Abstract
With projected increase in storms, sea-level rise and saltwater intrusion in low-lying terrestrial areas, compositional changes that favor more salt tolerant species are likely to occur. Wetland species are expanding into declining forested communities, primarily dominated by trees. Higher salinities for germination may preclude establishment of tree species. We examined the capacity for coastal tree species (Acer rubrum, Liquidambar styraciflua, Pinus taeda, Celtis occidentalis, Persea borbonia) and a wetland shrub (Morella cerifera) to germinate at salinity concentrations of 0, 2, 5, 10, and 20 ppt. A growth chamber experiment was established examining the effect of salinity on germination of common species found in mixed forests throughout the mid-Atlantic coastal plain and Gulf of Mexico, USA. The study revealed that regeneration from seed will be difficult for most of the selected species at salinities >5 ppt with implications for community composition with continued saltwater intrusion. Germination of A. rubrum was not impacted at higher salinities, with Pinus taeda not as affected other species. Morella cerifera did not have an advantage at the germination stage over selected tree species. Knowing threshold limits of germination response to salinity is critical for identifying future community trajectories.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Factors that facilitate the formation of ghost forests in coastal ecosystems are on the rise and forests are being replaced by more salt-tolerant communities (Kirwan and Gedan 2019; Fagherazzi et al. 2019). Increases in soil salinity play a major role in forest decline and transitioning at early stages of plant development (Williams 2013). Soil salinity increases in plant communities through several factors such as storm surges, drought, salt intrusion through land subsidence, and sea-level rise (Hayden and Hayden 2003; DeSantis et al. 2007; Fernandes et al. 2018; Kirwan et al. 2016; Schieder et al. 2018). The long-term ecological implications for regeneration of forested coastal plant communities is not well understood, particularly in temperate coastal forests (Williams 2013) as plant response to disturbance can shift species distributions (Ash et al. 2017). Disturbances increase heterogeneity within the landscape, resulting in visible (e.g. short-term structural damage) and invisible effects (e.g. long-term community regeneration) on community dynamics (Lugo 2008). Long-lived tree species die over time due to higher salinity; however, the extent to which coastal forests can regenerate is unclear.
Coastal communities are highly driven by environmental filtering (i.e. salinity and wind) and plants assemble based on their capacity to withstand environmental stresses (Ehrenfeld 1990). The response to increased salinity can be species-specific and decrease overall total germination (Paudel and Battagilia 2013). Species-specific responses can restructure species dominance in a community when species with lower salt tolerances are impeded from colonizing areas with higher salinities (Langston et al. 2017; Liu et al. 2017). The reduction and replacement of coastal forests due to changes in the environment has been reported throughout the Atlantic and Gulf coast regions (Conner et al. 2005; Schieder et al. 2018), particularly, along forest/marsh boundaries (DeSantis et al. 2007; Kirwan et al. 2016; Schieder et al. 2018). Sea-level rise, storm events, and saltwater intrusion result in marsh expansion into upland with differential consequences for long-lived trees that depend on freshwater for survival. A recent study shows that trees may resist tidal marsh expansion (Field et al. 2016); however, little is known about the long-term regeneration ability of trees in areas exposed to higher salinities.
Examining coastal forest response to elevated salinity is essential for understanding and predicting community regeneration. The niche in which a seed germinates plays a fundamental role in community regeneration at later life stages (Bochet 2015). The abiotic environment can have a strong influence on when germination occurs in coastal ecosystems, and the timing of germination has consequences for later life stages. If species are plastic in their response, they may be able to withstand perturbations in the environment (Paudel and Battagilia 2013); however, for some species disturbance may delay germination which alters the competitive landscape for interacting species (Walck et al. 2011). If a species cannot recruit, then colonization cannot occur.
Relatively little is known about the contribution of early life stages to species distributions in communities (Fraaije et al. 2015). When considering community regeneration an examination of the entire life cycle of species is important because levels of vulnerability to disturbance change with ontogeny. Seed germination and emergence are vital phases of the plant life cycle that are highly dependent on environmental filters and can be a bottleneck for community regeneration (Donohue et al. 2010). Since the extent to which plants respond to increases in salinity is unclear, exploring species’ tolerances to the post disturbance environment during recruitment and the potential for compositional change is necessary.
