et al. (2015) compared the relative accumulation to soluble Hg in the soil. Similarly, Meng et al. (2014) observed that Hg in rice tissues had a strong positive correlation with water soluble Hg in paddy soil. Therefore, if much of the Hg in USLR wetland hydric soil is bound to organic particulate, thus not available in a soluble form, a lower BCF would be expected. The high Hg concentrations in the detritus at Coles Creek, relative to soil and root concentrations, support the possibility for most Hg in Coles Creek to be unavailable for bioaccumulation by living Typha as well. The low BCF values may also be attributed to the evolutionary history of the Typha in the USLR. Ye et al. (1997) observed that T. latifolia originating from point source contaminated sites were more efficient at bioaccumulation of metals (Zn, Pb, Cd) than those originating from uncontaminated sites. Although atmospheric deposition of mercury has occurred over the last several decades in the New York region (Risch et al. 2017; Driscoll et al. 2007) most Typha in this region has not experienced point source mercury pollution in the USLR. Therefore, it may be that evolutionary history has not selected for adaptation to mercury pollution in the form of bioaccumulation in USLR T. angustifolia. Moreover, results here indicate T. angustifolia should not be used for phytoremediation or phytostabilization, particularly in regions lacking significant point-source pollution.
The hypotheses and methods of this study provide results that reveal the limited application and potentially adverse outcomes of phytoaccumulation. Bonanno and Cirrelli (2017) suggest that T. angustifolia is effective for phytoremediation due to high uptake ability and high biomass production. Here, the high [Hg]T of detritus in conjunction with other recent work on organic matter increasing Hg exposure risk (Mahar et al. 2016), suggests that high biomass production may instead increase Hg cycling and ecosystem exposure. By focusing on only the living biomass tissue, researchers may be missing a key factor in determining the phytoremediation capabilities of plant species. Although those with higher biomass have the potential to uptake more of the desired contaminant, the dead organic matter resulting from high growth may have unintended consequences, particularly in cases where the contaminant has a complex cycle in the environment, such as Hg. Applications of phytoremediation should be limited to highly controlled and managed situations, such as treatment of wastewater with a hyperaccumulator species that is frequently maintained and disposed of safely. Additionally, in context of the relative contributions of biomass to overall wetland Hg burden (Table 2), criteria determining a specie’s ability to hyperaccumulate or phytostabilize, should be determined on a percent removal basis, considering all major wetland components (soil and different biomass types) on a mass or volume per unit area basis to avoid false positives. For example, here, roots had a BCF > 1.0, but only contributed 0.12% to the overall wetland Hg burden.
Fate of Hg in riparian wetlands
Whereas 83% of total Hg in the Coles Creek wetland resides in the soil, organic detritus makes the next largest contribution (13%), confirming the hypothesis that biomass constitutes a significant fraction (> 5%) of total Hg burden in the wetland. The scale of Hg burden in hydric soil is in part due to the soil volume estimate used in this model, which is dependent on the depth of the soil core. We assume here that the soil most likely to erode is that which was easily cored (Brahmstedt et al. 2019). Based on field observations, the average soil core depth was 20 cm. While the actual volume eroded may vary depending on location in the wetland and erosive conditions, for the purposes of creating a simple model to determine Hg burden within a wetland, 20 cm is functional. In contrast, the contribution made by detritus is not due to its large volume or mass, but rather its high [Hg]T, 110 ± 53 ng/g d.w. (Fig. 3), which is significantly greater than the [Hg]T of the soil (p < 0.001). He et al. (2019) suggest soil organic matter increases risk of Hg exposure, which counters previous work suggesting organic matter acts as more of a Hg sink and lowers bioavailability. Increased Hg methylation may occur in the presence of organic matter due to namely, the characteristics of the organic matter and its ability to stimulate the activity of Hg methylating microbes (He et al. 2019). Considering the overall life cycle of T. angustifolia, as well as the Hg burden in each wetland component present in the Coles Creek wetland, it is evident that T. angustifolia is not good for phytoremediation or phytostabilization. The large amount of detritus biomass resulting from T. angustifolia, may instead increase the risk of Hg exposure to biota within these wetlands by retaining Hg, creating anoxia in hydric soils upon biomass decay, and stimulating Hg-methylation.
From 1958, the year the hydroelectric power dam began production, until 2017, the year a new water level management plan began operating, water levels in the USLR were unnaturally stable and ranged from 73.92—75.68 m elevation above mean sea level, compared to the natural range of 73.88—76.18 m elevation (International Joint Commission 2016). The lack of disturbance to the shoreline allowed for Typha spp. to dominate riparian wetlands and decrease overall wetland vegetation biodiversity (Farrell et al. 2010; Wilcox et al. 2008). The new water level management plan that began in 2017, known as Plan 2014, allows water level fluctuations to simulate natural fluctuations more closely with water levels ranging from 73.72—75.74 m elevation (International Joint Commission 2016). The goal of Plan 2014 is to restore biodiversity within the USLR by increasing disturbance to Typha spp., that will promote an increase in vegetation diversity, and a concomitant increase in the biodiversity of other species, including fish (International Joint Commission 2016).
