Abstract
Dredged materials from navigation channel maintenance represent a potentially valuable resource for wetland creation and restoration. In the northern Chesapeake Bay, fine-grained sediments from Baltimore Harbor approach channels are transported by barge southward for creation of wetlands on the site of an eroded island. High concentrations of ammonium, soluble reactive phosphorus, dissolved iron, and iron sulfide minerals in channel deposits are altered by the transport and drying of these materials prior to wetland development. The oxidation of iron sulfide minerals results in low pH, with the initiation of tidal inundation removing sulfuric acid from near-surface soil horizons and moderating the soil pH. Despite the loss of ammonium during dewatering and soil processing, the resultant soils retained high concentrations of dissolved and adsorbed ammonium. Iron-associated inorganic phosphorus represented a large pool of potentially labile phosphorus and along with the high ammonium, resulted in high nutrient concentrations for plant growth. Combined with results on plant growth presented elsewhere, these data suggest that fine-grained dredged materials from non-contaminated environments are well suited for the creation of tidal wetlands after placement and seasoning 1–2 years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The dredging of navigation channels in estuarine and coastal environments often results in large quantities of sediment that must be disposed of, either by re-deposition in subtidal environments, placement in upland containment facilities (Coch 1996), or for beneficial use (Hamons and Young 2000; Yozzo et al. 2004; Maher et al. 2013). In the upper Chesapeake Bay, containment facilities for contaminated dredged sediments (aka dredged materials) and bottom placement away from navigation channels for “clean” materials were the primary placement options utilized in the past. In recent decades, particularly in the face of wetland and shoreline losses, clean dredged materials have been increasingly viewed as a resource for wetland restoration. In Maryland, placement of dredged materials in sub-tidal locations was disallowed by law, after a contentious debate (Poltrack 2000). The scientific literature and political discussion regarding dredged materials have generally focused on the negative impacts of dredging on affected benthic communities, often because of chemical contamination (Cappuyns et al. 2006) and other effects on living resources (Carter III 1986).
Losses of Chesapeake Bay islands, wetlands and coastal land via inundation and/or erosion have been observed throughout the northern Chesapeake Bay (Kearney and Stevenson 1991; Kearney et al. 2002; Wilson et al. 2007) and sediment infill has limited navigation since the time of European land clearing (Gottschalk 1945). While conversion of upland to wetland may help mitigate future tidal wetland losses (Schieder et al. 2018), sea- level rise has contributed to considerable marsh degradation throughout the Chesapeake Bay (Kearney et al. 2002; Beckett et al. 2016). Because of the value of coastal wetlands for capturing sediment, mitigating storm surges, providing essential habitat for aquatic, terrestrial and avian animals, and removal/storage of nutrients and carbon (Costanza et al. 1989; Merrill and Cornwell 2000; Wilson et al. 2007; Hopkinson et al. 2008), increased acreage of wetlands has been cited as a performance metric for the ecological restoration of the Chesapeake Bay (Chesapeake Bay Program 2014). Consequently, efforts to restore and create wetland acreage are considered extremely valuable to Chesapeake Bay ecosystem restoration. While many opportunities for large scale restoration are limited by financial resources, the requirement for maintenance of navigation channels commerce can provide resources for the beneficial use of dredged materials, including wetland restoration and creation.
In many cases, wetland creation and restoration projects have utilized coarse grain sediments, primarily because of their ease of handling and local availability. However, navigation channel deposits in the upper Chesapeake consist of fine-grain materials whose relative mobility, high water content, and in some cases, elevated concentrations of contaminants, pose environmental and logistical challenges. In addition, estuarine sediments can be highly enriched in nitrogen, phosphorus, hydrogen sulfide and iron sulfide minerals (Cornwell and Sampou 1995; Cornwell and Owens 2011). The oxidation of iron sulfide minerals results in the production of sulfuric acid (Portnoy and Giblin 1997; Demas et al. 2004) and represents a potential challenge to wetland plant success. In this paper, defining sediments as the material collected from the bottom of the bay and soils as the same material after placement in wetlands, we describe the transformation of sediments dredged from the upper Chesapeake Bay to wetland soils placed on the footprint of an eroded island. This paper characterizes both estuarine sediments prior to dredging and the wetland soils created from the placement of these materials. Although not an emphasis in this paper, the fertility-related effects of high nutrients are described in a companion paper (Staver et al. In review).
