Abstract
The aims of the present investigation are to evaluate the chemical controlling factors of the water resources and identify the source, as well as assess its quality with respect to domestic and irrigation criteria, to establish long-term environmental measures and a sustainable water resources management plan in the mountain regions. For these purposes, 28 water resources (14 drainage; 3 piezometric; 7 Arminaz Stream; 2 spring and 2 Dora River) samples were collected from different locations of the Mont de la Saxe region and analyzed for pH, electrical conductivity, total dissolved solids (TDS), total hardness, and major cations and anions. The pH of the water samples ranged from 7.6 to 11.6, indicating the alkaline nature of the water in the study area. The TDS ranged from 142.1 to 995.3 mg L−1 and exceeded the desirable limit of 500 mg L−1 at some sites. Ca–Mg–HCO3 and Ca–Mg–SO4 were the dominant hydrogeochemical facies; Ca2+ and Mg2+ were the dominant cations and HCO3− and SO42− were the dominant anions in the Mont de la Saxe region. High concentrations of SO42− were attributed to the dissolution of gypsum in the study area. The hydrogeochemical data show the mixing process from the springs pass through higher altitudes drains samples to lower drains samples as well as piezometer. The chemistry of water resources of the study area is mainly controlled by the rock-weathering phenomenon and ion exchange processes as well as by water flow direction of the area. High concentrations of TDS, Ca2+, SO42−, and TH and high salinity in many samples of the drainage and Arminaz Stream water make it unsuitable for domestic and irrigation uses in the study area. This study could be useful for future water resources management in the mountain regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freshwater resources are very limited in the world; only 3% of the Earth’s water is freshwater and it is very necessary for the survival of life. Most of the freshwater is in icecaps and glaciers (69%) and groundwater (30%), while all lakes, rivers, and swamps combined only account for a small fraction (0.3%) of the Earth’s total freshwater reserves. Preserving freshwater quality is important for public health and also for aquatic life. Unfortunately, deteriorating water quality and depleting water resources are major environmental issues in many parts of the world. In the last few decades, there has been a tremendous increase in the demand for freshwater due to the rapid growth of population and the accelerated rate of industrialization (Chandra et al. 2015).
Geochemistry assessment helps to obtain an insight into the contributions of rock/soil water interaction and anthropogenic influences on water resources. These processes are responsible for the spatiotemporal variations in water chemistry, which in turn depends on a number of factors, such as general geology, the degree of chemical weathering of the various rock types, quality of recharge water and inputs from sources other than water–rock interaction. Such factors and their interactions are responsible for changing water quality. Babiker et al. 2007 suggested that the water quality required would depend on the desired use of water; hence, different uses require different criteria of water quality assessment as well as a standard method for reporting and comparing the result of water analysis. Further, it is possible to understand the change in quality due to water–rock interaction (weathering) or any type of anthropogenic influence (Todd 1980). Nowadays, understanding of the proper hydrogeochemical processes, the source of pollutant entering and monitoring of water quality and quantity status are very much essential for sustainable development and effective management of water resources for any area of the globe. Recently, many researchers from several countries of the world have shown much interest in understanding the geochemistry and quality of water resources by using hydrogeochemical techniques (De Montety et al. 2008; Prasanna et al. 2010; Zhu and Schwartz 2011; Gaofeng et al. 2010; Alexakis 2011; Ryzhenko and Cherkasova 2012; Ahmed et al. 2013; Zhang et al. 2015; Nair et al. 2015; Nematollahi et al. 2016; Barzegar et al. 2017; Sánchez et al. 2017; Madhav et al. 2018) and multivariate statistical tools (Shrestha and Kazama 2007; Zhou et al. 2007; Srivastava and Ramanathan 2008; Huang et al. 2010; Chapagain et al. 2010; Oyebog et al. 2012; Wang et al. 2013; Marimon et al. 2013; Arslan 2013; Nasri et al. 2015; Singh et al. 2015, 2017; Voutsis et al. 2015; Arulbalaji and Gurugnanam 2016; Al-Omran et al. 2017). In a similar context, a number of Italian researchers have evaluated the water resources quality and its geochemistry using the above discussed methods (Nisi et al. 2008; Giménez-Forcada et al. 2010; Ruggieri et al. 2011; Albanese et al. 2013; Mongelli et al. 2013; Corniello and Ducci 2014; Zuzolo et al. 2017; Biddau et al. 2017).
In the Aosta Valley region (present research is a portion of the Aosta Valley region), several environmental studies (landslide, soil erosion, snow avalanche test, water-inflow discharge and hydrographs and time series analysis) have been conducted by many researchers in the recent time period (Barla et al. 2010; Freppaz et al. 2010; Viglietti et al. 2010; Maggioni et al. 2013; Barbero et al. 2013; Russo et al. 2013; Stanchi et al. 2014; Lo Russo et al. 2015). However, only a few researchers have conducted studies on water resource quality and its geochemistry in the Aosta Valley region (De Maio et al. 2010; Filippa et al. 2010; Bonomi et al. 2015; Tiwari et al. 2017). Moreover, in the mountain areas of Italy, three important strategies (water monitoring, water use and protection of water) are essential for a sustainable management of the water resources (Christe et al. 2013). Based on the above suggested points, the primary objective of this study is to evaluate the geochemistry of water resources (drainage water, piezometric water, stream water, spring water and river water) and their quality for suitability for drinking and domestic as well as irrigation uses in the Mont de la Saxe region through an integrated statistical and hydrogeochemical approach. The targeted outcome of this study is the generation of baseline information on the water resources geochemistry and its quality status in the study area that will help the mountain planners in managing their water resources for community use.