The water level management plan (Plan 2014) should consider the effects that fluctuating water levels may pose to detritus-bound Hg in this region. The wetland we studied is small (13.2 ha), but it is representative of a protected embayment wetland, of which there are over 6,300 ha in the Upper St. Lawrence River and Lake Ontario, representing 23% of all riparian wetland types present (Wilcox et al. 2005). Although living T. angustifolia biomass did not accumulate Hg extensively, the detritus that originated as T. angustifolia, traps a significant amount of total Hg. Fluctuating water levels are predicted to restore wetland meadows at the terrestrial margin of the wetland and restore submerged aquatic vegetation at the near-channel edge. At the near-channel edge, Typha has recently (July 2019) been observed being uplifted and eroded away in mats by high water levels combined with wave energy during extreme high-water events. Impacts to the terrestrial margin Typha have not yet been observed, although, it is predicted that repeated flooding and drying of Typha at the terrestrial margin will cause marsh dieback, allowing the wetland meadow to reestablish. Once the Typha dies, it will generate detritus, thus creating a region at the terrestrial wetland margin where there is not only periodic wetting and drying of wetland—likely stimulating Hg methylating microbes—but also a thick surface layer of detritus further promoting microbial growth and retention and transformation of Hg.
Conclusions
The initial hypotheses that biomass contributed > 5% to total wetland Hg burden and the use of Typha as a biomonitor due to tissue Hg correlation with soil Hg, were supported by the results of this study, but with some caveats and unexpected conclusions. Although root tissue correlated significantly with soil [Hg]T (p = 0.045) (Table 1), it is not recommended as a useful biomonitor due to the extensive cleaning and sample processing involved. While biomass contributed 17% (Table 2) to the overall wetland burden, the majority was due to detritus Hg content (13% of the total wetland Hg burden), rather than living Typha biomass Hg content. The root tissue presented a BCF of 1.2, which indicates minor bioaccumulation; although, the living biomass in the wetland did not constitute much Hg (1.1%) relative to the soil, detritus, and dry leaf. Granted that Typha in the Coles Creek wetland did not present similar bioaccumulation results as Typha in other studies assessing phytoremediation, the results here necessarily shift perspective toward a total wetland view that suggests potentially harmful implications of detritus and the full life cycle of vegetation in wetlands subjected to legacy Hg contamination.
Since legacy Hg in the landscape is a global issue that will be present for many years into the future (Chen et al. 2018), it is important to understand how it is cycling and threating regions that are plagued with legacy Hg from atmospheric deposition. Such understanding is increasingly critical when there are other human influences imposed on the system that may enhance Hg cycling. Brahmstedt et al. (2019) confirmed conditions for Hg methylation were present in the USLR wetlands in 2016. Given the results here, Plan 2014 may increase detritus and thus Hg exposure to biota at the terrestrial wetland margin under fluctuating water levels. Until the wetland meadow fully reestablishes at the margin, Hg may have heightened bioavailability, further substantiating the presence of conditions that promote Hg mobilization in USLR wetlands under water level fluctuations, such as those implemented in Plan 2014 and expected with extreme events from climate change.
A mercury budget for all USLR wetlands would be an appropriate next step, although considerations must be made regarding differences among wetlands. Farrell et al. (2010) observed other cattail species, T. latifolia and the hybrid T. × glauca, in addition to T. angustifolia in USLR wetlands. Hybrid Typha species have greater biomass (height) relative to the other two cattail species in this region. Thus, monotypic hybrid cattail wetlands may have much different proportions of biomass materials than that found at Coles Creek State Park wetland, which was dominated by native T. angustifolia. The different species of Typha may accumulate Hg differently as well, as discussed previously with other studies that have observed Hg accumulation in Typha.
Data Availability
The datasets generated and analyzed during the current study are available in the Mendeley Data repository, http://dx.doi.org/10.17632/4xtg543hdj.1
Code Availability
Not applicable.