Methods
Study Area
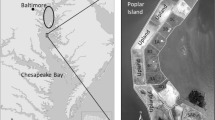
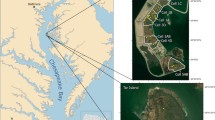
The Port of Baltimore, located 260 km north of the mouth of the Chesapeake Bay, utilizes the deep Chesapeake Bay paleo channel (Colman et al. 1990) for most of the traverse of marine ships plying waters north of Norfolk, Virginia (Fig. 1). Above the William Preston Lane Jr. Memorial Bridge near Annapolis, Maryland, a series of approach channels are incised in the bay bottom leading to the Patapsco River sub-estuary (Baltimore Harbor). Above the Patapsco River, channels extend northward to the Chesapeake and Delaware Canal. Sediment sources in this upper part of the Chesapeake Bay are dominated by Susquehanna River fluvial inputs from the 71,000 km2 watershed, supplemented by shoreline erosion (Hobbs III et al. 1992). Interception of sediment by three dams in the lower Susquehanna River is thought to have decreased because of dam infill (Zhang et al. 2016). Dredging in the approach channels is carried out using clamshell dredges, with sediments placed in barges for transport southward to Poplar Island. Dredging volumes vary year to year, but upper bay dredging averages ~3.5 × 106 yd3 (2.7 × 106 m3) of wet sediment per year.
Location of Baltimore approach channels and Poplar Island in the northern Chesapeake Bay. The triangles show the locations where gravity cores were collected to characterize sediment deposits prior to dredging (Cornwell and Owens 2010). The west side of Poplar Island facing the mainstem Chesapeake Bay is split into a series of “cells” that will be developed into upland habitat while a series of cells on the eastern side have been developed into wetlands
The Paul S. Sarbanes Environmental Restoration Project at Poplar Island (Poplar Island), a 694 ha restoration project, is the major site for dredged material placement from the Port of Baltimore approach channels, with placement initiated in 2001. Poplar Island is split between upland and wetland restoration areas and is surrounded by an exterior containment dike. The interior of the island consists of a series of wetland and upland “cells” separated by dikes. Dredged materials are off-loaded from the barges by slurrying the sediment with adjacent bay water and the material is pumped into the various cells where the material disperses by gravitational flow. After the sediment settles, water is removed from the soil surface, repeatedly pumped from incised drainage ditches, and allowed to dry for 3–8 years, with the soil becoming highly consolidated. Prior to cell development, the cell surface is incised with marsh creeks and graded to elevations appropriate for high (Spartina patens) and low marsh (S. alternifora) communities. The connections to tidal waters were made 3–13 months prior to planting in the calendar year in which planting occurred; culverts connected the wetland cell and tidal waters. The data shown here are generated from the first year of cell development, typically during or soon after spring planting of the marshes. Planting occurred on 0.46 m centers for Spartina patens and 0.91 m centers for S. alternifora; soil sampling was not influenced by the slow spreading first-year plants.
Field Sampling
Sediments were collected in 1998 and 1999 from navigation channels that were scheduled for dredging. The cores collected here have been described previously for the concentrations of adsorbed and pore water ammonium (Cornwell and Owens 2011). Cores were collected at the bottom “notch” at the edge of the channel, locations that generally accumulate fine-grained sediments most rapidly. A gravity corer was used with a 7 cm inner diameter polycarbonate liner and cores were extruded using a N2-filled glove bag to minimize oxidation of dissolved iron (Bray et al. 1973) and co-precipitation of soluble reactive phosphorus (SRP). For pore water, sections up to 50 cm were homogenized and 50 mL centrifuge were filled. Samples were centrifuged for 20 min at 2000 g at room temperature, filtered with a 0.45 μm syringe filter, and stored frozen until analysis. Samples for pore water iron and SRP concentration were separated immediately into the final analysis vials and hydrogen sulfide reagent added immediately to a 1 mL sample. The sediment remaining in the centrifuge tube was frozen until analyzed.
Pore water chemistry was determined in Poplar Island wetland soils using equilibrators (Hesslein 1976). For the initial part of this study when surface soils were very compacted from drying, a pilot-hole was established by inserting into the soil a 50 cm long rectangular metal form in the shape of the equilibrator. The equilibrators had multiple 2.5 cm wells covered with a polycarbonate membrane. In the latter part of the study, a round equilibrator was inserted into the soil after insertion of a power auger, with similar size wells. The solid phase analyses from wetlands sampling presented here are generally from the 0–10 cm soil horizon and were collected by hand insertion of acrylic cores or use of a trowel. Soil samples were kept moist for grain size analysis or dried at 65 °C for chemical analyses. In addition to the sampling of surface soils, deeper profiles (> 1 m) in wetland cells were collected using a soil auger and analyzed for solid phase constituents. Additionally, vertical (0–75 cm) pH and chromium-reducible sulfur (CRS) (Canfield et al. 1986) samples were collected from a cell during the dewatering process, with vibracoring employed for sample collection.