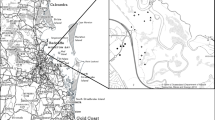
Morella. cerifera (L.) is a moderately salt-tolerant shrub (Tolliver et al. 1997) that is encroaching in many coastal areas throughout the Atlantic and Gulf coast regions (Battaglia et al. 2007; Huang et al. 2018). Specifically, M. cerifera has expanded over 40% in the last 30 years along the Virginia Coast Reserve barrier islands (Zinnert et al. 2011). The current study is motivated in part by the decrease in coastal forests on Parramore Island, VA, where discontinuous portions of forests are located along a marsh boundary and M. cerifera is expanding (Fig. 1). It is unknown if there is an advantage for M. cerifera at the germination stage of development over coastal species. Through this research we aim to answer the following questions: (1) To what extent does elevated soil salinity impact the percent total germination of selected coastal forest species? (2) What is the effect of elevated soil salinity on the timing of germination for the selected species? and (3) What is the probability of species not germinating due to higher salinities? To increase the accuracy of modelling the future trajectory of coastal communities, it is vital to know how species will respond to the altered post-disturbance environment particularly regarding soil salinity during early stage development.
Methods
Plant Species
A growth chamber experiment was set up to examine the effect of soil salinity on the germination of common species that can be found in mixed forests throughout the mid-Atlantic coastal plain and Gulf coast regions. Due to high seed availability and germination rates, Acer rubrum (L.), Liquidambar styraciflua (L.), Pinus taeda (L.), Celtis occidentalis (L.), Persea borbonia (L.) and M. cerifera were the selected species in this study. Additional species (i.e. Quercus rubra (L.), Ilex opaca (L.), and Juniperus virginiana (L.) were tested but failed to germinate due to seed viability or germinated during cold stratification, and thus were not included in the analysis. Acer rubrum, L. styraciflua and C. occidentalis are all deciduous species. Pinus taeda, P. borbonia, and M. cerifera are all evergreen species.
The species used in this study are broadly distributed in eastern and southeastern US coastal environments. Most of the study species are tolerant to aerosol salt spray in their adult forms including Morella spp. (formerly Myrica spp.) (Flint 1985; Gilman and Watson 1993; Gilman and Watson 1994; Schultz 1997). In coastal areas, salt sensitive species are found more inland where salinity is relatively low (Ehrenfeld 1990). Celtis occidentalis, P. borbornia and M. cerifera are bird dispersed. Pinus taeda, L. styraciflua and A. rubrum all are wind dispersed but are capable of dispersing seed away from the parent canopy (Kormanik 1990; Schultz 1997). Capacity for distant dispersal is a vital factor for seedling establishment in favorable habitats (Rey and Alcántara 2000). The seedling stage is the most vulnerable stage of development and will succumb more readily to perturbations in the freshwater habitat. Salinity has been shown to adversely impact establishment of woody species (Pezeshki 1992; Middleton 2016). The seed stage of development is more tolerant to perturbation than the seedling stage because seeds can delay germination if conditions are unfavorable (Donohue et al. 2010). The extent to which high salinity precludes the germination of woody species is unclear.