References
Afrous A, Manshouri M, Liaghat A, Pazira E, Sedghi H (2011) Mercury and arsenic accumulation by three species of aquatic plants in Dezful. Iran Afr J Agric Res 6:5391–5397. https://doi.org/10.5897/AJAR10.818
Anning AK, Akoto R (2018) Assisted phytoremediation of heavy metal contaminated soil from a mined site with Typha latifolia and Chrysopogon zizanioides. Ecotoxicol Environ Saf 148:97–104. https://doi.org/10.1016/j.ecoenv.2017.10.014
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar N (2019) Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol Environ Saf 174:714–727. https://doi.org/10.1016/j.ecoenv.2019.02.068
Baker AJM, Brooks RR (1989) Terrestrial higher plants which hyperaccumulate metallic elements – a review of their distribution, ecology, and phytochemistry. Biorecovery 1:81–126. https://doi.org/10.1080/01904168109362867
Beauford W, Barber J, Barringer AR (1977) Uptake and distribution of mercury within higher plants. Physiol Plant 39:261–265. https://doi.org/10.1111/j.1399-3054.1977.tb01880.x
Beauvais-Fluck R, Slaeykova VI, Skyllberg U, Cosio C (2018) Molecular effects, speciation, and competition of inorganic and methyl mercury in the aquatic plant Elodea nuttallii. Environ Sci Technol 52:8876–8884. https://pubs.acs.org/doi/abs/10.1021/acs.est.8b02124
Bonanno G, Cirelli GL (2017) Comparative analysis of element concentrations and translocation in three wetland congener plants: Typha domingensis, Typha latifolia and Typha angustifolia. Ecotoxicol Environ Saf 143:92–101. https://doi.org/10.1016/j.ecoenv.2017.05.021
Brahmstedt ES, Zhou H, Eggleston EM, Holsen TM, Twiss MR (2019) Assessment of mercury mobilization potential in Upper St. Lawrence River riparian wetlands under new water level regulation management. J Great Lakes Res 45:735–741. https://doi.org/10.1016/j.jglr.2019.03.001
Carranza-Alvarez C, Alonso-Castro AJ, La Torre MCA, La Cruz RFG (2008) Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial lagoon in San Luis Potosi, Mexico. Water Air Soil Pollut 188:297–309. https://doi.org/10.1007/s11270-007-9545-3
Chen CY, Driscoll CT, Eagles-Smith CA, Eckley CS, Gay DA, Hsu-Kim H, Keane SE, Kirk JL, Mason RP, Obrist D, Selin H, Selin NE, Thompson MR (2018) A critical time for mercury science to inform global policy. Environ Sci Technol 52:9556–9561. https://doi.org/10.1021/acs.est.8b02286
Driscoll CT, Han Y, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK (2007) Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. Bioscience 57:17–28. https://doi.org/10.1641/B570106
Esri Canada, Esri, Garmin, USGS, NGA, EPA, USDA, NPS, AAFC, NRCan, Maxar, Microsoft, Esri, GEBCO, DeLorme, NaturalVue, Esri, GEBCO, IHO-IOC GEBCO, DeLorme, NGS (2021)
Farrell JM, Murry BA, Leopold DJ, Halpern A, Rippke MB, Godwin KS (2010) Water-level regulation and coastal wetland vegetation in the upper St. Lawrence River: inferences from historical aerial imagery, seed banks, and Typha dynamics. Hydrobiologia 647:127–144. https://doi.org/10.1007/s10750-009-0035-z
Fay L, Gustin MS (2007) Investigation of mercury accumulation in cattails growing in constructed wetland mesocosms. Wetlands 27:1056–1065. https://doi.org/10.1672/0277-5212(2007)27[1056:IOMAIC]2.0.CO;2
Gomes M, de Souza R, Teles V, Mendes É (2014) Phytoremediation of water contaminated with mercury using Typa domingensis in constructed wetland. Chemosphere 103:228–233. https://doi.org/10.1016/j.chemosphere.2013.11.071
He M, Tian L, Braaten HFV, Wu Q, Luo J, Cai L, Meng J, Lin Y (2019) Mercury-organic matter interactions in soils and sediments: Angel or devil? B Environ Contam Tox 102:621–627. https://doi.org/10.1007/s00128-018-2523-1
International Joint Commission, (2016) Regulation Plan 2014 for the Lake Ontario and the St. Lawrence River. Washington, D.C. https://ijc.org/sites/default/files/201808/Plan2014_CompendiumReport_1.pdf. Accessed 20 April 2021
Krupp EM, Mestrot A, Wielgus J, Meharg AA, Feldmann J (2009) The molecular form of mercury in biota: identification of novel mercury peptide complexes in plants. Chem Commun 28:4257–4259. https://doi.org/10.1039/B823121D
Lominchar MA, Sierra MJ, Millan R (2015) Accumulation of mercury in Typha domingensis under field conditions. Chemosphere 119:994–999
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, Li R, Zhang Z (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol Environ Saf 126:111–121. https://doi.org/10.1016/j.ecoenv.2015.12.023
Meng B, Feng X, Qui G, Wang D, Liang P, Li P, Shang L (2012) Inorganic mercury accumulation in rice (Oryza sativa L.). Environ Toxicol Chem 31:2093–2098. https://doi.org/10.1002/etc.1913
Meng M, Li B, Shao J, Wang T, He B, Shi J, Ye Z, Jiang G (2014) Accumulation of total mercury and methylmercury in rice plants collected from different mining areas in China. Environ Pollut 184:179–186. https://doi.org/10.1016/j.envpol.2013.08.030
Muthusaravanan S, Sizarajasekar N, Vivek JS, Paramasivan T, Naushad M, Prakashmaran J, Gayathri C, Al-Duaij OK (2018) Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environ Chem Lett 16:1339–1359. https://doi.org/10.1007/s10311-018-0762-3
Olsen CI, Fakhraei H, Driscoll CT (2020) Mercury emissions, atmospheric concentrations, and wet deposition across the conterminous United States: Changes over 20 years of monitoring. Environ Sci Technol Lett 7:376–381. https://doi.org/10.1021/acs.estlett.0c00185
Panich-pat T, Srinives P, Kruatrachue M, Pokethitiyook P, Upatham S, Lanza GR (2005) Electron microscopic studies on localization of lead in organs of Typha angustifolia grown on contaminated soil. Sci Asia 31:49-53.