Study area
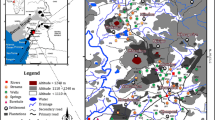
In the Aosta Valley region of Italy, the study area (Mont de la Saxe) (Fig. 1) covers about 50 km2. The Mont de la Saxe has a steep terrain with an elevation ranging from 1100 m above sea level (a.s.l.) in the northwest to 4100 m a.s.l. in the southeast, respectively. The slope of the study area region is composed of meta-granites to meta-rhyolites (Permian volcanic rocks) and belongs to the Mont de la Saxe Unit. The meta-granites are composed of grayish aplitic granites with porphyritic texture, locally foliated, with cataclastic and mylonitic bands and it dominated the study area. The complex is characterized by intense fracturing with numerous open joint sets. A number of landslide phenomena frequently occur in Mont de la Saxe, particularly in the southwestern slope (above the Entrèves village). The Aosta Valley (the study area is a part of the valley) is formed by the Dora River, which flows from east to west, and its 12 tributary valleys. The Aosta Valley is the smallest region in Italy and important alpine valley systems of the Po River, surrounded by the key peaks of the Graie and the Pennine Alps. The climate of the Aosta Valley region is cold in the winter season and cool in the summer season, respectively. Maximum annual rainfall occurs in the spring and autumn seasons and minimum during the summer and winter seasons in the valley. Mercalli et al. (2003) reported the highest mean precipitation value by month is roughly 140 mm and the minimum is 30 mm in the region of Aosta Valley.
The Aosta Valley geological structure (from NW to SE) is a natural cross section through the structural block of the Alps. According to Dal Piaz (1992), the stacked relics of the African continental margins and ancient European region, with wrecked portions of the ocean floor between them, emerge in this area of the Alps. Moreover, the valley has several structural geological domains, for example, the Austroalpine Domain, Elvetico–Ultraelvetico Domain, Pennidic Domain and Piemontese Zone.
The Mont de la Saxe (the study area of the present research) geological and structural setting is strictly influenced by the Ultrahelvetic–Helvetic front and the Penninic frontal thrust, two local compressive structures oriented NE–SW which dispersed the key tectonostratigraphic units (Fig. 1). The unit of the Helvetic contains prominent crystalline duplexes, décollement nappes as well as sedimentary cover units. It is noticeable by a blueschist-to-eclogite-facies imprint of Cretaceous–Eocene age, followed by a Barrovian overprint and consists of minor oceanic and continental nappes. The collisional wedge was later accreted by the Helvetic basement and cover units and depressed by the Southalpine lithosphere, which in turn was deformed as an antithetic fold-and-thrust belt. The Helvetic–Dauphinois domain was powerfully malformed from the Late Oligocene onward when the orogeny spread onto the proximal European margin. The Helvetic Domain contains migmatites, granites, orthogneisses, paragneisses and porphyries in the southeast part of the massif (Val Veny–Val Ferret) and belongs to the Monte Bianco Massif and Mont Chètif wedge complex. The Ultrahelvetic units are a meta-sedimentary sequence which predominantly contains closely foliated carbonate-bearing argillaceous schists and arenaceous limestones with quartz arenites levels. The age of the sequence can be referred to as the Middle Jurassic. The Penninic units cover the lower distal clastics and pelagic Cretaceous deposits of the Courmayeur Zone, the more internal ocean–continent transition zone and the middle Penninic nappes comprising Zone Huillère Permo-Carboniferous deposits (black schist, quartzites and conglomerates) and a Pre-Permian crystalline basement complex (paragneiss and mica schists with amphibolites and metabasites intercalations). These units dip toward SE developing an imbricated structure and large belts oriented northeast–southwest. The Quaternary alluvial, glacial and debris deposits are in unconformity and related. The recent and actual alluvial deposits lie on the valleys’ bottom and consist of gravels, pebbles and boulders in a coarse-grained sandy matrix along the river channels and finer-grained deposits (sandy silts and silts) in floodplain areas. Old glacial outcrop deposits occur irregularly along the slopes, while the recent ones become particularly widespread along secondary tributary valleys at altitudes higher than 2000 m.a.s.l. The debris deposits include moraines, alluvial fan and flow deposits, along with the stream incision and at the outlet of the minor valleys.
Materials and methods
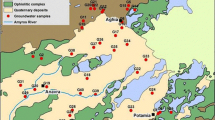
A total of 28 water resources, including 19 groundwater samples (14 drainage, 3 piezometric and 2 spring) and 09 surface water samples (7 Arminaz Stream and 2 Dora River) were collected in pre-washed polyethylene bottles during the year 2015 in the Mont de la Saxe area for determination of the quality status of water and its geochemistry (Fig. 1 and Table 1). Sampling site elevation and coordinate values were determined using a Differential Global Positioning System (DGPS) during the sampling period in the study area. The pH, temperature, electric conductivity (EC) and total dissolved solids (TDS) were measured in the field using the Multiparameter Instrument (HI 9829). In the laboratory, the water samples were filtered through 0.45 µm Millipore membrane filters to separate suspended particles. Appropriate quality assurance procedures and precautions were carried out to ensure reliability, and samples were carefully handled to avoid contamination. Glassware was properly cleaned and analytical-grade reagents were used. Milli-Q water was used throughout the study. Acid titration was used to determine the bicarbonate (HCO3−) of the water samples. Major anions (F−, Cl−, SO42−, and NO3−) were analyzed by ion chromatography (Dionex ICS-5000, Thermo Scientific). Major cations (Ca2+, Mg2+, Na+ and K+) were measured by inductively coupled plasma mass spectrometry (ICP-MS 7500ce, Agilent). In Italy, reference analytical methods for the determination of water chemical parameters follow the Legislative Decree No. 152 (2006). Moreover, the APAT (Protection Agency of Environment and Technical Services) and IRSA-CNR (Institute for Water Research, National Research Council) established the guidelines (No. 29/2003) for water sampling and physical, chemical, biological and microbiological water characterization (Apat—Irsa/Cnr 2003). Cationic and anionic charge balance (< 10%) was achieved for all analyzed water samples.
Evaluation of geochemical and solute acquisition processes
Piper diagram, Gibbs diagram and saturation index (SI) were computed using AqQA, Grapher and PHREEQC software.
The ion exchange between the groundwater and its host environment during residence or in movement processes is the important controlling factor for the water chemistry of the region. The ion exchange process can be understood by chloro-alkaline indices, also known as Schoeller indices (Schoeller 1977) and expressed as:
Multivariate statistical analysis
A correlation coefficient is a commonly used measure to establish the relationship between two variables. It is simply a measure to exhibit how well one variable predicts the other. Cluster analysis (CA) is an important technique for analyzing hydrogeochemical data. A number of researchers have successfully used it to classify the water samples and formulate geochemical models (Alberto et al. 2001; Meng and Maynard 2001; Singh et al. 2005, 2012). According to Singh et al. (2012), the main aim of the CA is to group objects into statistically distinct groups or clusters, so that objects within a cluster are similar to each other but different from those in another cluster. In the present study, the inter-elemental correlations and CA of the analyzed physico-chemical parameters of the water resource samples were computed to understand the possible sources of ions in the study area.