Growth Chamber Germination Experiment
All seeds for this study were collected along the Atlantic coastal plain from Virginia and North Carolina and most can also be in Gulf coast regions. Seeds were stratified according to protocols described in Young and Young (1992). In addition to stratification, M. cerifera was scarified to remove the waxy coat. Seed flotation tests were used to determine seed quality for all seeds. To examine the effect of salinity on germination, twenty-five seeds of each of the six species were exposed to five salinity treatments (0, 2, 5, 10, and 20 ppt) with four replicates per treatment for each species. Seeds were placed in five rows and five columns on top of the substrate. Germination experiments for each replicate per species were performed in 6 × 6 × 3 clam shell plastic containers with perforations in the bottom and filled with a 3:1 sand: soil mixture. The salinity treatments were made using dilutions of a commercial mixture that approximates ocean salts (Instant Ocean, Aquarium Systems). Hoagland solution was added to each salinity treatment to approximate nutrients in the natural soil environment. Hoagland solution and specific nutrient concentrations were prepared exactly as described by Hoagland and Arnon (1950). There were no differences in nutrients among the five treatments and salinity treatments were verified before application during the experiment. Salinity treatments (containing Hoagland solution) were added to the respective replicates every five days to account for evaporation. Replicates were placed in a single growth chamber (model E15, Conviron, Pembina, N. D.) with a 14-h photoperiod and a 25:20 °C temperature regime at a photon flux density of 400 μmol m−2 s−1. A seed that contained a radicle of 2 mm was counted as germinated. Seeds were treated and observed for 55 days.
Statistical Analysis
The process of germination was analyzed by determining total germination, mean germination time, mean germination rate and Kaplan-Meier survival curves. To determine the effect of soil salinity on total germination we calculated total percent germination by summing all seeds that germinated per treatment over the course of the experiment. To determine seed vigor, we calculated mean germination time (MGT), the time to 50% germination for each species. To determine if there was a delay in germination relative to the control treatment, we calculated mean germination rate (MGR), which measures the speed of germination. MGR was calculated by taking the reciprocal of the mean germination time (Ranal et al. 2009). We analyzed these indices using two-way ANOVA and post-hoc Tukey tests to determine significant differences among treatments and species. Kaplan Meier curves were used to determine the probability of not germinating as described by McNair et al. (2012). For analysis of the Kaplan Meier survival curves, we performed multiple log-rank tests to determine significant differences in survival times of species among treatments. Salinity and species were fixed effects in the model and were categorical variables. Each species was modelled separately to examine the effect of salinity. We adjusted the alpha for the multiple comparisons using a Bonferroni correction (α = 0.005). JMP Version 13 (SAS Institute, Inc., 2016) software was used for the statistical analyses.
Results
Total Percent Germination
On average total percent germination declined with increasing salinity for all species except A. rubrum, Supplementary Fig. 1); A. rubrum had the highest percent germination and C. occidentalis the least, regardless of salinity treatment. There was a significant treatment (F = 111.4, p < 0.0001) and species (F = 222.7, p < 0.0001) effect for total % germination. There was also a significant species x treatment interaction (F = 5.2, p < 0.0001, Fig. 2). Acer rubrum and P. taeda were the only species to germinate at 20 ppt. Pinus taeda germination did not differ from A. rubrum at 0, 2, and 5 ppt; however, at 10 ppt its germination became comparable to all other species germinating at lower levels of salinity (0 ant 2 ppt). Only L. styraciflua at 5 ppt had comparable germination to P. taeda at 10 ppt. Germination for M. cerifera was not different from P. borbonia and C. occidentalis with the control or with increases in salinity (2, 5, 10 ppt).
Total Percent Germination graphs show that total percent germination decreased for L. styraciflua, P. taeda, C. occidentalis, P. borbonia and M. cerifera with increased salinity. Only A. rubrum and P. taeda germinated at 20 ppt. Different letters represent significant differences in salinities at which species germinated. These results are from two-way ANOVA analysis
Mean Germination Time.
Increased salinity differentially affected the MGT of the selected species. It took longer for P. borbonia to reach MGT than all other species (p < 0.0001) and it took less time for A. rubrum to reach MGT (p < 0.0001) (Supplementary Fig. 2). Mean germination time had a treatment (F = 158.5, p < 0.0001), species (F = 116.0, p < 0.0001), and interaction effect (F = 25.0, p < 0.0001; Fig. 3). Salinities of 2, 5 and 10 ppt increased MGT relative to the control (Supplementary Fig. 2). When averaged across all salinities 10 ppt significantly increased mean germination time. Twenty ppt decreased MGT because P. borbonia, L. styraciflua, M. cerifera, and C. occidentalis did not germinate at this salinity. Acer rubrum was the only species that did not experience a significant decrease in MGT due to salinity.