Analyses
Pore water SRP and ammonium (NH4+) were analyzed colorimetrically (Parsons et al. 1984), dissolved chloride and sulfate were analyzed on an ion chromatograph, hydrogen sulfide was analyzed colorimetrically (Cline 1969) and dissolved iron was analyzed using a Ferrozine colorimetric procedure (Gibb 1979). Adsorbed ammonium was analyzed colorimetrically after KCl extraction (Cornwell and Owens 2011) and chromium reducible sulfur, a measure of iron mono-sulfide and pyrite sulfur concentrations, was analyzed on dry samples using a chromium (II) acid extraction (Canfield et al. 1986). Dried samples have been shown to provide similar data to analyses on frozen samples (Morse and Cornwell 1987). Total carbon and nitrogen concentrations were analyzed on a CHN analyzer (Cornwell et al. 1996). Grain size was analyzed for sand, silt and clay fractions by sieving and pipet analysis (Sweet et al. 1993). Total and inorganic phosphorus were extracted in HCl on ashed/un-ashed sediments and soils and analyzed colorimetrically (Aspila et al. 1976) and HCl-extractable iron was analyzed on the un-ashed HCl extract (Leventhal and Taylor 1990).
Statistics
Box plots were used to present the data from individual wetland cells. For the solid phase data, 0–10 cm sections were used to compare the initial condition in each wetland cell or from dredged channel sediment collections. Because the data failed Shapiro-Wilk normality tests, a Kruskal-Wallis One Way Analysis of Variance on Ranks was carried out and pairwise comparisons made. Significance was set at P < 0.05. Linear regressions were employed on selected data sets and significance of slopes set at 0.01. All statistical data are included in supplemental materials. In all cases, when means are presented in the text and tables, the standard deviation is shown. Median concentrations are presented because they are shown in box plots and for many analytes the data are not normally distributed.
Results
A key element of the processing of dredged materials is the removal of water from the original channel deposits as well as the water used to slurry the sediment and pump it into the cells. Considerable effort was expended to dry the soils to 1) enhance the capacity of the cell via surficial soil compaction and 2) allow heavy equipment to traverse the cells to incise channels and set final elevations relative to tide. The wetland cells had variable times after sediment addition in which the soils developed under dry conditions prior to tidal inundation (Table 1). The timing of the initial geochemical assessment after inundation also was variable, with soils exposed to tidal waters 3–8 months prior to pore water sampling. The channel sediments had median and average water content of 58%, with the compacted wetland soils having median and average water content of 37% (Fig. 2). Assuming a soil solid phase density of 2.65 g cm−3, the median bulk density of Poplar Island soils (1.09 g cm−3) is about twice that of the channel sediments (0.56 g cm−3).
Water content in channel (Bay; N = 86) and Poplar Island wetland sediments (N = 176). In Poplar Island wetland soils, the median bulk density was 0.56 g cm−3 in channels and 1.09 g cm−3 in Poplar Island soils. Water content is significantly higher in the channels (Kruskal-Wallis; P < 0.001). The water content box plot shows the median value (horizontal line), the 25–50 and 50-75th percentile of data within the lower and upper box segments, and the 10–25 and 75–90 data percentiles between the box and the horizontal bar. Outliers are shown as symbols
While in this study sediment grain size was not measured in channel deposits in the Upper Chesapeake Bay, sand was generally not observed to be an important part of the sediment matrix in maintenance dredging and is more commonly associated with channel widening and deepening. Although the dredged materials were dominantly fine-grained, the marsh surface was often amended with sand dredged near Poplar Island, often in a non-uniform pattern within a cell, with a goal of matching the wetland cell elevation design specifications (Fig. 3a). Elevation was a function of inputs, dewatering of soils, deformation of underlying original “bay bottom” by the weight of the added materials, and sand additions. The variable sand content is illustrated by data from Cell 3D (Fig. 3b), with many samples being dominated either by mud (silt + clay) or by sand. Adjustment to elevation varied from cell to cell and was reflected in the distribution of sand in the wetland cells.
The drying of the surface soils after the removal of overlying water results in formation of large cracks, with eventual smoothing of the soil after a year or two (Fig. 4a). After drying of the upper soil horizons (Fig. 4b), low pH (4–5) was observed in the top 20 cm of soil (Fig. 4c), with very low concentrations of CRS in the upper layer (Fig. 4d). Below 40 cm, the CRS concentrations were ~ 0.5%. Similar observations are shown in a profile from Cell 5AB (Fig. 5g), with the upper meter of soil having CRS concentrations <0.025% and deep horizons approaching 0.5%. Average concentrations of CRS in 0–10 cm soil horizons ranged from 0.015 to 0.043% S, considerably smaller than the channel sediment (Fig. 6b) average of 0.395 ± 0.225% S.
Sediment characteristics of a wetland cell (5AB) after channel incision and exposure to bay water. Decreased concentrations of C, N, P and HCl-Fe in the surface 20 cm reflect dilution by sand added to meet elevation targets. The concentrations of inorganic P (IP) and organic P (OP) are summed to sediment total P
Box plots of 0–10 cm sediment chemistry in the wetland cells after tidal inundation commenced and during the year the wetlands were planted. The total P (TP) and chromium reducible S (CRS) are box plots of all data from the wetland cell, with between 17 and 104 observations per box. The box plots of pH and HCl-extractable Fe represent data from all wetland cells and have 172 and 140 observations respectively. The channel sediment is referred to as “Bay”
Profiles of pH in the Cell 5AB core (Fig. 5h) showed a pH minima at 40–60 cm, suggesting that tidal flooding 10 months prior to sampling had partially flushed the soil of acid, resulting in higher pH in the upper soil. Higher pH deep in the 5AB profile is consistent with soil that has not been altered by oxidation. Overall, the surficial soil pH conditions observed during planting activities (Fig. 6c) showed a pH range from 4.2 to 7.9, with median and average pH of 6.2 and 6.2 ± 0.7 respectively.