Evaluation of the suitability of water for irrigation
Salinity, sodicity, toxicity and related parameters are generally considered when evaluating the suitability of water for irrigation (Shainberg and Oster 1976; Todd 1980). Specific parameters indicating the suitability of water for irrigation (Table 2) are based on the effects of dissolved major ions on plant growth and soil properties.
Results and discussion
The analyzed physico-chemical data of the water resources and the computed chloro-alkaline indices (CAI), sodium adsorption ratio (SAR), percent sodium (%Na), residual sodium carbonate (RSC) and permeability index (PI) in the year 2015 of the Mont de la Saxe region in the Aosta Valley are given in Table 1. Moreover, a statistical summary for the analyzed 28 water resources samples compared with the drinking water quality standard established by the World Health Organization (WHO 2006) is presented in Table 4.
pH, temperature and EC
The pH of the analyzed water samples varied from 7.6 to 11.6, indicating the alkaline nature of the water samples in the study area. The measured temperature of the water samples ranged from 0.93 to 9.75 °C (avg. 5.84 °C), and EC ranged from 206 to 1253 µS cm−1 (avg. 541 µS cm−1) in the study area. A study of the pH, electrical conductivity and temperature (Figs. 2, 3) shows that the Dora River and its tributary Arminaz Stream have high values of pH, between 10 and 11.6, and low to high values of temperature and low conductivity except for a few locations. However, the drainage and the piezometric samples, although maintain temperature values close to those of the surface with a very narrow variability, clearly deviate from low pH values of about 8 and have a higher conductivity (Figs. 2, 3). This phenomenon can be explained by the natural process of mineralization and altered water regime that lowers the differences in the study area. The FD1, FD2 and TA1 sites (the River Dora and the Arminaz Stream) represent the less mineralized areas, typical for high-altitude drained systems, where the high-water regime and turbulence result in low mineralization (Fig. 3). Particularly, TA1 site of the Arminaz stream was taken at high altitude (next to the snowfield) probably due to this reason it has less mineralized as compared with other Arminaz stream sites (Fig. 3). Surface waters correspond to the limit just mineralized and formed by the waters of the River Dora and the Arminaz Stream. Also fall into this group of the water sources, confirming their familiarity and the low interaction with the rocks and their subterranean circuit. Along the Arminaz Stream, the high concentration of EC may be due to the presence of small deposits of “Carniole” (sulfate enrichment, i.e., gypsum), substantially modifying the composition of these waters with an obvious increase in the sulfate component.
Hydrogeochemical relations and water type
The Piper (1944) diagram is very useful in determining relationships of different dissolved constituents and classification of water on the basis of its chemical characters. The entire water samples are plotted in the Ca2+-dominant zone in the cation facies and most of the water samples in the HCO3− and SO42− zones in the anion facies, respectively (Fig. 4). The plot of the geochemical data for both years on a diamond-shaped field reveals that the majority of the plotted points fall in zones 1, 3, 4, 5 and 6 (Fig. 4). All of the water samples are plotted in zone 1, which indicates the dominance of alkaline earths (Ca2+ + Mg2+) over alkalis (Na+ + K+). In most of the water resources samples, weak acids (HCO3−) exceed strong acids (SO42− + Cl−) and are plotted in zone 3. However, in the few samples of the Arminaz Stream and drainage, strong acids exceed weak acids and are plotted in zone 4. The plotted points of the water samples fall in zone 5, suggesting carbonate hardness, while some samples of the Arminaz Stream and drainage lie in zone 6, indicating that non-carbonate hardness exceeds 50% (Fig. 4). Based on the dominance of different cations and anions in the water resources, two hydrogeochemical water types can be defined as Ca–Mg–HCO3 and Ca–Mg–SO4 in the study area.
Statistical analysis
Statistical analysis is an important tool used to process large amounts of data and report overall trends. The computed correlation matrix of the 13 measured parameters of the water resources samples is given in Table 3. It is observed that the EC and TDS show a high positive correlation with SO42−, Ca2+, Mg2+ and TH and moderate correlation with HCO3− and NO3−. Good correlations were also observed between Ca2+–Mg2+ (0.851), Mg2+–HCO3− (0.861) and Ca2+–HCO3− (0.587) in the water samples, suggesting that a significant fraction of Ca2+, Mg2+ and HCO3− was derived from weathering and dissolution of carbonate minerals. The lithology of the study area supports this observation. The positive correlations between Na+–Cl− (0.852), K+–Cl− (0.816), Na+–K+ (0.633), Ca2+–TH and Mg2+–TH in the water samples indicates similar sources and/or geochemical behavior during ionic mobilization. The good correlation of Ca2+–SO42− (0.982) indicates that gypsum dissolution could be the major contributor for these dissolved ions in the water of the study area.
The results of the cluster analysis are shown in Fig. 5. Each observation is labeled with the sample code to which it was assigned. We found four cluster groups in the study area (Fig. 5). It is apparent that the observations assigned to each group share similar geochemical characteristics. The first cluster group was associated with the spring (SB, SA) and five high-altitude drainage water samples (DC, DG, DH1, DH1C, and DHF), which shows that the drainage water intersects the alimentation system of the springs. The second cluster group was associated with the three drainage water samples (DHB1, DHB2, DH4), one piezometric sample (P3), one stream sample (TA1) and the Dora River samples (FD2 FD1), and occur in this group maybe due to a hydraulic connection between drainage, piezometric, stream and the Dora River. The third cluster group was associated with the two drainage water samples (DHB5, DHB3), three samples from the Arminaz Stream (TA5, TA6, TA7) and two samples from piezometric (P1, P2), and occur in this group because the buried sulfate deposits intersect the water flow net of these aquifers. The last cluster group (fourth) was associated with the rest of the drainage water samples (DL, DLB, DLT2, DLT1) and rest of the Stream Arminaz water samples (TA2, TA3, TA4) at high altitude, and occur there maybe due to a similar hydrogeochemical behavior.