Mean germination time (MGT) graphs show that P. borbonia took longer to reach mean time germination that any other species at 5 and 20 ppt. Of all species A. rubrum took the least amount of time to reach MGT. Different letters represent significant differences in salinities at which species germinated. These results are from two-way ANOVA analysis
Mean Germination Rate.
As seen in total germination and MGT, there was a treatment (F = 101.4, p < 0.0001) and species (F = 722.4, p < 0.0001) effect for mean germination rate (MGR). There was also a species x treatment interaction (F = 8.02, p < 0.0001, Fig. 4). MGR did not differ at low salinities, 0 and 2 ppt but was slowed at all other salinities (Supplementary Fig. 3). Across all treatment levels, MGR was fastest in A. rubrum, followed by P. taeda and C. occidentalis (Supplementary Fig. 3). There were no differences between L. styraciflua and M. cerifera. Persea borbonia had the slowest MGR. MGR was the same for A. rubrum at all salinities and faster than all other treatment and species combinations (Fig. 4). Pinus taeda germination at higher salinities (5 ppt and 10 ppt) were significantly different from mean germination rates at lower salinities (0 ppt and 2 ppt). Persea borbonia had the lowest mean germination rate, across all salinity levels, but was not significantly different from M. cerifera.
Mean germination rate (MGR) shows that there is a delay in germination at higher salinities for L. styraciflua, P. taeda, C. occidentalis, and M. cerifera. Acer rubrum at all salinities had a higher MGR. Different letters represent significant differences in salinities at which species germinated. These results are from two-way ANOVA analysis
Kaplan-Meier Probability
The probability of not germinating increased with salinity for all species except A. rubrum. Kaplan-Meier curves and log-rank tests revealed significant differences in seed germination based on soil salinity for most of the selected species in this study. All species L. styraciflua, P. taeda, C. occidentalis, P. borbonia and M. cerifera (p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, respectively) showed a response to salinity except A. rubrum. Most of its germination was completed by day 5. However, for L. styraciflua most germination occurring between days 10–30 days (Fig. 5). Pinus taeda experienced early germination with most germination occurring between days 5–30. Celtis occidentalis also experienced early germination, but most germination occurred over a shorter period, 5–15 days, after which, most seeds stopped germinating. Persea borbonia had the longest germination time with the first germination at day 15 and most of the germination occurring between days 25–50. The shrub, M. cerifera, started germinating on day 15 as well and most of its germination occurred between days 15 and 40. There were differences in the patterns of germination for all species affected by higher salinity. Pairwise comparisons show that the threshold for salinities too high for germination to occur appear to be reached between salinities of 2 and 5 ppt for all species responding to the treatments. A salinity of 10 ppt is too high for most of the selected species to germinate (Table 1).
Discussion
With projected increases in sea-level rise and saltwater intrusion in low-lying terrestrial and tidal freshwater environments (Sallenger et al. 2012), compositional changes that favor more salt-tolerant species are likely to occur (Conner 2007; Doyle et al. 2010) in coastal forests. In this study we set out to determine the potential for coastal tree species to regenerate at higher salinities and determine if a moderately salt-tolerant shrub that is found in freshwater wetlands, M. cerifera, has advantages during germination that may contribute to its expansion in coastal forests. This study showed that M. cerifera does not have an advantage at the germination stage over the tree species in this study. Its total germination was equally affected by salinity as P. borbonia and C. occidentalis and reduced relative to A. rubrum and P. taeda. Salinity increased mean time to germination for M. cerifera, P. borbonia, L. styraciflua, and P. taeda. With increased salinity (> 5 ppt) it took longer for these species to reach mean time germination relative to the controls.