The HCl-extractable Fe is generally related to the concentration of iron oxide minerals plus any iron monosulfide minerals present (Leventhal and Taylor 1990), though this extraction may underestimate the concentration of crystalline iron oxides (Raiswell et al. 1994). This analysis does not include most pyrite-associated Fe (Cornwell and Morse 1987). Below the top 40 cm of soil in Cell 5AB, the concentration of extractable Fe was in excess of 20 mg g−1 (Fig. 5f) and generally similar to observations in the upper Chesapeake Bay (Cornwell and Sampou 1995). The decreased concentrations in the top 40 cm reflect the addition of sand to raise the marsh elevation. There was a broad range of HCl-Fe concentration in the top 10 cm of new cells, reflecting the effects of dilution by sand (Fig. 6d).
The low pH of dredge soil results in a loss of any CaCO3 from the channel sediments, and the total C present is organic C. In the deeper, fine grained soil horizons in Cell 5AB, concentrations of C approached 3% (Fig. 5b). Considerable variability was observed in C concentrations in surficial soils at the outset of cell development (Fig. 7a; Table 2). All but Cell 1B concentrations were lower than the average dredge channel C concentrations (3.40 ± 0.84%). Using all data from Cell 3D, the site with the greatest number of initial analyses (104), the percent mud (silt + clay) is positively correlated (P < 0.01) with C concentrations (Fig. 8a). Cells 1B, 1C and 3A have the lowest median sand (no grain size data for Cell 1B; Fig. 3b), and these three cells have the highest concentration of carbon. Thus, the carbon concentrations appear to be related to the overall soil grain size and the dilution of dredged materials by sand has a strong influence on C concentration. Carbon oxidation during drying and the fallow period prior to planting may result in surficial soil C losses, and C inputs from “volunteer” plants naturally recruited to the soils surface under non-flooded conditions may also contribute organic matter.
Parameter plots of sediment composition versus the percent mud (silt + clay) are presented in a-d, panel e shows a plot of inorganic P versus HCl-Fe, Panels f and g show plots of N and organic P versus C, and panel h shows organic P versus N. All correlations are significant (P < 0.05) and regression lines are plotted. All data are from Cell 3D
Total N concentrations had the same general pattern as C concentrations, with lower concentrations (< 0.2% N) in the upper horizons of Cell 5AB (Fig. 5c) resulting from dilution by sand. This dilution is evident from a plot of N versus mud in Fig. 8b. Surficial concentrations of N in wetland cells were lower (P < 0.01) than upper Bay channel sediments in Cells 1A, 3A, 3B, 3C, and 5AB (Fig. 7b). The adsorbed ammonium in the upper part of Cell 5AB (Fig. 5d) was generally <0.02 mg N g−1 and in the top 1.6 m of soil, the adsorbed NH4+ averaged 1.0 ± 0.3% of the total N. In the 1.6–1.8 m horizon, ~ 5% of the total N occurred as adsorbed NH4+. The adsorbed NH4+ in the deepest section (0.15 mg N g−1) was similar to observations in dredged channels (0.16 ± 0.05 mg N g−1) using identical techniques (Cornwell and Owens 2011).
Ratios of C:N can be somewhat useful for distinguishing sources of organic matter, with higher ratios suggesting less labile organic matter sources (Thornton and McManus 1994). Average molar C:N ratios of the surficial organic matter ranged from 10.8 to 14.2 (Fig. 7c; Table 2), with the highest average ratio in Cell 1B. The Cell 1B C:N ratios were higher than all other cells and channel sediments, with the exceptions of Cells 1A and 3A. The high outliers evident in the channel sediments likely result from fluvially-derived coal from deposits in the Susquehanna watershed (Goldberg et al. 1978). Overall these ratios are somewhat higher than those in algal-dominated mesohaline sediments (Cornwell et al. 1996) and are consistent with a mixture of both terrestrial and algal organic matter (Cornwell and Sampou 1995).
Total P concentrations were affected by dilution from sand, as shown in a vertical profile (Fig. 5e). Inorganic P is the dominant form of P in these soils. Average total P concentrations in surficial soils varied from 0.44 to 0.94 mg g−1 in the wetland cells, with channel sediments averaging 1.24 mg g−1 (Table 2; Fig. 6a). Channel sediments were significantly higher (P < 0.01) than surficial wetland soils from Cells 3D, 1A, 3C and 5AB and were significantly correlated with the percent mud (Fig. 8c) in Cell 3D.