Hydrogeochemical processes and solute acquisition processes
The chemical quality of water depends on some important controlling factors like geological structure and mineralogical composition of the aquifer, duration of water–rock interaction, dissolution, precipitation of mineral species and seawater and anthropogenic influences (Hounslow 1995). Gibbs’s (1970) diagram represents the ratio of Na+ + K+/(Na+ + K+ + Ca2+) and Cl− + NO3−/(Cl− + NO3− + HCO3−) as a function of TDS and helps to understand the functional sources of dissolved chemical constituents, such as precipitation/rock/evaporation dominance. The plot of geochemical data on Gibbs’s diagrams suggests rock weathering as a major driving force, which controls the water resources chemistry of the Mont de la Saxe region (Fig. 6).
The plot of (Ca2++Mg2+) versus (HCO3−) and (Ca2++Mg2+) versus (SO42−) indicates that the weathering and mineralization of carbonate minerals and gypsum are the dominant reactions with the minor addition of silicate dissolution in the study area and the lithology of the area is adding proof (Figs. 7, 8).
For the evaluation of geochemical affinity of the study area, the normalized ratios of K/HCO3 versus SO4/Ca were plotted (Fig. 9). The plotted samples indicate that the composition of the Stream Arminaz waters is controlled by the leaching of “Carniole deposits”, with a sulfate enrichment (i.e., gypsum), as in the landslide area samples. This can be justified by the partial leaching of the same deposits buried very close to the landslide area, intercepted by the water network flow. The spring samples represented the terms less involved in this process probably due to the different water regime. The drainage sample DG and DC had geochemical characteristics close to those of spring water; this could be due to their partial mixing (Fig. 9). Moreover, we can observe that how the DH drainage system is intersecting the groundwater mixing area and it allows defining its extension up to the silicate terms of the piezometric P2 and P3 (Fig. 9).
Figure 10 shows that the groundwater flow directions of the study area were reconstructed by observation of geochemical data, and we observed two potentially overlapping water circulation circuits: the karst and superficial. The karst system aquifer is characterized by a network of conduits and caves set along the planes of shrinkage detained within the NE–SW direction. However, the superficial aquifer (discontinuous aquifer) is in the altered subsurface area with directions that roughly follow the slope. During the summer period when the water capacity rises, the overflowing phenomena occur along the north side of the Mont de la Saxe, causing both travertine deposition and instability and triggering landslide processes. In particular, the analyzed data confirm that the geochemistry affinity between the springs (SA and SB) and the Arminaz Stream is probably due to a hydraulic connection, and the Arminaz water feeds these springs, linked under-bed stream by the karst aquifer system (Fig. 10). The hydrogeochemical data show the mixing process from the springs pass through higher altitudes drains samples (DG and DC) to lower drains samples (DL and DH) as well as piezometer water samples. It is also probable that the presence of buried Carniole sulfate deposits influences the mineralization process of the water sampled in the drains DL and DLT and partly the drains DH in the study area.
Chloro-alkaline indices (CAI)
The positive or negative values of the chloro-alkaline indices (CAI-I and CAI-II) depend on whether the exchange of Na+ and K+ is from water with Mg2+ and Ca2+ in rock/soil or vice versa. The positive value of CAI ratio can be due to the exchange of Na+ and K+ in water with Mg2+ and Ca2+, and this phenomenon indicates base-exchange processes. However, if CAI values are negative, the reaction will be a cation–anion exchange reaction and indicates chloro-alkaline disequilibrium phenomenon. Throughout this process, the host rocks are the prime source of dissolved solids in water. In the present study, all of the tested water samples had negative chloro-alkaline indices values, revealing chloro-alkaline disequilibrium phenomenon and the reaction as a cation–anion exchange in the study area (Table 1).
Saturation indices
By using the saturation indices (SI), it is possible to predict the reactive mineralogy of the subsurface from water data without collecting the samples of the solid phase and analyzing mineralogy (Deutsch 1997). Figure 11 shows the comparison between the calcite saturation index and pH; the weathering process of silicate causes calcite saturation in the spring and river samples. High pH values about 11 activates a continuous uptake of the atmospheric CO2 that dissolves. The sudden CO2 outgassing, availability of calcium and magnesium and the oxygenation process cause the travertine inside fractures and flowing of springs in the site. Travertine deposits located along the mountainside are proofs also of the seasonal springs of this area.
Suitability for drinking and domestic uses
The statistical summary of the physico-chemical parameters of the analyzed water samples was compared with the standard guideline values recommended by the World Health Organization (WHO 2006) for drinking and public health (Table 4). The pH values were above 10 for the Arminaz Stream, spring and the Dora River water samples. The concentration of TDS ranged from 142.1 to 995.3 mg L−1 (avg. 395.3 mg L−1) and the values of TDS exceeded the desirable limit of 500 mg L−1 in 25% analyzed samples (Table 1). We found a high concentration of TDS in the drainage water samples as well as in few samples of the Arminaz Stream of the study area (Table 1). The total hardness (TH) is an important parameter of water quality whether it is to be used for domestic, industrial or agricultural purposes. Water can be classified into soft (< 75 mg L−1), moderately hard (75–150 mg L−1), hard (150–300 mg L−1) and very hard (> 300 mg L−1) based on hardness (Sawyer and McCarty 1967). The TH of the analyzed water samples ranged between 102.7 and 717.8 mg L−1 with an average value of 288 mg L−1. The analytical data indicated that 17.8% of the samples were moderately hard, 53.6% were hard and 28.6% were very hard in the study area (Table 1). We observed the high concentration of TH in the drainage water samples as well as in few sample of the Arminaz Stream. Long-term consumption of extremely hard water might lead to an increased incidence of urolithiasis, anencephaly, prenatal mortality, some types of cancer and cardiovascular disorders (Agrawal and Jagetia 1997).
The concentrations of F−, NO3− and Cl− were within the respective permissible limits of 1.5, 50 and 250 mg L−1 (WHO 2006) in all of the water resources samples in the study area (Table 4). Sulfate concentration in the drainage water samples and in few samples of the Arminaz Stream water sample exceeded the limit of 200 mg L−1 (WHO 2006) (Table 1). However, the concentrations were within the respective limit of 200 mg L−1 in the rest of the water resources samples. Higher sulfate concentrations are associated with respiratory disorders (Subba 1993).