Soil salinity is an important factor for early stages of development for many species. Paudel and Battaglia (2015) and Middleton (2016) showed that germination decreased for trees and shrubs as salinity increases. However, soil salinity does not always control the distribution of adult vegetation. Martin and Young (1997) documented spatial and temporal differences in soil salinity across Hog Island, VA and noted that J. virginiana patterns were not established in accordance with soil salinity. For some species, zonation patterns are set at earlier stages of development. Adult trees and shrubs are still affected by salt in the form of aerosols, which may contribute to their overall persistence (Wells and Shunk 1938; Bellis and Keough 1995). Morella cerifera appears limited by soil salinity at earlier life stages (Woods et al. 2019) but can withstand saltwater flooding at the adult stage (Naumann et al. 2008; Liu et al. 2017). The extent to which M. cerifera persists at earlier life stages relative to neighboring tree species may determine overall community trajectory.
In the current study, A. rubrum and P. taeda, both wind-dispersed species, experienced greater total germination than all other species with increasing levels of salinity. Acer rubrum was not affected at all at the germination stage of development by increasing levels of salinity. A recent study showed that after Hurricane Sandy, A. rubrum trees were negatively impacted by saltwater flooding but were able to recover over time (Hallett et al. 2018). Total germination of P. taeda decreased at higher salinities; however, even at 10 ppt germination capacity was higher than all other selected species, except A. rubrum and L. styraciflua at low salinities. Records show that P. taeda was a dominant species on Virginia barrier islands seven years before Hurricane Isabel in 2003 (unpublished data). Both A. rubrum and P. taeda appear to have the capacity to be resilient to soil salinity disturbance at the germination stage but they may be affected by salinity as seedlings and adults (Johnson and Young 1993; Donohue et al. 2010; Hallett et al. 2018). As the environment changes to one with variable soil salinity, these species may be able to persist through regeneration.
Total germination of L. styraciflua, C. occidentalis, P. borbonia and M. cerifera were all affected by >10 ppt soil salinities, with no germination at 20 ppt. Liquidambar styraciflua appears to be more salt-tolerant than C. occidentalis, P. borbonia and M. cerifera at low salinities; however as salinity increased its germination became comparable to species over which it may have had a competitive advantage in a low salinity environment. Total germination of L. styraciflua would be the most impacted by increased salinity because at low salinities it had germination comparable to that of A. rubrum and P. taeda; however, L. styraciflua experienced the greatest loss with increased salinity (58% decrease from 2 to 5 ppt). This reduction of regeneration potential in L. styraciflua could cause compositional changes forests where it is dominant if the adult species experience a high mortality event.
Altered patterns of regeneration can cause compositional changes in forests (Johnstone et al. 2016). Increased soil salinity delayed germination of L. styraciflua, P. taeda, C. occidentalis and M. cerifera. As P. taeda germination was high, a delay in germination had less impact overall. Conversely, for species with low germination rates C. occidentalis, a delay in germination, due to soil salinity, reduced its regeneration potential. Persea borbonia and M. cerifera, common species in wet maritime forests throughout the Atlantic and gulf coasts (Gresham 1985), experienced the lowest MGRs. They were slow to start germinating without increased salinity. They are both broadleaf evergreen species that are often found in association with one another, P. borbonia dominant in the canopy layer while, M. cerifera dominant in the shrub layer. While increased salinity did not delay the germination of P. borbonia, it delayed the germination of M. cerifera at 10 ppt. A delay in germination could cause nutrients to be usurped by species that can germinate at higher salinities, which could impact competitive dynamics at the seedling stage of development. The result of species experiencing delayed germination or being prevented from germinating due to their level of salinity tolerance is community transitioning or retreating (Williams 2013; Langston et al. 2017).
Kaplan-Meier curves showed that P. borbonia took the longest to germinate, but germination was steady and consistent once it occurred. Slow germination was not due to higher soil salinities, except at 20 ppt. This indicates that P. borbonia can regenerate up to 10 ppt. However, a delay in germination may affect future forest composition, especially in competition with an expanding shrub population. Conner and Askew (1993) showed that P. borbonia and A. rubrum seedlings were not able to persist after up to five days of flooding with a high salinity treatment (20–27 ppt). Even though A. rubrum germinates at salinities as high as 20 ppt, the seedling stage of development may limit its relative abundance in coastal forests under saline flooded conditions. Tolerance to an altered environment at early developmental stages is essential for persistence in a community.
