Abstract
Recent studies suggest that Rab11-family interacting proteins (Rab11-FIPs) play an important role in tumorigenesis and progression. Among the Rab11-FIPs, Rab11-FIP4 has been reported to be significantly upregulated in various cancers, including hepatocellular carcinoma (HCC). However, the possible effect on HCC stemness and the underlying mechanism has never been characterized. Here, we found that Rab11-FIP4 was dramatically increased in HCC cell lines and tissues, and had a positive correlation with cancer stemness. Functional studies revealed that elevated expression of Rab11-FIP4 in HCC cells significantly promoted sphere formation, and enhanced the mRNA and protein levels of stemness-associated markers, ALDH1A1, CD133, NANOG, and OCT4. Conversely, the knockdown of Rab11-FIP4 suppressed the cancer stem cell (CSC)-like characteristics of HCC cells. Moreover, silencing of Rab11-FIP4 obviously increased the sensitivity of HCC cells to sorafenib. Mechanistically, Rab11-FIP4 was shown to interact with ADP-ribosylation factor 5 (ARF5) to influence cell cycle-related proteins, CDK1/cyclin B, thereby promoting HCC stemness. Taken together, our results uncovered an essential role for Rab11-FIP4 in regulating CSC-like features of HCC cells and identified Rab11-FIP4 as a potential target for HCC therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most malignant cancer in the digestive system with high incidence and mortality rates [1]. Despite the great advances in diagnosis and therapeutic modalities, the 5-year survival rate of HCC remains less than 10% [30]. In recent years, some emerging inhibitors (such as sorafenib and lenvatinib) have been widely implicated in advanced or metastatic HCC, but the efficacy is extremely limited [2, 9]. Therefore, it is crucial to explore novel targets for treating HCC and elucidate the underlying molecular mechanisms.
HCC is regarded as a stem cell disease because it contains a specific population of cancer stem cells (CSCs) [28]. CSCs are associated with self-renewal, proliferation, differentiation, drug resistance, relapse, and metastasis of tumors [10, 29]. CSCs in HCC can be distinguished by expressing several biomarkers, such as NANOG, SOX2, KLF4, and OCT4 [3, 4, 14]. In recent years, despite numerous studies investigating the role of CSCs in HCC, therapeutic approaches for CSC eradication have not been established. Considering that CSCs have a crucial function in HCC occurrence and progression, clarifying the characteristics and regulatory mechanisms of CSCs may help identify novel targets for therapeutic intervention in HCC.
Rab11-family interacting protein 4 (Rab11-FIP4) is localized to the endosomal recycling compartment and involved in vesicle trafficking mediated cytokinesis [8]. Previous studies have reported that Rab11-FIP4 plays a key role in the retinal development of mice and zebrafish, which can regulate the retinal progenitor cell proliferation and differentiation [23, 24]. More recently, Rab11-FIP4 has been identified as an oncogene in various tumors. It has been reported that depletion of Rab11-FIP4 in pancreatic cancer cells represses cell growth, metastasis, and arrests the cell cycle [11]. Aberrant Rab11-FIP4 expression was negatively correlated with the clinical outcomes in pancreatic cancer patients [11]. Rab11-FIP4 was also reported to promote tumor progression in colorectal cancer by increasing the phosphorylation of ERK1/2 and AKT [31]. Additionally, Rab11-FIP4 has been demonstrated to play a role in facilitating tumor metastasis by modulating the phosphorylated PRAS40 in HCC [12]. However, whether Rab11-FIP4 affects the stemness of HCC and its underlying mechanism remains unclear.
In this study, we investigated the biological function of Rab11-FIP4 in modulating the stemness of HCC cells. Increased expression of Rab11-FIP4 in HCC cells could enhance the CSC-like phenotype and sorafenib resistance. Mechanistic studies identified ADP-ribosylation factor 5 (ARF5) had an interaction with Rab11-FIP4. This interaction leads to the alteration of the CDK1/cyclin B complex. These data suggest that Rab11-FIP4 may be a novel target for HCC treatment.
Materials and methods
Cell culture
Human HCC cell lines (HepG2, HCCLM3, Huh7, and Hep3B) and normal hepatocytes (LO2) were purchased from Fenghui Biotechnology Co. Ltd. (Hunan, China). HepG2, HCCLM3, and Huh7 cells were cultured in DMEM (Biological Industries, Beit HaEmek, Israel), Hep3B cells were cultured in MEM (Biological Industries) with 1% non-essential amino acids (Gibco, USA), and LO2 cells were cultured in RPMI-1640 medium (Biological Industries). All media were supplemented with 10% FBS (Gibco, USA) and 1% penicillin-streptomycin (Sangon Biotech Co., Ltd., Shanghai, China). All cells were maintained in an incubator with 5% CO2 at 37 °C.