The concentrations of Ca2+ exceeded the desirable limits of 75 mg L−1 established by the WHO (2006) in 52.8% of the water samples in the study area. Maragella et al. (1996) reported that the consumption of high level of Ca2+ concentration for long periods may cause risk of kidney stones. Moreover, 10.7% of the water samples had high concentrations of Mg2+ (> 30 mg L−1) as per the WHO standards in the study area. The level of Na+ concentrations in the water resources samples was less than the recommended limit (200 mg L−1) by the WHO (2006) for drinking water uses.
Suitability for irrigation uses
The statistical summary of calculated sodium adsorption ratio (SAR), percentage sodium (%Na), residual sodium carbonate (RSC) and permeability index (PI) are presented in Table 4.
The concentration of sodium and electrical conductivity play a vital role in classifying irrigation water. The high salt concentrations in the water directly affect plant growth, soil structure, permeability and aeration. For assessment of the suitability of water for irrigation purposes, tests based on electrical conductivity and sodium adsorption ratio were proposed by the US Salinity Laboratory (1954). The US Salinity Laboratory (1954) diagram classifies four salinity classes for irrigation uses, such as low (EC = < 250 µS cm−1), medium (EC = 250–750 µS cm−1), high (EC = 750–2250 µS cm−1) and very high (EC = 2250–5000 µS cm−1). A high sodium concentration leads to the development of an alkaline soil, while a high salt concentration (EC) in water leads to the formation of saline soil.
Water is classified into four alkali classes, such as low (SAR < 6), medium (SAR 6–12), high (SAR 12–18) and very high (SAR 18) as per the SAR value. The calculated SAR values of the water resources samples ranged from 0.02 to 0.35 (avg. 0.7) in the study area (Table 4). The plot of the data on the US salinity diagram (USSL 1954) in which EC is taken as the salinity hazard and SAR as the alkalinity hazard shows that the entire samples of the river water and that of piezometer, and most of the drainage and Arminaz Stream samples fall in the C2S1 categories, indicating good to permissible quality of water for irrigation uses (Fig. 12). Due to low sodium and medium salinity, the water of C2S1 class can be used for irrigation purpose on almost all soil with little danger of problem from sodium. However, some samples of the drainage water and the Arminaz Stream fall into the categories C3S1, indicating high-salinity and low-alkali water. Special salinity control measures are required before using the high-salinity water (C3) water on soils. High-salinity water can be used to irrigate semi-tolerant and salt-tolerant crop under favorable drainage conditions. Only the spring water samples fall in the class C1S1, indicating these to be good for irrigation purposes in the study area.
US salinity diagram for classification of irrigation waters (after Richards 1954)
Wilcox (1955) reported that the percent sodium (%Na) is an important parameter to evaluate the suitability of water quality for irrigation purposes. Singh et al. (2008) suggested that the high percentage of Na+ with respect to (Ca2+, Mg2+, Na+) in irrigation water causes impairment of soil permeability and deflocculation. The percent sodium in the water resources samples ranged from 0.53 to 13.43% (avg. 2.58%) in the study area. The plot of analytical data on the Wilcox (1955) diagram relating EC and %Na shows that water samples fall into two classes. Most of the water resources samples are of excellent to good and few are of good to permissible quality and may be used for irrigation purposes without any hazard (Fig. 13).
Plot of sodium percent versus electrical conductivity (after Wilcox 1955)
The Doneen (1964) proposed a permeability index (PI) for assessing the suitability of water quality for irrigation uses. Long-term use of water rich in Na+, Ca2+, Mg2+ and HCO3 concentrations affects the permeability of the soil. The PI diagram classifies irrigation water into three classes (Doneen 1964): Class-I and Class-II water types are suitable for irrigation with 75% or more of maximum permeability. However, Class-III with 25% of maximum permeability is unsuitable for irrigation uses. On the basis, all of the water resources samples fall in Class-I and Class-II in the Doneen’s chart implying that the water is of good quality for irrigation purposes with 75% or more of maximum permeability (Domenico and Schwartz 1990) (Fig. 14).
Classification of irrigation water based on the permeability index (after Doneen 1964)
Summary and conclusions
The water resources of the study area were alkaline in nature, while the Dora River and Arminaz Stream water samples had high values of pH, between 10 and 11.6, and lowest value of temperature and electrical conductivity in the study area. Ca–Mg–HCO3 and Ca–Mg–SO4 were the dominant hydrogeochemical facies in the Mont de la Saxe region. In most of the water resources samples, concentrations of alkaline earths (Ca2+ + Mg2+) exceeded alkali cations (Na+ + K+) and weak acids (HCO3−) dominated over strong acids (SO42− + Cl−). Moreover, in the few samples of the Arminaz Stream and drainage, strong acids exceeded weak acids in the study area. Weathering and dissolution of carbonate, sulfate, and silicate minerals, as well as a cation–anion exchange reaction were the major processes controlling the water resources chemistry of the Mont de la Saxe region.
In particular, the analyzed data confirm that the geochemistry affinity between the springs (SA and SB) and the Arminaz Stream, probably due to a hydraulic connection and the Arminaz water feed these springs, linked under-bed stream by the Karst system. The hydrogeochemical data show the mixing process from the springs pass through higher altitudes drains samples (DG and DC) to lower drains samples (DL and DH) as well as piezometer. It is also probable that the presence of buried Carniole sulfate deposits influences the mineralization process of the water sampled in the drains DL and DLT and partly the drain DH. The cluster analysis of the analyzed water resources samples supported these observations in the study area.
All of the tested water of the piezometric, spring and Dora River was suitable for domestic and drinking uses and can safely be used for irrigation propose without any hazard. However, high concentrations of TDS, Ca2+, SO42− and TH and high salinity in many samples of the drainage and Arminaz Stream water make it unsuitable for drinking and irrigation uses and a suitable treatment and special management plan in the study area are required.