With the timing of nor’easters coinciding with the germination of many coastal forest tree species, salinity will be a major factor affecting future tree regeneration. Trees at the forest/marsh boundary are particularly susceptible to loss of habitat as sea-level rises and upland migration of marshes advances. Forests understory tree species are being replaced by halophytic species (Williams 2013). Empirically, the formation of ghost forests and changes in vegetation along many mid-Atlantic and Gulf coast regions suggest that salinities of 5 ppt are regularly exceeded (Williams 2013; Thomas et al. 2015; Kirwan and Gedan 2019). Entire forests in the mid-Atlantic and southeast are either retreating or transitioning in favor of more salt tolerant species (Williams 2013; DeSantis et al. 2007; Kirwan et al. 2016). It is hard to project changes in salinity for coastal forests with continued sea-level rise and saltwater intrusion because these systems are dynamic. Salt intrusion may increase soil salinity temporarily and prevent germination, but a pulse of freshwater may decrease salinity allowing species to regenerate. The current study reveals that if trees succumb to salt intrusion, regeneration from seed will be difficult for most species at higher (i.e. >5 ppt) salinities, potentially altering community composition. Some species (i.e. A. rubrum, P. taeda) can germinate quickly at multiple salinity levels but may not grow to adult form with increased salinity. Other species (i.e. C. occidentalis, L. styraciflua, P. borbonia and M. cerifera) are more vulnerable at the seed stage to salinity. Knowing threshold limits of germination response to salinity is critical for future modeling of forest response to sea-level rise and storm events. Regeneration from seed is slow, but also determines future community trajectory after disturbance events.
References
Ash JD, Givnish TJ, Waller DM (2017) Tracking lags in historical plant species’ shifts in relation to regional climate change. Global Change Biology 23:1305–1315
Battaglia LL, Denslow JS, Hargis TG (2007) Does woody species establishment alter herbaceous community composition of freshwater floating marshes? Journal of Coastal Research 23:1580–1587
Bellis VJ, Keough JR (1995) Ecology of maritime forests of the southern Atlantic Coast: a community profile. U.S. Department of Interior, Washington, DC
Bochet E (2015) The fate of seeds in the soil: a review of the influence of overland flow on seed removal and its consequences for the vegetation of arid and semiarid patchy ecosystems. Soil 1:131–146
Conner WH (2007) Ecology of tidal freshwater forested wetlands of the southeastern United States Dordrecht: springer pp 448-450
Conner WH, Askew GR (1993) Impact of saltwater flooding on red maple, redbay, and Chinese tallow seedlings. Castanea pp 214-219
Conner WH, Mixon WD, Wood GW (2005) Maritime forest habitat dynamics on bulls island cape Romain National Wildlife Refuge, S. C., following hurricane Hugo. Forest Ecology and Management 212:127–134
DeSantis LR, Bhotika S, Williams K, Putz FE (2007) Sea-level rise and drought interactions accelerate forest decline on the Gulf coast of Florida, USA. Global Change Biology 13:2349–2360
Donohue K, de Cosas RR, Burghardt L, Kovach K, Willis CG (2010) Germination, post germination, adaptation and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics 41:293–319