Plasmids and small interfering RNA (siRNA) transfection
For plasmid transfection, human pCDH-Rab11-FIP4, pCDH-ARF5, and pCDH-vector expression plasmids were purchased from WZ Biosciences, Inc. (Shandong, China). Transfection was performed using Lipofectamine 2000 (Thermo Fisher, Waltham, MA, USA) according to the manufacturer’s instructions. For siRNA transfection, siRNAs were synthesized by RiboBio (Guangzhou, China). The sequences of the siRNAs used were as follows: Rab11-FIP4, 5′-CUCAAGCAGGAGAAUUAUAAG-3′ (#1) and 5′-ACGACUUGAATGGGCAGAUUU-3′ (#2); ARF5, 5′-GAUGCAGUGCUGCUGGUAUUU-3′ (#1) and 5′-GUCCAAGAAUCUGCUGAUGAA-3′ (#2). The transfection procedure was conducted using jetPRIME reagent (Polyplus, NY, USA) according to the manufacturer’s instructions. After transfection for 48 h, cells were collected for further analysis.
RT-qPCR
Total RNA was prepared and detected as previously described [27]. The primers used for RT-qPCR were listed in Supplementary Table S1. The results were normalized to that of human GAPDH.
Western blot analysis
Western blot analysis was performed as described previously [27]. Cells were washed twice with cold phosphate-buffered saline (PBS), and added the RIPA lysis buffer with protease inhibitors (Beyotime, Shanghai, China). After cells lysed for 30 min on ice, samples were centrifuged at 12,000 rpm for 15 min at 4 °C. Supernatants were collected and protein concentration was measured using Bradford’s method. Equal amounts of protein lysates (20 μg/lane) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes (PVDF) (Merck Millipore, St. Louis, MO, USA). The primary and secondary antibodies used in Western blotting are listed in Table S2. Quantification of band intensities was performed using ImageJ software (Wayne Rasband, USA).
CCK-8 assay
CCK-8 (FUDE Biological Technology Co., Ltd, Hangzhou, China) was used to evaluate cell viability. Cells were cultured in a 96-well plate at 5 × 103 cells/well. Then, various concentrations of sorafenib (0, 0.5, 1.0, 2.5, 5.0, 7.5 μg/mL) (Selleck, China) were used. After 24 h for treatment, 10 μL of CCK-8 reagent was added to each well and incubated for 1 h. Absorbance was measured at 450 nm using a microplate reader. All experiments were performed in triplicate.
Sphere formation assay
HCCLM3, Huh7, and Hep3B cells were transfected with the indicated plasmids or siRNAs for 48 h. Thereafter, cells (2 × 103 cells/well) were plated in ultralow attachment six-well plates (Corning, New York, NY, USA) and grown in DMEM/F12 medium containing 10% B27, 10 ng/mL basic fibroblast growth factor (bFGF), and 20 ng/mL epidermal growth factor (EGF). The cells were cultured for seven days and photographed using an CKX53-inverted fluorescence microscope.
Immunoprecipitation (IP) assay
To measure the interaction between Rab11-FIP4 and ARF5, Hep3B cells were cultured in a 10-cm dish before transfection. After cells transfected with pCDH-ARF5-his plasmid for 48 h, cells were harvested and immunoprecipitated with an anti-His-tag antibody (1:400, cat no. 66005-1-Ig; Proteintech, Wuhan, China). IP assay was performed according to standard protocols. Firstly, cells were washed with cold PBS and added the IP lysis buffer containing protease inhibitors. After cells lysed for 30 min on ice, samples were collected and centrifuged at 12,000 rpm for 15 min at 4 °C. Then, the precipitates were discarded, and the supernatants were incubated with normal IgG or anti-His-tag antibodies and protein A/G magnetic beads (BioLegend, San Diego, CA, USA) overnight at 4 °C. The next day, the precipitated complexes were washed with lysis buffer five times at RT. Finally, protein samples were eluted with SDS lysis buffer and analyzed by Western blot.
Immunofluorescence (IF)
To detect the co-expression of Rab11-FIP4 and ARF5, Hep3B cells were cultured in a 15-mm glass-bottom confocal dish before transfection. After cells transfected with indicated plasmids for 48 h, cells were washed with PBS and fixed with ice-cold methanol for 10 min. Then, cells were blocked with 10% bovine serum albumin containing 0.1% Triton X-100 for 1 h at RT, and incubated with primary antibodies against His (1:800) and Rab11-FIP4 (1:500) at 4 °C overnight. The cells were then incubated in the dark with iFluor 488- or 594-conjugated secondary antibody (1:250; HUABIO, Woburn, MA, USA) for 1 h. DAPI (Beyotime, Shanghai, China) was used to stain the nuclei for 5 min before visualization under a TCS SP8 confocal microscope (Leica, Germany).
Immunohistochemical (IHC) analysis
HCC and non-tumorous paraffin sections were obtained from Zhejiang Provincial People’s Hospital (Hangzhou, China). All patients included in this study provided signed consent, and the study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital. IHC analysis was performed as previously described [26]. The anti-Rab11-FIP4 and anti-ARF5 antibody was used at a 1:50 and 1:100 dilution, respectively.
Cell cycle analysis
Huh7 cells transfected with the indicated plasmids or siRNAs were collected and washed with PBS. Then, the cells were resuspended in a staining buffer with 10 μg/mL propidium iodide (PI; Sigma, USA) and 25 μg/mL RNase. After staining, the cell cycle was analyzed by the Cytoflex flow cytometer (Beckman-Coulter, USA).