References
Agrawal V, Jagetia M (1997) Hydrogeochemical assessment of groundwater quality in Udaipur city, Rajasthan, India. In: Proceedings of national conference on dimension of environmental stress in India, vol 151154. Department of Geology, MS University, Baroda, India
Ahmed MA, Samie SA, Badawy HA (2013) Factors controlling mechanisms of groundwater salinization and hydrogeochemical processes in the Quaternary aquifer of the Eastern Nile Delta, Egypt. Environ Earth Sci 68(2):369–394. https://doi.org/10.1007/s12665-012-1744-6
Albanese S, Iavazzo P, Adamo P, Lima A, De Vivo B (2013) Assessment of the environmental conditions of the Sarno river basin (south Italy): a stream sediment approach. Environ Geochem Health 35(3):283–297. https://doi.org/10.1007/s10653-012-9483-x
Alberto WD, Del PDM, Valeria AM, Fabiana PS, Cecilia HA, De Los ABM (2001) Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquia river basin (Cordoba-Argentina). Water Res 35:2881–2894. https://doi.org/10.1016/S0043-1354(00)00592-3
Alexakis D (2011) Assessment of water quality in the Messolonghi–Etoliko and Neochorio region (West Greece) using hydrochemical and statistical analysis methods. Environ Monit Assess 182(1):397–413. https://doi.org/10.1007/s10661-011-1884-2
Al-Omran AM, Aly AA, Al-Wabel MI, Al-Shayaa MS, Sallam AS, Nadeem ME (2017) Geostatistical methods in evaluating spatial variability of groundwater quality in Al-Kharj Region, Saudi Arabia. Appl Water Sci 7(7):4013–4023. https://doi.org/10.1007/s13201-017-0552-2
APAT—IRSA/CNR (2003) Metodi analitici per le acque, Manuali e linee guida, 29/2003 ISBN: 88-448-0083-7
Arslan H (2013) Application of multivariate statistical techniques in the assessment of groundwater quality in seawater intrusion area in Bafra Plain, Turkey. Environ Monit Assess 185(3):2439–2452. https://doi.org/10.1007/s10661-012-2722-x
Arulbalaji P, Gurugnanam B (2016) Groundwater quality assessment using geospatial and statistical tools in Salem District, Tamil Nadu, India. Appl Water Sci. https://doi.org/10.1007/s13201-016-0501-5
Babiker IS, Mohamed MAA, Hiyama T (2007) Assessing groundwater quality using GIS. Water Resour Manag 21:699–715. https://doi.org/10.1007/s11269-006-9059-6
Barbero M, Barpi F, Borri-Brunetto M, Bovet E, Chiaia B, De Biagi V, Ceaglio E (2013) A new experimental snow avalanche test site at Seehore peak in Aosta Valley (NW Italian Alps)—part II: engineering aspects. Cold Reg Sci Technol 86:14–21. https://doi.org/10.1016/j.coldregions.2012.10.014
Barla G, Antolini F, Barla M, Mensi E, Piovano G (2010) Monitoring of the Beauregard landslide (Aosta Valley, Italy) using advanced and conventional techniques. Eng Geol 116(3):218–235. https://doi.org/10.1016/j.enggeo.2010.09.004
Barzegar R, Moghaddam AA, Tziritis E, Fakhri MS, Soltani S (2017) Identification of hydrogeochemical processes and pollution sources of groundwater resources in the Marand plain, northwest of Iran. Environ Earth Sci 76(7):297. https://doi.org/10.1007/s12665-017-6612-y
Biddau R, Cidu R, Lorrai M, Mulas MG (2017) Assessing background values of chloride, sulfate and fluoride in groundwater: a geochemical-statistical approach at a regional scale. J Geochem Explor 181:243–255. https://doi.org/10.1016/j.gexplo.2017.08.002
Bonomi T, Fumagalli L, Stefania GA, Rotiroti M, Pellicioli F, Simonetto F, Capodaglio P (2015) Groundwater contamination by Cr(VI) in the Aosta Plain (northern Italy): characterization and preliminary modeling. Rendiconti Online Della Società Geologica Italiana 35:21–24. https://doi.org/10.3301/ROL.2015.54
Chandra S, Singh PK, Tiwari AK, Panigrahy BP, Kumar A (2015) Evaluation of hydrogeological factor and their relationship with seasonal water table fluctuation in Dhanbad district, Jharkhand, India. ISH J Hydraul Eng 21(2):193–206. https://doi.org/10.1080/09715010.2014.1002542
Chapagain SK, Pandey VP, Shrestha S, Nakamura T, Kazama F (2010) Assessment of deep groundwater quality in Kathmandu Valley using multivariate statistical techniques. Water Air Soil Pollut 210(1–4):277–288. https://doi.org/10.1007/s11270-009-0249-8
Christe P, Amanzio G, Suozzi E, Mignot E, Ornstein P (2013) Global warming in the Alps: vulnerability and climatic dependency of alpine springs in Italy, Regione Valle d’Aosta (Italy) and Canton Valais (Switzerland). Eur Geol 35:64–69
Corniello A, Ducci D (2014) Hydrogeochemical characterization of the main aquifer of the “Litorale Domizio-Agro Aversano NIPS” (Campania—southern Italy). J Geochem Explor 137:1–10. https://doi.org/10.1016/j.gexplo.2013.10.016
Dal Piaz GV (1992) Alpi dal Monte Bianco al Lago Maggiore. Guide geologiche regionali. Soc Geol It BE-MA ed 3(2)
De Maio M, Minucci F, Nocerino G (2010) Evaluation of the groundwater global pollution risk: relationships with territorial planning. Am J Environ Sci 6(2):103–114. https://doi.org/10.3844/ajessp.2010.103.114
De Montety V, Radakovitch O, Vallet-Coulomb C, Blavoux B, Hermitte D, Valles V (2008) Origin of groundwater salinity and hydrogeochemical processes in a confined coastal aquifer: case of the Rhône delta (Southern France). Appl Geochem 23(8):2337–2349. https://doi.org/10.1016/j.apgeochem.2008.03.011
Deutsch WJ (1997) Groundwater geochemistry: fundamentals and application to contamination. CRC Press, Boca Raton
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology, 2nd edn. Wiley, New York
Doneen LD (1964) Notes on water quality in agriculture. Department of Water. Science and Engineering, University of California, Davis, USA
Filippa G, Freppaz M, Williams MW, Zanini E (2010) Major element chemistry in inner alpine snowpacks (Aosta Valley Region, NW Italy). Cold Reg Sci Technol 64(2):158–166. https://doi.org/10.1016/j.coldregions.2010.07.005
Freppaz M, Godone D, Filippa G, Maggioni M, Lunardi S, Williams MW, Zanini E (2010) Soil erosion caused by snow avalanches: a case study in the Aosta Valley (NW Italy). Arct Antarct Alp Res 42(4):412–421. https://doi.org/10.1657/1938-4246-42.4.412