Doyle TW, Krauss KW, Conner WH, From AS (2010) Predicting the retreat and migration of tidal forests along the northern Gulf of Mexico under sea-level rise. Forest Ecology and Management 259:770–777
Ehrenfeld JG (1990) Dynamics and processes of barrier-island vegetation. Reviews in Aquatic Sciences 2:437–480
Fagherazzi S, Anisfeld SC, Blum LK, Long E., Feagin RA, Fernandes A, Kearney, WS, Williams K. (2019) Sea level rise and the dynamics of the marsh-upland boundary. Frontiers in environmental science 7 article 25
Fernandes A, Rollinson CR, Kearney WS, Dietze MC, Fagherazzi S (2018) Declining radial growth response of coastal forests to hurricanes and nor'easters. Journal of Geophysical Research: Biogeosciences 123:832-849
Field CR, Gjerdrum C, Elphick CS (2016) Forest resistance to sea-level rise prevents landward migration of tidal marsh. Biological Conservation 201:363–369
Flint HL (1985) Plants showing tolerance of urban stress. Journal of Environmental Horticulture 3:85–89
Fraaije RG, ter Braak CJF, Veryuyn B, Breeman LBS, Verhoven JTA, Soons MB (2015) Early plant recruitment stages set the template for the development of vegetation patterns along a hydrological gradient. Functional Ecology 29:971–980
Gilman, EF and Watson, DG, 1993. Celtis occidentalis common hackberry. Available: n http://hort. Ufl. Edu/database/documents/pdf/tree_fact_sheets/celocca. Pdf
Gilman, EF; Watson, DG, 1994. Persea borbonia Redbay. Available: n http://hort.ufl.edu/trees/PERBORA.pdf
Hallett R, Johnson ML, Sonti NF (2018) Assessing the tree health impacts of saltwater flooding in coastal cities: a case study in New York City. Landscape and Urban Planning 177:171–177
Hayden BP, Hayden NR. (2003) Decadal and century-long changes in storminess at long- term ecological research sites. Climate Variability and Ecosystem Response at Long- Term Ecological Research Sites. Oxford University Press, New York, NY, USA, pp.262–285
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station 347 2nd ed.
Huang H, Zinnert JC, Wood LK, Young DR, D’Odorico P (2018) Non-linear shift from grassland to shrubland in temperate barrier islands. Ecology 99:1671–1681
Johnson SR, Young DR (1993) Factors contributing to the decline of Pinus taeda on a Virginia barrier island. Bulletin of the Torrey Botanical Club 120:431–138
Johnstone JF, Allen CD, Franklin JF, Frelich LE, Harvey BJ, Higuera PE, Mack MC, Meentemeyer RK, Metz MR, Perry GL, Schoennagel T (2016) Changing disturbance regimes, ecological memory, and forest resilience. Frontiers in Ecology and the Environment 14:369–378
Kirwan ML, Gedan KB (2019) Sea-level driven land conversion and the formation of ghost forests. Nature Climate Change 9:450–457
Kirwan ML, Temmerman S, Skeehan EE, Guntenspergen GR, Fagherazzi S (2016) Overestimation of marsh vulnerability to sea level rise. Nature Climate Change, 6:253. Kormanik P. 1990. Liquadambar styraciflua, sweetgum. In: Silvics of North America: 2. Hardwoods. Burns, R.M., and B.H. Honkala, Tech. Coords. http://www.na.fs.fed.us/ Arboriculture & Urban Forestry 33(2): March 2007 95 ©2007 International Society of Arboriculture spfo/pubs/silvics_manual/table_of_contents.htm (accessed 3/8/06) Agriculture Handbook 654 Vol. 2 of the USDA Forest Service, Washington, DC. 877 pp.