Bioinformatics analysis
The RNA-sequencing dataset of patients with HCC was obtained from The Cancer Genome Atlas (TCGA; https://tcga-data.nci.nih.gov/tcga/). The correlation between Rab11-FIP4 and stemness was analyzed by Pearson statistics. Survival analysis based on gene expression was conducted using the Kaplan-Meier plotter (https://kmplot.com/analysis/). Protein-protein interaction (PPI) was analyzed using String database (https://cn.string-db.org/), and data format was performed using R software (version v4.0.3, the R Project for Statistical Computing, 2020; Vienna, Austria). A P < 0.05 was considered statistically significant.
Statistical analysis
All data are expressed as the mean ± SD unless otherwise specified. Statistical comparisons between two groups or multiple groups were determined using the two-tailed Student’s t-test and one-way analysis of variance (ANOVA), with the results indicated as ***p < 0.001, **p < 0.01, *p < 0.05, and not significant (n.s.). GraphPad Prism 8 (GraphPad, Inc., La Jolla, CA, USA) was used for analysis.
Results
Rab11-FIP4 is upregulated in HCC and highly correlated with HCC stemness
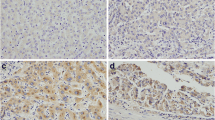
As previously reported, Rab11-FIP4 was significantly upregulated in HCC tissues, and patients with high Rab11-FIP4 expression displayed a worse prognosis [12]. To investigate the alteration of Rab11-FIP4 in HCC, we first conducted TCGA data analysis and found that Rab11-FIP4 expression was markedly increased in HCC tissues (Fig. 1A). Then, the prognostic significance of Rab11-FIP4 in HCC was performed by Kaplan-Meier plotter, and data showed that patients with high Rab11-FIP4 expression had shorter relapse-free survival (RFS) and progression-free survival (PFS), but no effect on overall survival (OS) (Fig. 1B). Additionally, we determined the Rab11-FIP4 expression in normal hepatocytes and four HCC cell lines. The results displayed that Rab11-FIP4 was obviously increased in HepG2, HCCLM3, Huh7, and Hep3B HCC cells than in LO2 normal hepatocytes (Fig. 1C). Immunohistochemical results also revealed a higher level of Rab11-FIP4 in HCC tissues than that in non-cancerous tissues (Fig. 1D). To further investigate whether Rab11-FIP4 influence HCC stemness, we compared the stemness index of HCC and normal tissues based on TCGA database and found that HCC is a high-stemness tumor (Fig. 1E). The correlation analysis demonstrated that Rab11-FIP4 expression was highly correlated with HCC stemness (Fig. 1F). Therefore, our results indicated that high Rab11-FIP4 expression is closely associated with HCC stemness.
Expression of Rab11-FIP4 in HCC and its correlation with HCC stemness. A mRNA expression of Rab11-FIP4 in HCC and normal tissues based on TCGA database. B Survival analysis of HCC patients with high and low Rab11-FIP4 expression based on Kaplan-Meier Plotter. C mRNA and protein expression of Rab11-FIP4 in normal and HCC cell lines. D Expressions of Rab11-FIP4 in HCC and adjacent non-tumorous tissue samples detected using IHC. E Stemness index analysis of HCC based on TCGA database. F Correlation analysis of Rab11-FIP4 expression and stemness index. Data are shown as mean ± SD (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar, 250 μm
Rab11-FIP4 facilitates the stemness of HCC cells
To further assess the role of Rab11-FIP4 in HCC stemness, we overexpressed or knocked down Rab11-FIP4 in the HepG2, Huh7, and Hep3B HCC cell lines. Compared to normal controls, HCC cells with Rab11-FIP4 overexpression had higher sphere formation capacity. In contrast, the depletion of Rab11-FIP4 greatly decreased the self-renewal ability of HCC cells (Fig. 2A, B). Furthermore, we measured the stemness markers, such as CD133, ALDH1A1, OCT4, and NANOG, in different groups at both the transcriptional and translational levels. As expected, overexpression of Rab11-FIP4 dramatically upregulated the expression of these markers but were drastically downregulated in the Rab11-FIP4 depletion group (Fig. 2C, D). These data suggest that Rab11-FIP4 is essential for the maintenance of stemness of HCC cells.
Effect of Rab11-FIP4 on the maintenance of stem cell-like properties in HCC cells. A Transfection efficiency was evaluated using qRT-PCR and western blotting 48 h post-transfection. B Left, photomicrographs depict the sphere. C, D Rab11-FIP4 influences the transcriptional and translational levels of HCC stem cell-related markers, including CD133, ALDH1A1, OCT4, and NANOG. Data are shown as mean ± SD (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar, 250 μm
Rab11-FIP4 enhances resistance to sorafenib therapy in HCC cells
CSCs is commonly regarded as one important factor of the therapeutic failure in clinic, which is mainly due to its highly resistance to drugs by aberrant changes of several ATP-binding cassette (ABC) transporters [7]. To evaluate whether the alterations of Rab11-FIP4 have an effect on drug therapy, we performed a cell proliferation assay to determine the IC50 values of HCC cells treated with sorafenib. As shown in Fig. 3A, the ablation of Rab11-FIP4 obviously improved the sensitivity to sorafenib in all three HCC cell lines. Considering the role of ABC drug transporters in drug resistance, we sought to elucidate whether Rab11-FIP4 induced sorafenib resistance by regulating ABC transporter. We determined the expression of ABCB1 and ABCC1 in HCC cells with high or low Rab11-FIP4 expression at the mRNA level. Surprisingly, the expression of both ABCB1 and ABCC1 was not changed in the Rab11-FIP4 overexpression or depletion group, indicating that neither of them participate in Rab11-FIP4 mediated HCC stemness (Fig. 3B, C). Rab11-FIP4 may regulate HCC stemness and enhance sorafenib resistance by a specific molecular mechanism.