Gaofeng Z, Yonghong S, Chunlin H, Qi F, Zhiguang L (2010) Hydrogeochemical processes in the groundwater environment of Heihe river basin, northwest China. Environ Earth Sci 60(1):139–153. https://doi.org/10.1007/s12665-009-0175-5
Gibbs RJ (1970) Mechanism controlling world water chemistry. Science 170:1088–1090. https://doi.org/10.1126/science.170.3962.1088
Giménez-Forcada E, Bencini A, Pranzini G (2010) Hydrogeochemical considerations about the origin of groundwater salinization in some coastal plains of Elba Island (Tuscany, Italy). Environ Geochem Health 32(3):243–257. https://doi.org/10.1007/s10653-009-9281-2
Hounslow AW (1995) Water quality data: analysis and interpretation. CRC Lewis Publishers, New York
Huang F, Wang X, Lou L, Zhou Z, Wu J (2010) Spatial variation and source apportionment of water pollution in Qiantang river (China) using statistical techniques. Water Res 44(5):1562–1572. https://doi.org/10.1016/j.watres.2009.11.003
Legislative Decree (2006) 3 Aprile 2006 n 152—Norme in materia ambientale—(G.U. n. 88 del 14 aprile 2006) Allegato 4/14—Allegato 5 al Titolo V della Parte quarta: Valori di concentrazione limite accettabili nel suolo e nel sottosuolo riferiti alla specifica destinazione d’uso dei siti da bonificare
Lo Russo S, Amanzio G, Ghione R, De Maio M (2015) Recession hydrographs and time series analysis of springs monitoring data: application on porous and shallow aquifers in mountain areas (Aosta Valley). Environ Earth Sci 73(11):7415–7434. https://doi.org/10.1007/s12665-014-3916-z
Madhav S, Ahamad A, Kumar A, Kushawaha J, Singh P, Mishra PK (2018) Geochemical assessment of groundwater quality for its suitability for drinking and irrigation purpose in rural areas of Sant Ravidas Nagar (Bhadohi), Uttar Pradesh. Geol Ecol Landscapes 2(2):127–136. https://doi.org/10.1080/24749508.2018.1452485
Maggioni M, Freppaz M, Ceaglio E, Godone D, Viglietti D, Zanini E, Chiaia B (2013) A new experimental snow avalanche test site at Seehore peak in Aosta Valley (NW Italian Alps)—part I: conception and logistics. Cold Reg Sci Technol 85:175–182. https://doi.org/10.1016/j.coldregions.2012.09.006
Maragella M, Vitale C, Petrarulo M, Rovera L, Dutto F (1996) Effects of mineral composition of drinking water on risk for stone formation and bone metabolism in idiopathic calcium nephrolithiasis. Ciln Sci 91:313–318
Marimon MPC, Roisenberg A, Suhogusoff AV, Viero AP (2013) Hydrogeochemistry and statistical analysis applied to understand fluoride provenance in the Guarani aquifer system, Southern Brazil. Environ Geochem Health 35(3):391–403. https://doi.org/10.1007/s10653-012-9502-y
Meng SX, Maynard JB (2001) Use of statistical analysis to formulate conceptual models of geochemical behavior: water chemical data from Butucatu aquifer in Sao State, Brazil. J Hydrol 250:78–97. https://doi.org/10.1016/S0022-1694(01)00423-1
Mercalli L, Cat Berro D, Montuschi S (2003) Atlante climatico della Valle d’Aosta. Società Meteorologica Subalpina, Turin, p 405
Mongelli G, Monni S, Oggiano G, Paternoster M, Sinisi R (2013) Tracing groundwater salinization processes in coastal aquifers: a hydrogeochemical and isotopic approach in the Na-Cl brackish waters of northwestern Sardinia, Italy. Hydrol Earth Syst Sci 17(7):2917. https://doi.org/10.5194/hess-17-2917-2013
Nair HC, Padmalal D, Joseph A (2015) Hydrochemical assessment of tropical springs—a case study from SW India. Environ Monit Assess 187(2):48. https://doi.org/10.1007/s10661-014-4164-0
Nasri N, Bouhlila R, Riadh A (2015) Multivariate statistical analysis of saline water—a case study: Sabkha Oum Lekhialate (Tunisia). Int J Environ Sci Dev 6:40–43. https://doi.org/10.7763/IJESD.2015.V6.558
Nematollahi MJ, Ebrahimi P, Razmara M, Ghasemi A (2016) Hydrogeochemical investigations and groundwater quality assessment of Torbat-Zaveh plain, Khorasan Razavi, Iran. Environ Monit Assess 188(1):2. https://doi.org/10.1007/s10661-015-4968-6
Nisi B, Buccianti A, Vaselli O, Perini G, Tassi F, Minissale A, Montegrossi G (2008) Hydrogeochemistry and strontium isotopes in the Arno river basin (Tuscany, Italy): constraints on natural controls by statistical modeling. J Hydrol 360(1):166–183. https://doi.org/10.1016/j.jhydrol.2008.07.030
Oyebog SA, Ako AA, Nkeng GE, Suh EC (2012) Hydrogeochemical characteristics of some Cameroon bottled waters, investigated by multivariate statistical analyses. J Geochem Explor 112:118–130. https://doi.org/10.1016/j.gexplo.2011.08.003
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928. https://doi.org/10.1029/TR025i006p00914
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2010) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168(1):63–90. https://doi.org/10.1007/s10661-009-1092-5
Richards LA (1954) Diagnosis and improvement of saline and alkali soils, hand book, No. 60. US Department of Agriculture, Washington, DC
Ruggieri N, Castellano M, Capello M, Maggi S, Povero P (2011) Seasonal and spatial variability of water quality parameters in the Port of Genoa, Italy, from 2000 to 2007. Mar Pollut Bull 62(2):340–349. https://doi.org/10.1016/j.marpolbul.2010.10.006
Russo SL, Gnavi L, Peila D, Suozzi E (2013) Rough evaluation of the water-inflow discharge in abandoned mining tunnels using a simplified water balance model: the case of the Cogne iron mine (Aosta Valley, NW Italy). Environ Earth Sci 70(6):2753–2765. https://doi.org/10.1007/s12665-013-2335-x
Ryzhenko BN, Cherkasova EV (2012) Chemical composition of natural waters and brines as a result of hydrogeochemical processes in water–rock–gas systems. Geochem Int 50(13):1101–1150. https://doi.org/10.1134/S0016702912130058