The pore water NH4+ profile derived from all Cell 3D data (Fig. 9a) showed an increase with depth, averages increased from 406 ± 319 μmol L−1 at 2.5 cm to 1638 ± 949 μmol L−1 at 45 cm. These high concentrations represent a large source of NH4+ for initial plant growth. The NH4+ channel data from Cornwell and Owens (2011) averaged 2651 ± 2544 μmol L−1 and was significantly higher (P < 0.05) than the data from all wetland cells except Cells 1C and 3C (Table 2). Cells 1B and 3A had lower concentrations of NH4+ than all other cells (P < 0.01).
The pore water SRP concentration (Fig. 9b) profiles had median concentrations almost two orders of magnitude lower than the NH4+ concentrations, with little vertical structure. The data range at each depth was quite high, with the highest concentrations exceeding 100 μmol L−1. Wetland and channel SRP showed similar variability (Fig. 10b), with significant differences (P < 0.05) observed between the channel data and data from Cells 3A, 3B, 3C and 1A. Cell 1B pore water SRP is not significantly different from channel data, but also is higher than cells 3A, 3B, 3C and 1A. The lowest concentrations were observed in Cells 1A and 3A, which were significantly lower (P < 0.05) than the data from the channel and the other wetland cells. The pattern of cell to cell SRP concentrations differences did not mirror that of NH4+, with the exception that the channel data was among the highest concentration grouping.
Pore water iron concentrations were generally very high (Table 2; Fig. 10c) and in most cells was highly variable. The Fe data from the channel and Cells 3D, 1C, and 5AB were all significantly higher (P < 0.05) than data from cells 1A, 1B and 3A. Median pore water Fe concentrations in wetland cells ranged from 1.9 to 140 mg L−1, with a median channel concentration of 191 mg L−1. Although pore water H2S was observed at one channel site, no measureable H2S concentrations were observed in Poplar Island wetland sediments.
The accumulation of SO42− from pyrite oxidation is best expressed as a molar ratio of SO42− to Cl−, with ratios higher than the sea water ratio (0.052) indicating a sediment enrichment of SO42− and lower values resulting from SO42− reduction. In Poplar Island wetland cells, the mean and median ratios of SO42− to Cl− were all higher than the sea water ratio, with the exception of the data from Cell 3C. Median wetland cell molar SO42− to Cl− ratios ranged from 0.052 to 0.117, a substantial enrichment of SO42−.
Discussion
The changes in sediment chemistry described here are specific to the techniques employed at Poplar Island, including dredging, placement, drying, and reworking of soils in preparation of planting. Other restoration approaches, such as thin-layer marsh enhancement (La Peyre et al. 2009), may have different amounts of drying and re-oxidation than observed in the multi-year approach to marsh development at Poplar Island. In this study, the dewatering of sediment initiates the major changes in sediment chemistry, with the most obvious change associated with the oxidation of iron sulfide minerals such as pyrite:
Because of the ready availability of atmospheric oxygen in these shallow systems, the consumption of oxygen likely has few ecological consequences, except at the heads of creeks during warm summer months. The low pH from iron sulfide mineral oxidation during the drying of dredged materials is also a concern for release of metals (Cappuyns et al. 2004). The introduction of tidal exchange prior to planting provides a mechanism for the removal of sulfuric acid, with median pH values >6 at the time of planting (Fig. 6c); a pH minima in sediment pH profiles (Fig. 5h) well below the soil surface results from such exchange, with pH above the minima increased by exchange with bay water and pH below the minima resulting from incomplete pyrite oxidation. Although iron sulfide oxidation of recently placed dredged materials can be an issue for upland fertility (Bramley and Rimmer 1988), the pH in Poplar Island soils are similar to the pH of 6 shown to be well suited to Spartina alterniflora success (Linthurst and Blum 1981).
Pore water iron concentrations are high in both dredged channels and in the pore waters of Poplar Island wetland cells (Fig. 10c). Re-wetting of the soils, after drying and oxidation of iron sulfide minerals, results in re-establishment of anaerobic conditions and iron reduction, resulting in dissolved Fe. The presence of high concentrations of extractable Fe poises the redox of these wetlands at the Fe(III) – (Fe II) couple (Johnston et al. 2011). The re-introduction of diffusive transport under flooded conditions at the soil surface, combined with reactive estuarine organic matter and abundant iron oxides, results in a substantial buffer against the development of pore water hydrogen sulfide. This buffer occurs because of the high concentrations of Fe(III) oxides as well as the solubility limitation of dissolved sulfide when dissolved iron concentrations are high (Morse et al. 1987).