Rab11-FIP4 inhibits the sensitivity of HCC cells to sorafenib. A Knockdown of Rab11-FIP4 in HCC cells was cultured with the indicated concentrations of sorafenib. B, C Overexpression or knockdown of Rab11-FIP4 had no effect on the transcriptional levels of transporter genes (ABCB1 and ABCC1) in HCC cells. Data are shown as mean ± SD (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001
Rab11-FIP4 interacts with ARF5 to influence cancer stemness and sorafenib sensitivity in HCC cells
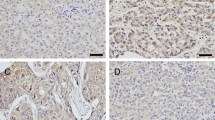
It has been reported that Rab11-FIP4 and other FIPs have a common Rab11 binding domain at the C-terminal, which participates in vesicle trafficking by selectively interacting with Rab11 [32]. However, several studies demonstrated that Rab11-FIP4 influences cell proliferation and differentiation in a Rab11-independent manner [23, 24]. To elucidate the regulatory mechanism of Rab11-FIP4-mediated HCC stemness, we used the String database to analyze the protein-protein interaction (PPI) network of Rab11-FIP4. The PPI data showed that seven proteins could directly interact with Rab11-FIP4, including ARF5, DYTN, EXOC7, MYO1D, Rab11a, Rab11-FIP3, and Rab11-FIP2 (Fig. 4A). Further survival analysis revealed that only ARF5 displayed a similar association to prognosis in HCC patients as Rab11-FIP4; these patients exhibited a shorter PFS and RFS with high expression of ARF5 (Fig. 4B and Supplementary Fig. S1). Therefore, we speculate that Rab11-FIP4 may interact with ARF5 to modulate HCC stemness and sorafenib sensitivity. To test this hypothesis, co-IP and IF assays were performed using Hep3B cells transfected with ARF5-his or vector plasmid. As shown in Fig. 4C and D, Rab11-FIP4 formed a complex with ARF5. Moreover, IHC results showed an obviously increased expression of ARF5 in HCC tissues as compared to non-tumorous tissues (Fig. 4E). As expected, ARF5 overexpression or knockdown also influences mRNA and protein expression of Rab11-FIP4 (Fig. 4F).
ARF5 interacts with Rab11-FIP4 in HCC cells. A PPI analysis based on the String database. B Survival analysis of HCC patients with high and low ARF5 expression based on Kaplan-Meier plotter. C, D The interaction between Rab11-FIP4 and ARF5 was detected using IP and IF assays. E Expression of ARF5 in HCC and adjacent non-tumorous tissue samples detected using IHC. F Transfection efficiency and Rab11-FIP4 expression were evaluated using qRT-PCR and Western blotting. Data are shown as mean ± SD (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar, 250 μm
To further investigate whether stemness and sorafenib sensitivity were affected by ARF5 in HCC cells with increased Rab11-FIP4 expression, stemness-related gene expression, sphere formation, and sorafenib sensitivity assays were conducted. qRT-PCR and Western blot results revealed that HCC cells with high level of ARF5 markedly increased the transcriptional and translational levels of stemness markers, such as CD133, ALDH1A1, OCT4, and NANOG, but did not influence the expression of ABCB1 and ABCC1 (Fig. 5A-C). Moreover, ARF5 overexpression greatly increased the number and size of HCC spheres than in controls (Fig. 5D). Simultaneously, depletion of ARF5 dramatically decreased the stemness of HCC cells and improved their sensitivity to sorafenib (Fig. 6). Overall, these results indicate that Rab11-FIP4 promotes stemness of HCC cells, which is mediated by ARF5.
Overexpression of ARF5 promotes stem cell-like properties in HCC cells. A, B ARF5 increases transcriptional levels of HCC stem cell-related markers (CD133, ALDH1A1, OCT4, NANOG) and does not affect efflux transporters (ABCB1, ABCC1). C ARF5 increases translational levels of HCC stem cell-related markers. D Left, photomicrographs depict the sphere. Data are shown as mean ± SD (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar, 250 μm
Knockdown of ARF5 suppressed stem cell-like properties in HCC cells. A, B ARF5 decreases transcriptional levels of HCC stem cell-related markers (CD133, ALDH1A1, OCT4, NANOG) and does not affect efflux transporters (ABCB1, ABCC1). C ARF5 reduces translational levels of HCC stem cell-related markers. D Left, photomicrographs depict the sphere. E Knockdown of ARF5 in HCC cells was cultured with the indicated concentrations of sorafenib. Data are shown as mean ± SD (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001. Scale bar, 250 μm
Rab11-FIP4 interacts with ARF5 to influence HCC stemness by regulating CDK1/cyclin B
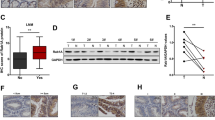
To understand the mechanism of Rab11-FIP4-induced HCC stemness, we performed the gene set enrichment analysis (GSEA) by defining the high and low Rab11-FIP4 expression groups. GSEA GO analysis showed that cell cycle-related processes were enriched in the high Rab11-FIP4 expression group (Fig. 7A). Moreover, we found that the cell cycle, oocyte meiosis, and p53 signaling pathways were positively correlated with Rab11-FIP4 high expression based on the KEGG analysis (Fig. 7B), indicating that Rab11-FIP4 may influence HCC stemness by regulating the cell cycle pathway. To test this hypothesis, the protein expression of enriched genes that emerged in the three pathways was detected, including CDK1, CCNB1, and CCNB2 (Fig. 7C). As expected, Western blot analysis showed that the expression of CDK1, CCNB1, and CCNB2 was markedly decreased in HCC cells transfected with Rab11-FIP4 or ARF5 siRNAs (Fig. 7D). Since Rab11-FIP4 is involved in cytokinesis, we next monitored whether Rab11-FIP4 or ARF5 depletion influences the cell cycle. Consistent with KEGG analysis, overexpression of Rab11-FIP4 or ARF5 arrested HCC cells in the G0/G1 phase, whereas depletion of Rab11-FIP4 or ARF5 reversed cell cycle arrest (Fig. 7E). Taken together, these findings indicated that cell cycle arrest is involved in Rab11-FIP4-induced HCC stemness.