Sánchez ERS, Hoyos SEG, Esteller MV, Morales MM, Astudillo AO (2017) Hydrogeochemistry and water–rock interactions in the urban area of Puebla Valley aquifer (Mexico). J Geochem Explor 181:219–235. https://doi.org/10.1016/j.gexplo.2017.07.016
Sawyer CN, McCarty PL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw Hill, New York
Schoeller H (1977) Geochemistry of groundwater. In: Groundwater studies—an international guide for research and practice, Chap 15. UNESCO, Paris, France, pp 1–18
Shainberg I, Oster JD (1976) Quality of irrigation water. International Irrigation Information Center, publication no. 2
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Softw 22(4):464–475. https://doi.org/10.1016/j.envsoft.2006.02.001
Singh KP, Malik A, Singh VK, Mohan D, Sinha S (2005) Chemometric analysis of groundwater quality data of alluvial aquifer of Gangetic plain, north India. Anal Chim Acta 550:82–92. https://doi.org/10.1016/j.aca.2005.06.056
Singh AK, Mondal GC, Kumar S, Singh TB, Tewary BK, Sinha A (2008) Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar river basin, India. Environ Geol 54:745–758. https://doi.org/10.1007/s00254-007-0860-1
Singh AK, Mondal GC, Singh TB, Singh S, Tewary BK, Sinha A (2012) Hydrogeochemical processes and quality assessment of groundwater in Dumka and Jamtara districts, Jharkhand, India. Environ Earth Sci 67(8):2175–2191. https://doi.org/10.1007/s12665-012-1658-3
Singh SK, Srivastava PK, Singh D, Han D, Gautam SK, Pandey AC (2015) Modeling groundwater quality over a humid subtropical region using numerical indices, earth observation datasets, and X-ray diffraction technique: a case study of Allahabad district, India. Environ Geochem Health 37(1):157–180. https://doi.org/10.1007/s10653-014-9638-z
Singh CK, Kumar A, Shashtri S, Kumar A, Kumar P, Mallick J (2017) Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J Geochem Explor 175:59–71. https://doi.org/10.1016/j.gexplo.2017.01.001
Srivastava SK, Ramanathan AL (2008) Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environ Geol 53(7):1509–1528. https://doi.org/10.1007/s00254-007-0762-2
Stanchi S, Freppaz M, Ceaglio E, Maggioni M, Meusburger K, Alewell C, Zanini E (2014) Soil erosion in an avalanche release site (Valle d’Aosta: Italy): towards a winter factor for RUSLE in the Alps. Nat Hazard Earth Syst 14(7):1761. https://doi.org/10.5194/nhess-14-1761-2014
Subba Rao N (1993) Environmental impact of industrial effluents in groundwater regions of Visakhapatnam industrial complex. Indian J Geol 65:35–43
Tiwari AK, Ghione R, De Maio M, Lavy M (2017) Evaluation of hydrogeochemical processes and groundwater quality for suitability of drinking and irrigation purposes: a case study in the Aosta Valley region, Italy. Arab J Geosci 10(12):264. https://doi.org/10.1007/s12517-017-3031-z
Todd D (1980) Ground water hydrology, 2nd edn. Wiley, New York, p 535
USSL (US Salinity Laboratory) (1954) Diagnosis and improvement of saline and alkali soils. US Department of Agriculture Hand Book, No. 60
Viglietti D, Letey S, Motta R, Maggioni M, Freppaz M (2010) Snow avalanche release in forest ecosystems: a case study in the Aosta Valley Region (NW-Italy). Cold Reg Sci Technol 64(2):167–173. https://doi.org/10.1016/j.coldregions.2010.08.007
Voutsis N, Kelepertzis E, Tziritis E, Kelepertsis A (2015) Assessing the hydrogeochemistry of groundwaters in ophiolite areas of Euboea Island, Greece, using multivariate statistical methods. J Geochem Explor 159:79–92. https://doi.org/10.1016/j.gexplo.2015.08.007
Wang Y, Wang P, Bai Y, Tian Z, Li J, Shao X, Mustavich LF, Li BL (2013) Assessment of surface water quality via multivariate statistical techniques: a case study of the Songhua river Harbin region, China. J Hydroenviron Res 7(1):30–40. https://doi.org/10.1016/j.jher.2012.10.003
WHO (2006) Guidelines for drinking-water quality, vol 1, recommendations, 3rd edn. World Health Organization, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. US Department of Agriculture, Circular 969, Washington, DC, USA
Zhang F, Jin Z, Yu J, Zhou Y, Zhou L (2015) Hydrogeochemical processes between surface and groundwaters on the northeastern Chinese Loess Plateau: implications for water chemistry and environmental evolutions in semi-arid regions. J Geochem Explor 159:115–128. https://doi.org/10.1016/j.gexplo.2015.08.010
Zhou F, Liu Y, Guo H (2007) Application of multivariate statistical methods to water quality assessment of the watercourses in Northwestern New Territories, Hong Kong. Environ Monit Assess 132(1):1–13. https://doi.org/10.1007/s10661-006-9497-x
Zhu C, Schwartz FW (2011) Hydrogeochemical processes and controls on water quality and water management. Elements 7(3):169–174. https://doi.org/10.2113/gselements.7.3.169
Zuzolo D, Cicchella D, Catani V, Giaccio L, Guagliardi I, Esposito L, De Vivo B (2017) Assessment of potentially harmful elements pollution in the Calore river basin (Southern Italy). Environ Geochem Health 39(3):531–548. https://doi.org/10.1007/s10653-016-9832-2
Acknowledgements
The data were collected as part of a project drawn up between Politecnico di Torino and the Aosta Valley regional authority. The authors are grateful to the Department of Environment and Territory and the Department of Public work, Soil conservation and Public housing of the Aosta Valley regional authority for their support and assistance during the field survey and for allowing publication of the data. We thank the editors and anonymous reviewers for their valuable suggestions in improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pisciotta, A., Tiwari, A.K. & De Maio, M. An integrated multivariate statistical analysis and hydrogeochemical approaches to identify the major factors governing the chemistry of water resources in a mountain region of northwest Italy. Carbonates Evaporites 34, 955–973 (2019). https://doi.org/10.1007/s13146-018-0452-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13146-018-0452-z