Although pore water NH4+ concentrations are generally lower than the pore water concentrations observed in dredged channels in the upper Chesapeake (Fig. 10a), they are relatively high in most wetland cells at Poplar Island. We observed no relationship between the duration of tidal flooding prior to sampling and the NH4+ concentrations in the soil. At the outset of this restoration project, fertilizer was added to the plantings to ensure plant success, but was shown to be unnecessary; the high NH4+ concentrations result in exceptionally high above ground biomass (Staver et al. In review). The processing of dredged materials prior to wetland cell development results in losses of nitrogen to the soils (compared to upper bay channel sediments). The addition of water to the dredged material during off-loading from barges results in the dilution of pore water NH4+ and exchange of adsorbed NH4+ to the added water. This elutriation of the adsorbed NH4+ by added water releases NH4+ similar to the KCl extraction commonly used for adsorbed NH4+ characterization (Cornwell and Owens 1999). Comparing the KCl-extractable NH4+ in both channel and wetland environments suggests a loss of NH4+ of 85–90% of the amount dredged from the channel. Inputs of NH4+ from decomposition of organic matter in these estuarine-derived particulates may also have an effect on NH4+ concentrations. Losses result from dewatering of the soil and discharge to surrounding bay waters, uptake by volunteer upland plants prior to flooding and uptake by algae; low pH in ponded water suggest losses as NH3 to the atmosphere are likely minimal due to a predominance of NH4+ over NH3 (Larsen et al. 2001).
The resulting pore water SRP concentrations likely result from interactions with the abundant solid phase Fe. A strong correlation between extractable Fe and inorganic P (Fig. 8e) is consistent with the importance of Fe to P cycling in Chesapeake Bay sediments (Jordan et al. 2008). The high variability of pore water SRP suggests that the controls on SRP solubility are complex and variable, and the abundant solid phase inorganic P represents a large pool of potentially bioavailable P. While high N:P ratios in pore water suggests that plants might be P limited, these large inorganic P concentrations in soils suggests that neither N or P would limit plant growth during the initial phases of marsh establishment. The retention of Fe-bound P likely will decrease with marsh development, as the production of iron sulfide minerals usually results in loss of P-adsorbing surface area (Roden and Edmonds 1997; Lehtoranta et al. 2009). Sulfate reduction would likely proceed for a considerable period without sulfate limitation because of the large excess of pyrite-derived sulfate in the soils at Poplar Island.
The high nutrient concentrations observed at the time of planting generally resulted in extremely high above-ground biomass (Staver et al. In review) in the year after planting, with S. alterniflora reaching 2 m in height (Fig. 11a). The high plant biomass results in the rapid attenuation of NH4+ concentrations in these soils (Fig. 11b).
Photo (a) shows the growth of Spartina alterniflora in the year after planting. Blade heights often reach 2 m. Box plots (b) of the concentrations of NH4+ in surficial sediments (0–10 cm) and deep sediments (20–47 cm) are shown, with the bar showing median concentrations. The year 2005 shallow and deep NH4+ concentrations are > than the 2007 and 2009 NH4+ concentrations (P < 0.05) for each depth range
Conclusions
Poplar Island wetland soil chemistry is shaped by 1) the character of the sediment dredged from navigation channels, 2) the dilution of sediments with bay water during offloading and transport of sediments to wetland cells, 3) the removal of overlying water and drying of soils resulting in both the removal of NH4+ and oxidation of iron sulfides, and 4) tidal inundation and drainage. Bay channels receive inorganic sediment from fluvial and shoreline erosion and their character in the channels is influenced by inputs of organic matter and microbial processes that result in the accumulation of NH4+ (Cornwell and Owens 2011), SRP (Bray et al. 1973), and conversion of iron oxide minerals into iron sulfide minerals (Cornwell and Sampou 1995). The processing of channel deposits between dredging and planting does not convert these soils to the condition of their terrestrial origins, but instead results in the accumulation of sulfuric acid and ammonium.
At the time of wetland development after tidal exposure, fine-grained dredged materials at Poplar Island are nutrient-rich, have pH that are well-suited to plant success, and anaerobic microbial processes that are dominated by iron reduction. These conditions favor the successful growth of wetland plants and result in luxuriant growth of Spartina alterniflora and Spartina patens (Staver et al. In review).
The conversion of aquatic sediments to wetland soils is strongly affected by the character of the dredged sediment, the processing of these materials during transport and placement, and the environmental setting into which they are placed. Consequently, assessment of wetland development using dredged materials will always have to consider site-specific characteristics; while generalizations from other sites may provide a useful guide, they may be of limited value until the numbers of relevant studies is sufficient for a broader generalization. In the case of Poplar Island, the examination of multiple discrete wetland planting cells allowed a generalization of soil transformation processes valid for using sediment dredged in the upper Chesapeake Bay for wetland restoration. These results are best used as a guide to potential transformation processes and approaches to assessment, with different results likely under different conditions of sediment and soil physical character, salinity, and nutrient concentrations.