Cell cycle is involved in Rab11-FIP4-induced HCC stemness. A GSEA was used to perform the GO annotation of biological process enrichment grouped by Rab11-FIP4 expression level in TCGA database. B GSEA was used to perform the KEGG pathway enrichment grouped by Rab11-FIP4 expression level in TCGA database. C Venn diagram of the enriched genes in the KEGG pathway. D Rab11-FIP4/ARF5 influences the protein levels of cell cycle-related genes, including CDK1, cyclin B1, and cyclin B2. E Knockdown or overexpression of Rab11-FIP4 or ARF5 in Huh7 cells was stained using PI, followed by flow cytometry to detect cell cycle distribution. Data are shown as mean ± SD (n = 3), *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
Recurrence, metastasis, and drug resistance are the leading causes of high mortality rate in various tumors, including HCC, which occurs in approximately 70% of all cases [18]. Although many tyrosine kinase inhibitors (TKIs) are implicated in advanced or metastatic HCC, the survival rate of these patients remains low, with an average 5-year OS less than 10% [34]. CSCs are a specific cell population that has a strong ability for tumor cell self-renewal, proliferation, and differentiation. To date, liver CSCs are considered to be one of the main contributors to the initiation and malignant progression of HCC [17]. Thus, to deal with HCC, intervention might be required in order to block the intrinsic characteristics of liver CSCs or directly eliminate the liver CSCs. Rab11-FIP4 has been demonstrated that play a role in regulating the proliferation and differentiation of retinal progenitor cells [24]. Moreover, it is closely related to the progression and worse prognosis of various tumors, such as pancreatic cancer, colorectal cancer, and HCC [11, 12, 31]. Rab11-FIP4 could promote the progression of tumors through several mechanisms, including influencing the metastasis, apoptosis, and cell cycle of tumor cells [11]. Nevertheless, its role in modulating the stemness of HCC and the underlying mechanism have never been clarified. In this study, we found that Rab11-FIP4, an oncogene, interacts with ARF5, which facilitates to CSC-like properties and sorafenib resistance in HCC by regulating CDK1/cyclin B. The above data highlight Rab11-FIP4 may as a novel target for HCC therapy.
Rab11-FIP4, a member of class II Rab11-FIPs, plays a key role in vesicle trafficking associated with cytokinesis regulation and human cytomegalovirus production [8, 15, 20]. However, no extensive evidence has been focused on its biological function in tumor initiation and progression until now. One study reported that the protein level of Rab11-FIP4 was greatly elevated in HCC tissues, which was closely correlated with the lower OS and disease-free survival in patients with HCC [12]. Fortunately, we also found that Rab11-FIP4 was significantly upregulated in HCC cells and tumor tissues. However, based on the survival analysis, we found that high Rab11-FIP4 expression had no effect on OS and only influenced RFS and PFS. Liver CSCs possess the abilities to self-renew and differentiate by activating several signaling pathways associated with proliferation, such as Wnt, Hedgehog, and Notch [13]. One important characteristic of liver CSCs is to induce the drug resistance of tumor cells, which is a main cause for the relapse and metastasis of HCC [19]. CD133, OCT4, NANOG, and ALDH1A1 serve as stem cell markers in liver tumors and participate in the growth, progression, and drug resistance of HCC [35]. Considering the significance of CSCs in HCC progression, it is necessary to seek novel targets regulate HCC stemness and understand the underlying molecular mechanism. In this study, we demonstrated that silencing of Rab11-FIP4 dramatically suppressed the sphere formation in HCC cells. Further analysis showed that all universal CSC markers (CD133, OCT4, NANOG, and ALDH1A1) were upregulated in HCC cells overexpressing Rab11-FIP4 but markedly downregulated in cells with Rab11-FIP4 knockdown. Moreover, Rab11-FIP4 knockdown greatly enhanced the response of HCC cells to sorafenib. Taken together, our data suggest that Rab11-FIP4 is essential for maintaining the CSC-like characteristics of HCC cells.