Kormanik PP (1990) Liquidambar styraciflua L. sweetgum. Silvics of North America 2:400–405
Langston AK, Kaplan DA, Putz F (2017) A casualty of climate change? Loss of freshwater forest islands on Florida’s Gulf Coast. Global Change Biology 5383–5397
Liu X, Conner WH, Son B, Jayakaran AD (2017) Forest composition and growth in a freshwater forested wetland community across a salinity gradient in South Carolina, USA. Forest Ecology and Management 389:211–219
Lugo AE (2008) Visible and invisible effects of hurricanes on forest ecosystems: an international review. Austral Ecology 33:368–398
Martin DW, Young DR (1997) Small-scale distribution and salinity response of Juniperus virginiana on an Atlantic Coast barrier island. Canadian Journal of Botany 75:77–85
McNair JN, Sunkara A, Frobish D (2012) How to analyse seed germination data using statistical time-to-event analysis: non-parametric and semi-parametric methods. Seed Science Research 22:77–95
Middleton BA (2016) Differences in impacts of hurricane Sandy on freshwater swamps on the Delmarva Peninsula, mid-Atlantic Coast, USA. Ecological Engineering 87:62–70
Naumann JC, Young DR, Anderson JE (2008) Leaf chlorophyll fluorescence, reflectance, and physiological response to freshwater and saltwater flooding in the evergreen shrub, Myrica cerifera. Environmental and Experimental Botany 63:402–409
Paudel S, Battagilia LL (2013) Germination responses of the invasive Triadica sebifera and two co-occurring native woody species to elevated salinity across a gulf coast transition ecosystem. Wetlands 33:527–535
Paudel S, Battaglia LL (2015) The role of light, soil and human factors on the probability of occurrence of an invasive and three native plant species in coastal transitions of coastal Mississippi, USA. Journal of Plant Ecology 8:491–500
Pezeshki SR (1992) Response of Pinus taeda L to soil flooding and salinity. In: Annales des sciences forestières EDP Sciences, vol 49, pp 149–159
Ranal MA, Santana DGD, Ferreira WR, Mendes-Rodrigues C (2009) Calculating germination measurements and organizing spreadsheets. Brazilian Journal of Botany 32:849–855
Rey PJ, Alcántara JM (2000) Recruitment dynamics of a fleshy-fruited plant (Olea europaea): connecting patterns of seed dispersal to seedling establishment. Journal of Ecology 88:622–633
Sallenger AH, Doran KS, Howd PA (2012) Hotspot of accelerated sea-level rise on the Atlantic coast of North America. Nature Climate Change 2:884–888
Schieder NW, Walters DC, Kirwan ML (2018) Massive upland to wetland conversion compensated for historical marsh loss in Chesapeake Bay, USA. Estuaries and Coasts 41:940–951
Schultz RP. 1997. Loblolly pine: the ecology and culture of loblolly pine (Pinus taedaL.). Agriculture handbook 713. Washington, DC: US Department of Agriculture, Forest Service. 493 p
Thomas BL, Doyle T, Krauss K (2015) Annual growth patterns of baldcypress (Taxodium distichum) along salinity gradients. Wetlands 35:831–839
Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P (2011) Climate change and plant regeneration from seed. Global Change Biology 17:2145–2161
Wells BW, Shunk IV (1938) Salt spray: an important factor in coastal ecology. Bulletin of the Torrey Botanical Club 65:485–493
Williams SJ (2013) Sea-level rise implications for coastal regions. Journal of Coastal Research 63:184–196
Woods NN, Dows BL, Goldstein EB, Moore LJ, Young DR, Zinnert JC (2019) Interaction of seed dispersal and environmental filtering affects woody encroachment patterns in coastal grassland. Ecosphere 10(7), p.e02818
Young JA, Young CG (1992) Seeds of Woody plants in North America. Dioscorides Press, Portland
Zinnert JC, Shiftlett SA, Vick JK, Young DR (2011) Woody vegetative cover dynamics in response to recent climate change on an Atlantic Coast barrier island: a remote sensing approach Geocarto International 26:595–612
Acknowledgments
This work was supported by the National Science Foundation Long-Term Ecological Research grants DEB-1237733 and DEB-1832221 to JC Zinnert and Ford Foundation Fellowship to NN Woods. The authors thank Amanda Faucette from North Carolina Botanical Garden at the University of North Carolina at Chapel Hill for providing seeds, the Virginia Coast Reserve staff for logistical support, Dot Field with the Virginia Department of Conservation and Recreation for permitted access to collect seeds on the eastern shore of Virginia and Caitlin Bishop, Joe Brown, Lauren Wood, Austin Tuley, Michael Sinclair, Audrey Kirschner, Eddie Long and Caroline Baucom for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Woods, N.N., Swall, J.L. & Zinnert, J.C. Soil Salinity Impacts Future Community Composition of Coastal Forests. Wetlands 40, 1495–1503 (2020). https://doi.org/10.1007/s13157-020-01304-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-020-01304-6