Patra M, Sharma A (2000) Mercury toxicity in plants. Bot Rev 66:379–422. https://doi.org/10.1007/BF02868923
Pirrone N, Cinnirella S, Feng X, Finkelman RB, Friedli HR, Leaner J, Mason R, Mukherjee AB, Stracher GB, Streets DG, Telmer K (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10:5951–5964. https://doi.org/10.5194/acp-10-5951-2010
Risch MR, DeWild JF, Gay DA, Zhang L, Boyer EW, Krabbenhoft DP (2017) Atmospheric mercury deposition to forests in the eastern USA. Environ Pollut 228:8–18. https://doi.org/10.1016/j.envpol.2017.05.004
Song U, Waldman B, Park JS, Lee K, Park S, Lee EJ (2018) Improving the remediation capacity of a landfill leachate channel by selecting suitable macrophytes. J Hydro-Environ Res 20:31–37. https://doi.org/10.1016/j.jher.2018.04.005
Taylor GJ, Crowder AA (1983) Uptake and accumulation of heavy metals by Typha latifolia in wetlands of the Sudbury, Ontario region. Can J Bot 61:63–73. https://doi.org/10.1139/b83-005
Weis J, Weis P (2004) Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ Int 30:685–700. https://doi.org/10.1016/j.envint.2003.11.002
Wilcox DA, Ingram JW, Kowalski KP, Meeker JE, Carlson ML, Xie Y, Grabas GP, Holmes KL, Patterson NJ (2005) Evaluation of water level regulation influences on Lake Ontario and Upper St. Lawrence River coastal wetland plant communities: Final Project Report. I-64, Report to the International Joint Commission, Washington, DC and Ottawa, ON.
Wilcox DA, Kowalski KP, Hoare HL, Carlson ML, Morgan HN (2008) Cattail invasion of sedge/grass meadows in Lake Ontario: photointerpretation analysis of sixteen wetlands over five decades. J Great Lakes Res 34:301–323. https://doi.org/10.3394/0380-1330(2008)34[301:CIOGMI]2.0.CO;2
Willis JM, Gambrell RP, Hester MW (2010) Growth response and tissue accumulation trends of herbaceous wetland plant species exposed to elevated aqueous mercury levels. Int J Phytoremediat 12:586–598. https://doi.org/10.1080/15226510903390460
Ye ZH, Baker AJM, Wong MH, Willis AJ (1997) Zinc, lead and cadmium tolerance, uptake and accumulation by Typha latifolia. New Phytol 136:469–480
Acknowledgements
We thank New York State Parks for permission to conduct this study at Coles Creek State Park.
Funding
This research was supported by the Great Lakes Research Consortium (New York). Support for C.N.A.C. was provided by the National Science Foundation Research Experience for Undergraduates program (NSF Award No. 1659623 to M.R.T.).
Author information
Authors and Affiliations
Contributions
ESB was responsible for conceptualization, investigation, methodology, project administration, formal analysis, and writing the original draft. CNAC assisted with the investigation and methodology; TMH provided resources, and manuscript review & editing. MRT was involved with project conceptualization, funding acquisition, supervision, and manuscript review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The authors have no known personal or financial competing interests influencing this work.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brahmstedt, E.S., Crespo, C.N.A., Holsen, T.M. et al. Mercury distribution in an Upper St. Lawrence River wetland dominated by cattail (Typha angustifolia). Wetlands 41, 119 (2021). https://doi.org/10.1007/s13157-021-01511-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-021-01511-9