To identify the downstream protein of Rab11-FIP4 that contributes to HCC stemness, we performed a PPI analysis to screen for potential molecules. The data showed that seven proteins (ARF5, DYTN, EXOC7, MYO1D, RAB11A, RAB11FIP3, and RAB11FIP2) may directly interact with Rab11-FIP4. Further analysis revealed that only ARF5 exerted a similar effect on the prognosis of HCC patients as did Rab11-FIP4, indicating that Rab11-FIP4 interacts with ARF5 to promote HCC stemness. Subsequently, we found that ARF5 had an interaction with Rab11-FIP4, and overexpression of ARF5 induced Rab11-FIP4 at both the mRNA and protein levels. ARF5 is a member of the ADP-ribosylation factor (ARFs) family of small GTPases responsible for vesicular transport [6]. To date, little is known about the biological functions of ARF5. It has been reported that activation of ARF5 promotes the internalization of clathrin-mediated integrin endocytosis [22]. A recent study found that ARF5 is essential for focal adhesion turnover, an important process for cell migration [5]. Simultaneously, a study demonstrated that ARF5 activates Rab35 to upregulate the transcription factor SPOCD1, thereby enhancing tumor growth and invasiveness of glioblastoma [16]. We observed that the protein level of ARF5 was increased in HCC tissues. Moreover, sphere formation, and stemness-related gene expression were significantly enhanced in HCC cells overexpressing ARF5 but inhibited in HCC cells with ARF5 depletion. Additionally, ARF5 knockdown also significantly increased the sensitivity of HCC cells to sorafenib. These findings indicate that ARF5 interacts with Rab11-FIP4 to promote HCC stemness.
The mechanism by which the Rab11-FIP4/ARF5 axis regulates stemness in HCC cells appears complicated, but potential results are mainly based on biological function. Our preliminary GSEA analysis revealed that the cell cycle process was positively correlated with high Rab11-FIP4 expression. Moreover, we identified three genes associated with the cell cycle: CDK1, cyclin B1, and cyclin B2. Using HCC cell models with Rab11-FIP4 or ARF5 knockdown, we showed that Rab11-FIP4 and ARF5 affect the translational levels of CDK1, cyclin B1, and cyclin B2. At the same time, insufficient Rab11-FIP4 or ARF5 expression arrested cell cycle progression. A report suggested that CDK1 could promote the initiation of melanoma by directly interacting with SOX2 (a CSC marker), whereas its blockade was responsible for inhibiting the phosphorylation, nuclear localization, and transcription of SOX2 [25]. Moreover, a recent study found that CDK1 is frequently augmented in HCC tissues and is an important prognostic factor for HCC patients. Blocking CDK1 may boost the antitumor response of sorafenib in vivo, which exerts its inhibitory effects on HCC stemness by influencing the CDK1/PDK1/β-Catenin signaling pathway [33]. Additionally, one study showed that embryonic stem cells display particularly high CDK1 activity [21]. These findings further suggest that cell cycle-associated proteins, such as CDK1 or cyclin B, are implicated in Rab11-FIP4/ARF5-mediated HCC stemness.
Conclusion
In summary, our results provide evidence that Rab11-FIP4 is pivotal for the maintenance of CSC-like features of HCC cells, like promoting the sphere formation, and enhancing the expression of stemness-associated markers. In addition, Rab11-FIP4 could increase the resistance of HCC cells to sorafenib, which is reflected by the silencing of Rab11-FIP4 in HCC cells with a smaller IC50 value. Mechanistically, Rab11-FIP4 might have an interaction with ARF5, which might lead to the upregulation of CDK1/cyclin B, thereby promoting HCC stemness. Taken together, our results advance the understanding of Rab11-FIP4 in tumor initiation and progression, and suggest novel targets for HCC therapy.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- NANOG:
-
Nanog homeobox
- SOX2:
-
SRY-Box transcription factor 2
- KLF4:
-
KLF transcription factor 4
- OCT4:
-
POU domain, class 5, transcription factor 1
- ERK1:
-
Mitogen-activated protein kinase 3
- ERK2:
-
Mitogen-activated protein kinase 1
- AKT:
-
AKT serine/threonine kinase
- PRAS40:
-
AKT1 substrate 1
- CDK1:
-
Cyclin-dependent kinase 1
- DMEM:
-
Dulbecco’s modified Eagle medium
- MEM:
-
Minimum essential medium
- RPMI-1640:
-
Roswell Park Memorial Institute 1640
- FBS:
-
Fetal bovine serum
- RT-qPCR:
-
Quantitative reverse transcription-polymerase chain reaction
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- RIPA:
-
Radio immunoprecipitation assay
- CCK-8:
-
Cell counting kit-8
- DMEM/F12:
-
Dulbecco’s modified Eagle media: nutrient mixture F-12
- IgG:
-
Immunoglobulin G
- RT:
-