References
Aspila KI, Agemian H, Chau ASY (1976) A semi-automated method for the determination of inorganic, organic and total phosphate in sediments. Analyst 101:187–197
Beckett LH, Baldwin AH, Kearney MS (2016) Tidal marshes across a Chesapeake Bay subestuary are not keeping up with sea-level rise. PLoS One 11:e0159753
Bramley R, Rimmer D (1988) Dredged materials–problems associated with their use on land. Journal of Soil Science 39:469–482
Bray JT, Bricker OP, Troup BN (1973) Phosphate in interstitial waters of anoxic sediments: oxidation effects durign the sampling procedure. Science 180:1362–1364
Canfield DE, Raiswell RR, Westrich JT, Reaves CM, Berner RA (1986) The use of chromium reduction in the analysis of reduced inorganic sulfur in sediments and shales. Chemical Geology 54:149–155
Cappuyns V, Swennen R, Verhulst J (2004) Assessment of acid neutralizing capacity and potential mobilisation of trace metals from land-disposed dredged sediments. Science of the Total Environment 333:233–247
Cappuyns V, Swennen R, Devivier A (2006) Dredged river sediments: potential chemical time bombs? A case study. Water Air and Soil Pollution 171:49–66
Carter WR III (1986) An argument for retaining periods of non-dredging for the protection of oyster resources in the upper Chesapeake Bay. American Malacological Bulletin 3:5–10
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnology and Oceanography 14:454–458
Coch CA (1996) Containment Islands: a solution for ports and the environment. Journal of Urban Technology 3:39–63
Colman SM, Halka JP, Hobbs C III, Mixon RB, Foster DS (1990) Ancient channels of the Susquehanna River beneath Chesapeake Bay and the Delmarva Peninsula. Geological Society of America Bulletin 102:1268–1279
Cornwell JC, Morse JW (1987) The characterization of Iron sulfide minerals in anoxic marine-sediments. Marine Chemistry 22:193–206
Cornwell J. C, Owens M. S (1999) The nutrient chemistry of sediment dredging: sediment nutrient inventories and fluxes. In: report to Maryland port administration TS-187-99. University of Maryland Center for environmental science, Cambridge, Maryland
Cornwell JC, Owens MS (2011) Quantifying sediment nitrogen releases associated with estuarine dredging. Aquatic Geochemistry 17:499–517
Cornwell J. C, Sampou P. A (1995) Environmental controls on iron sulfide mineral formation in a coastal plain estuary. In: Vairavamurthy MA, Schoonen MAA (eds) ACS symposium series 612
Cornwell JC, Stevenson JC, Conley DJ, Owens M (1996) A sediment chronology of Chesapeake Bay eutrophication. Estuaries 19:488–499
Costanza R, Farber SC, Maxwell J (1989) Valuation and management of wetland ecosystems. Ecological Economics 1:335–361
Demas SY, Hall AM, Fanning DS, Rabenhorst MC, Dzantor EK (2004) Acid sulfate soils in dredged materials from tidal Pocomoke sound in Somerset County, MD, USA. Australian Journal of Soil Research 42:537–545
Gibb MM (1979) A simple method for the rapid determination of iron in natural waters. Water Research 13:295–297
Goldberg ED, Hodge V, Koide M, Griffin J, Gamble J, Bricker OP, Matisoff G, Holdren GR Jr (1978) A pollution history of Chesapeake Bay. Geochimica et Cosmochimica Acta 42:1413–1425
Gottschalk LC (1945) Effects of soil erosion on navigation in upper Chesapeake Bay. Geograph Rev 35:219–238
Hamons F, Young W (2000) Beneficial uses of dredged material in the upper Chesapeake Bay. Journal of Dredging Engineering 2:1–40
Hesslein RH (1976) An in situ sampler for close interval pore water studies. Limnology and Oceanography 21:912–914
Hobbs CH III, Halka JP, Kerhin RT, Carron MJ (1992) Chesapeake Bay sediment budget. Journal of Coastal Research 8:292–300
Hopkinson CS, Lugo AE, Alber M, Covich AP, Van Bloem SJ (2008) Forecasting effects of sea-level rise and windstorms on coastal and inland ecosystems. Frontiers in Ecology and the Environment 6:255–263
Johnston SG, Keene AF, Bush RT, Burton ED, Sullivan LA, Isaacson L, McElnea AE, Ahern CR, Smith CD, Powell B (2011) Iron geochemical zonation in a tidally inundated acid sulfate soil wetland. Chemical Geology 280:257–270
Jordan TE, Cornwell JC, Boynton WR, Anderson JT (2008) Changes in phosphorus biogeochemistry along an estuarine salinity gradient: the iron conveyer belt. Limnology and Oceanography 53:172–184
Kearney MS, Stevenson JC (1991) Island land loss and marsh vertical accretion rate evidence for historical sea-level changes in Chesapeake Bay. Journal of Coastal Research:403–415
Kearney MS, Rogers AS, Townshend JR, Rizzo E, Stutzer D, Stevenson JC, Sundborg K (2002) Landsat imagery shows decline of coastal marshes in Chesapeake and Delaware bays. Eos, Transactions American Geophysical Union 83:173–178