Room temperature
- SD:
-
Standard deviation
- CD133:
-
Prominin 1
- ALDH1A1:
-
Aldehyde dehydrogenase 1 family member A1
- DYTN:
-
Dystrotelin
- EXOC7:
-
Exocyst Complex Component 7
- MYO1D:
-
Myosin ID
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- CDK1:
-
Cyclin-dependent kinase 1
- CCNB1:
-
Cyclin B1
- CCNB1:
-
Cyclin B2
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, Ikeda M, Lim HY, Ho GF, Choo SP, Ren Z, Malhotra H, Ueno M, Ryoo BY, Kiang TC, Tai D, Vogel A, Cervantes A, Lu SN et al (2020) Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol 31(3):334–351. https://doi.org/10.1016/j.annonc.2019.12.001
Chiba T, Iwama A, Yokosuka O (2016) Cancer stem cells in hepatocellular carcinoma: therapeutic implications based on stem cell biology. Hepatol Res 46(1):50–57. https://doi.org/10.1111/hepr.12548
Chiba T, Kamiya A, Yokosuka O, Iwama A (2009) Cancer stem cells in hepatocellular carcinoma: recent progress and perspective. Cancer Lett 286(2):145–153. https://doi.org/10.1016/j.canlet.2009.04.027
D'Souza RS, Lim JY, Turgut A, Servage K, Zhang J, Orth K, Sosale NG, Lazzara MJ, Allegood J, Casanova JE (2020) Calcium-stimulated disassembly of focal adhesions mediated by an ORP3/IQSec1 complex. Elife 9:e54113. https://doi.org/10.7554/eLife.54113
D'Souza-Schorey C, Chavrier P (2006) ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7(5):347–358. https://doi.org/10.1038/nrm1910
Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5(4):275–284. https://doi.org/10.1038/nrc1590
Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW (2005) Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J 24(19):3389–3399. https://doi.org/10.1038/sj.emboj.7600803
Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A et al (2020) Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol 38(36):4317–4345. https://doi.org/10.1200/JCO.20.02672
Gupta PB, Chaffer CL, Weinberg RA (2009) Cancer stem cells: mirage or reality? Nat Med 15(9):1010–1012. https://doi.org/10.1038/nm0909-1010
He Y, Ye M, Zhou L, Shan Y, Lu G, Zhou Y, Zhong J, Zheng J, Xue Z, Cai Z (2017) High Rab11-FIP4 expression predicts poor prognosis and exhibits tumor promotion in pancreatic cancer. Int J Oncol 50(2):396–404. https://doi.org/10.3892/ijo.2016.3828
Hu F, Deng X, Yang X, Jin H, Gu D, Lv X, Wang C, Zhang Y, Huo X, Shen Q, Luo Q, Zhao F, Ge T, Zhao F, Chu W, Shu H, Yao M, Fan J, Qin W (2015) Hypoxia upregulates Rab11-family interacting protein 4 through HIF-1alpha to promote the metastasis of hepatocellular carcinoma. Oncogene 34(49):6007–6017. https://doi.org/10.1038/onc.2015.49
Jeng KS, Chang CF, Sheen IS, Jeng CJ, Wang CH (2023) Cellular and molecular biology of cancer stem cells of hepatocellular carcinoma. Int J Mol Sci 24(2):1417. https://doi.org/10.3390/ijms24021417
Kim HJ, Jeong J, Park S, Jin YW, Lee SS, Lee SB, Choi D (2017) Establishment of hepatocellular cancer induced pluripotent stem cells using a reprogramming technique. Gut Liver 11(2):261–269. https://doi.org/10.5009/gnl15389
Krzyzaniak MA, Mach M, Britt WJ (2009) HCMV-encoded glycoprotein M (UL100) interacts with Rab11 effector protein FIP4. Traffic 10(10):1439–1457. https://doi.org/10.1111/j.1600-0854.2009.00967.x
Kulasekaran G, Chaineau M, Piscopo VEC, Verginelli F, Fotouhi M, Girard M, Tang Y, Dali R, Lo R, Stifani S, McPherson PS (2021) An Arf/Rab cascade controls the growth and invasiveness of glioblastoma. J Cell Biol 220(2):e202004229. https://doi.org/10.1083/jcb.202004229
Lee TK, Guan XY, Ma S (2022) Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications. Nat Rev Gastroenterol Hepatol 19(1):26–44. https://doi.org/10.1038/s41575-021-00508-3
Li XY, Shen Y, Zhang L, Guo X, Wu J (2022) Understanding initiation and progression of hepatocellular carcinoma through single cell sequencing. Biochim Biophys Acta Rev Cancer 1877(3):188720. https://doi.org/10.1016/j.bbcan.2022.188720
Man KF, Ma S (2022) Mechanisms of resistance to tyrosine kinase inhibitors in liver cancer stem cells and potential therapeutic approaches. Essays Biochem 66(4):371–386. https://doi.org/10.1042/EBC20220001
Meyers JM, Prekeris R (2002) Formation of mutually exclusive Rab11 complexes with members of the family of Rab11-interacting proteins regulates Rab11 endocytic targeting and function. J Biol Chem 277(50):49003–49010. https://doi.org/10.1074/jbc.M205728200
Michowski W, Chick JM, Chu C, Kolodziejczyk A, Wang Y, Suski JM, Abraham B, Anders L, Day D, Dunkl LM, Li Cheong Man M, Zhang T, Laphanuwat P, Bacon NA, Liu L, Fassl A, Sharma S, Otto T, Jecrois E et al (2020) Cdk1 controls global epigenetic landscape in embryonic stem cells. Mol Cell 78(3):459–476 e413. https://doi.org/10.1016/j.molcel.2020.03.010
Moravec R, Conger KK, D’Souza R, Allison AB, Casanova JE (2012) BRAG2/GEP100/IQSec1 interacts with clathrin and regulates alpha5beta1 integrin endocytosis through activation of ADP ribosylation factor 5 (Arf5). J Biol Chem 287(37):31138–31147. https://doi.org/10.1074/jbc.M112.383117
Muto A, Aoki Y, Watanabe S (2007) Mouse Rab11-FIP4 regulates proliferation and differentiation of retinal progenitors in a Rab11-independent manner. Dev Dyn 236(1):214–225. https://doi.org/10.1002/dvdy.21009
Muto A, Arai K, Watanabe S (2006) Rab11-FIP4 is predominantly expressed in neural tissues and involved in proliferation as well as in differentiation during zebrafish retinal development. Dev Biol 292(1):90–102. https://doi.org/10.1016/j.ydbio.2005.12.050
Ravindran Menon D, Luo Y, Arcaroli JJ, Liu S, KrishnanKutty LN, Osborne DG, Li Y, Samson JM, Bagby S, Tan AC, Robinson WA, Messersmith WA, Fujita M (2018) CDK1 interacts with Sox2 and promotes tumor initiation in human melanoma. Cancer Res 78(23):6561–6574. https://doi.org/10.1158/0008-5472.CAN-18-0330
Song F, Zhang Y, Pan Z, Hu X, Yi Y, Zheng X, Wei H, Huang P (2021) Identification of novel key genes associated with the metastasis of prostate cancer based on bioinformatics prediction and validation. Cancer Cell Int 21(1):559. https://doi.org/10.1186/s12935-021-02258-3
Song F, Zhang Y, Pan Z, Hu X, Zhang Q, Huang F, Ye X, Huang P (2021) The role of alcohol dehydrogenase 1C in regulating inflammatory responses in ulcerative colitis. Biochem Pharmacol 192:114691. https://doi.org/10.1016/j.bcp.2021.114691
Tsui YM, Chan LK, Ng IO (2020) Cancer stemness in hepatocellular carcinoma: mechanisms and translational potential. Br J Cancer 122(10):1428–1440. https://doi.org/10.1038/s41416-020-0823-9
Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8(10):755–768. https://doi.org/10.1038/nrc2499
Wang H, Lu Z, Zhao X (2019) Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J Hematol Oncol 12(1):133. https://doi.org/10.1186/s13045-019-0806-6
Wang JZ, Yang SX, Ye F, Xia XP, Shao XX, Xia SL, Zheng B, Xu CL (2018) Hypoxia-induced Rab11-family interacting protein 4 expression promotes migration and invasion of colon cancer and correlates with poor prognosis. Mol Med Rep 17(3):3797–3806. https://doi.org/10.3892/mmr.2017.8283
Welz T, Wellbourne-Wood J, Kerkhoff E (2014) Orchestration of cell surface proteins by Rab11. Trends Cell Biol 24(7):407–415. https://doi.org/10.1016/j.tcb.2014.02.004
Wu CX, Wang XQ, Chok SH, Man K, Tsang SHY, Chan ACY, Ma KW, Xia W, Cheung TT (2018) Blocking CDK1/PDK1/beta-Catenin signaling by CDK1 inhibitor RO3306 increased the efficacy of sorafenib treatment by targeting cancer stem cells in a preclinical model of hepatocellular carcinoma. Theranostics 8(14):3737–3750. https://doi.org/10.7150/thno.25487
Xiao Y, Li Y, Shi D, Wang X, Dai S, Yang M, Kong L, Chen B, Huang X, Lin C, Liao W, Xu B, Chen X, Wang L, Chen X, Ouyang Y, Liu G, Li H, Song L (2022) MEX3C-mediated decay of SOCS3 mRNA promotes JAK2/STAT3 signaling to facilitate metastasis in hepatocellular carcinoma. Cancer Res 82(22):4191–4205. https://doi.org/10.1158/0008-5472.CAN-22-1203
Yan Q, Fang X, Li C, Lan P, Guan X (2022) Oncofetal proteins and cancer stem cells. Essays Biochem 66(4):423–433. https://doi.org/10.1042/EBC20220025
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 82003853), Zhejiang Province Natural Science Foundation of China (No. LQ20H310005 and No. LYY21H310009), Medical and Health Research Program of Zhejiang (No. 2021KY046 and No. 2022KY047), and Leading Talent of “Ten Thousand Plan”—National High-Level Talents Special Support Plan and the Science Technology Plan Project of Zhejiang Province (No. 2020R52029).
Author information
Authors and Affiliations
Contributions
P. H. and F. S. designed the study. P. H. and Y. Z. supervised the study. F. S., Q. Z., X. L., and T. X. jointly performed the experiments. Q. H., X. H., and W. F. analyzed the data. F. S. wrote the manuscript. P. H. and Y. Z. revised the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital (approved code NO. KT2022041).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• RAB11-FIP4 promotes stemness and increases sorafenib resistance in HCC.
• Rab11-FIP4 interacts with ARF5 to promote stemness in HCC.
• Rab11-FIP4 promotes HCC stemness via influencing the CDK1/cyclin B complex.
Supplementary information
ESM 1
(DOCX 738 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, F., Zhang, Q., Lu, X. et al. Rab11-FIP4 interacts with ARF5 to promote cancer stemness in hepatocellular carcinoma. J Physiol Biochem 79, 757–770 (2023). https://doi.org/10.1007/s13105-023-00972-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00972-2