La Peyre MK, Gossman B, Piazza BP (2009) Short- and long-term response of deteriorating brackish marshes and open-water ponds to sediment enhancement by thin-layer dredge disposal. Estuaries and Coasts 32:390–402
Larsen RK, Steinbacher JC, Baker JE (2001) Ammonia exchange between the atmosphere and the surface waters at two locations in the Chesapeake Bay. Environmental Science & Technology 35:4731–4738
Lehtoranta J, Ekholm P, Pitkanen H (2009) Coastal eutrophication thresholds: a matter of sediment microbial processes. Ambio 38:303–308
Leventhal J, Taylor C (1990) Comparison of methods to determine degree of pyritization. Geochimica et Cosmochimica Acta 54:2621–2625
Linthurst RA, Blum U (1981) Growth modifications of Spartina alterniflora Loisel. By the interaction of pH and salinity under controlled conditions. Journal of Experimental Marine Biology and Ecology 55:207–218
Maher A, Douglas WS, Jafari F, Pecchioli J (2013) The processing and beneficial use of fine-grained dredged material. A Manual for Engineers, New Jersey. Rutgers University, In
Merrill J. Z, Cornwell J. C (2000) The role of oligohaline marshes in estuarine nutrient cycling. In: Weinstein MP, Kreeger DA (eds) concepts and controversies in tidal marsh ecology
Morse JW, Cornwell JC (1987) Analysis and distribution of Iron sulfide minerals in recent anoxic marine-sediments. Marine Chemistry 22:55–69
Morse JW, Millero FJ, Cornwell JC, Rickard D (1987) The chemistry of the hydrogen sulfide and iron sulfide systems in natural waters. Earth Science Reviews 24:1–42
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis, Vol. Pergamon Press, New York
Poltrack S (2000) The maritime industry and our environment: the delicate balance of economic and environmental concerns, globally, nationally, and within the port of Baltimore. U Balt J Envtl L 8:51
Portnoy JW, Giblin AE (1997) Effects of historic tidal restrictions on salt marsh sediment chemistry. Biogeochemistry 36:275–303
Raiswell R, Canfield DE, Berner RA (1994) A comparison of iron extraction methods for the determination of degree of pyritisation and the recognition ofiron-limited pyrite formation. Chemical Geology 111:101–110
Roden EE, Edmonds JW (1997) Phosphate mobilization in iron-rich anaerobic sediments: microbial Fe(III) oxide reduction versus iron-sulfide formation. Archiv für Hydrobiologie 139:347–378
Schieder NW, Walters DC, Kirwan ML (2018) Massive upland to wetland conversion compensated for historical marsh loss in Chesapeake Bay, USA. Estuaries and Coasts 41:940–951
Staver L. W, Cornwell J. C, Nidzieko N. J, Staver K. W, Owens M. S, Logan L, Kim C, Malkin S. Yn.d. (In review) tidal marsh restoration at Poplar Island: II. Elevation trends, vegetation development, and carbon dynamics. Wetlands
Sweet ST, Wong JM, Brooks JM, Wade TL (1993) Sediment grain size analysis. In: Lauenstein GG, Cantillo AY (eds) Sampling and analytical methods of the National Status and trends program, book II. NOAA, Silver Spring, Maryland
Thornton SF, McManus J (1994) Application of organic carbon and nitrogen isotope and C/N ratios as source indicators of organic matter provenance in estuarine ecosystems: evidence from the Tay estuary, Scotland. Est Coast Shelf Sci 38:219–233
Wilson MD, Watts BD, Brinker DF (2007) Status review of Chesapeake Bay marsh lands and breeding marsh birds. Waterbirds 30:122–138
Yozzo DJ, Wilber P, Will RJ (2004) Beneficial use of dredged material for habitat creation, enhancement, and restoration in New York–New Jersey Harbor. J Environ Mgmt 73:39–52
Zhang Q, Hirsch RM, Ball WP (2016) Long-term changes in sediment and nutrient delivery from Conowingo dam to Chesapeake Bay: effects of reservoir sedimentation. Environmental Science & Technology 50:1877–1886
Acknowledgements
The contracts funding this research were financially supported by the Maryland Department of Transportation Maryland Port Administration with project management by the Maryland Environmental Service. We thank the U.S. Army Corps of Engineers for additional guidance. Reviews by Michelle Osborn, Claire Ruark and two reviewers improved this manuscript. This is contribution 5748 from the University of Maryland Center for Environmental Science.
Author information
Authors and Affiliations
Contributions
JCC, MSO, LWS and JCS all contributed to study conception and design. All authors contributed field sampling and/or data analysis. JCC drafted the manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cornwell, J.C., Owens, M.S., Staver, L.W. et al. Tidal Marsh Restoration at Poplar Island I: Transformation of Estuarine Sediments into Marsh Soils. Wetlands 40, 1673–1686 (2020). https://doi.org/10.1007/s13157-020-01294-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-020-01294-5