Abstract
Rab25 was reported to be associated with several human cancers and malignant biological behavior of cancer cells. The goal of the present study was to determine its expression pattern and biological function in human hepatocellular carcinoma (HCC). We examined Rab25 protein in 92 cases of HCC tissues and 3 HCC cell lines. The results showed that Rab25 was upregulated in HCC tissues and cells compared with normal liver tissues and cell line. Rab25 overexpression correlated with advanced tumor stage and nodal metastasis. Rab25 small interfering RNA (siRNA) was employed in Bel7402 and SK-Hep-1 cell lines. Cell Counting Kit-8 (CCK-8) assay and colony formation assay showed that Rab25 depletion blocked cell growth rate and inhibited colony formation ability. Transwell assay showed that Rab25 depletion negatively regulated the invading ability of HCC cells. To explore the possible mechanisms, we checked several signaling pathways and found that Rab25 depletion downregulated AKT phosphorylation. In addition, luciferase reporter assay showed that Rab25 depletion inhibited the Wnt signaling pathway and its target genes such as cyclin D1, c-myc, and MMP7. In conclusion, Rab25 is overexpressed in human HCC and contributes to cancer cell proliferation and invasion possibly through regulation of the Wnt signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a common malignant cancer and the second main cause of cancer-related deaths [1–3]. Despite significant advances in therapeutic approaches during the past decades [4, 5], the prognosis still remains poor. High incidence of metastasis, tumor recurrence, and chemoresistance contribute to the poor prognosis of HCC patients [6]. So it is very important to explore oncogenes regulating malignant phenotype of HCC, which may contribute to the development of new targeted therapy.
Rab25 belongs to the Rab GTPases family proteins, which play roles in vesicular transportation [7, 8]. Rab25 controls various aspects of membrane trafficking from the cytoplasm to the membrane [9]. Recently, Rab family proteins including Rab25 were recently reported to play important roles in human cancer progression [10, 11]. Rab25 was reported to enhance the malignant feature of human breast and ovarian cancers [12]. Rab25 interacts with integrin signaling and promotes cancer progression [13, 14]. However, several recent reports showed that Rab25 depletion could result in a more aggressive phenotype in colorectal, esophageal, and breast (triple negative) cancers [15–18]. To date, expression pattern and biological function of Rab25 in HCC has not been explored. In this study, we examined Rab25 in 92 HCC tissues using immunohistochemistry. We also knocked down Rab25 expression in HCC cell lines and investigated its biological effects and potential mechanisms.
Materials and methods
Patients and specimens
This research was approved by the review board at the Shengjing Hospital of China Medical University. Ninety-two cases of HCC samples were obtained form Shengjing Hospital of China Medical University during the period of 2010 to 2012. All patients were treated with routine chemotherapy after the operation. The histological diagnosis and tumor grade were evaluated using H&E staining sections according to the WHO guidelines of classification. Patient information was retrieved from medical records.
Immunohistochemistry
Tumor specimens were fixed with neutral formalin, and 5-μm-thick paraffin sections were made. Immunostaining was previously described using the avidin–biotin–peroxidase complex method (S-P Kit, Maixin, Fuzhou, China). Tissue sections were incubated with Rab25 antibody (1:200 dilution, Sigma, USA). Counterstaining with hematoxylin was performed, and the sections were dehydrated in ethanol before mounting.
Two independent pathologists examined all tumor slides. At least five random fields were examined in each slide, and 100 cells were observed per view. Staining of Rab25 was scored following a semi-quantitative scale by evaluating in representative tumor areas the intensity and percentage of cells. Cytoplasmic and membrane staining was considered as positive. The intensity was also scored as 0 (none), 1 (weak), and 2 (strong). Percentage scores were designated as 1 (1–25 %), 2 (26–50 %), 3 (51–75 %), and 4 (76–100 %). The two scores were multiplied to get the final score from 0 to 8. Rab25 was determined as low expression, score <4, and overexpression (+), score ≥4.
Cell culture, transfection, and small interfering RNA treatment
LO2, Bel-7402, SK-Hep-1, and HepG2 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) containing 10 % fetal calf serum. siGENOME, SMARTpool, Rab25 small interfering RNAs (siRNAs) and non-targeting control were obtained from Dharmacon (ThermoFisher, USA). siRNA was transfected using DharmaFECT 1.
CCK-8 cell proliferation assay
Cell proliferation assay was performed using the Cell Counting Kit-8 (CCK-8) solution (Dojindo, Gaithersburg, MD, USA) according to the manufacturer’s protocol. Briefly, cells were seeded at a concentration of 3 × 103 cells each well in 96-well culture plates and treated with 10 μl CCK-8 solution during the last 4 h of the culture. Optical density of the wells was measured at 450 nm using a microplate reader.
Colony formation assay
Forty-eight hours after transfection, cells were plated into 6-cm culture dishes (about 1000 per dish). After 2 weeks, plates were washed with PBS and Giemsa staining was performed to visualize colony. The colonies with more than 50 cells were counted using a microscope.
Western blot analysis
Total proteins from cells were extracted in cell lysis buffer and quantified using the Bradford method. Forty micrograms of protein was separated by SDS-PAGE. Samples were transferred to PVDF membranes (Millipore, MA, USA) and incubated overnight at 4 °C with antibody against Rab25 (1:500; Sigma, USA), p-AKT, AKT, c-myc, cyclin D1, MMP7 (1:900, Cell Signaling Technology, Boston, USA), and GAPDH (1:2000; Santa Cruz, USA). After incubation with peroxidase-coupled anti-mouse or rabbit IgG antibody (1:2000 dilution, Cell Signaling Technology, USA) at 37 °C for 2 h, proteins on a membrane were visualized using the ECL kit and figures were captured using the DNR BioImaging System (DNR, Jerusalem, Israel).
Matrigel invasion assay
Matrigel invasion assay was performed using a 24-well Transwell chamber (Costar, MA, USA). The inserts were coated with 20 μl Matrigel (1:5 dilution, BD Bioscience, CA, USA). Forty-eight hours after the transfection, cells were trypsinized and suspended in 100 μl of serum-free medium and were transferred to the upper chamber. A medium supplemented with 10 % FBS was added to the lower chamber. After 16 h of incubation, the non-invaded cells on the upper membrane surface were removed with a cotton tip, and the cells that passed through the filter were stained using hematoxylin. The invading cell number was counted under the microscope.
Luciferase reporter assay
Luciferase activity assay was performed as follows: cells in a 24-well plate were co-transfected with pGL3-OT luciferase reporter (0.2 μg) along with the Renilla luciferase reporter (Promega Co) (0.02 μg) for 12 h using Attractene reagent and the reporter plasmids of TOP-Flash. The luciferase activity was measured using a Dual-Luciferase Assay System (Promega, CA, USA).
Wnt activity/TCF-mediated gene transcription activity was determined by using pGL3-OT activity normalized to Renilla luciferase activity.
Statistical analysis
SPSS version 12 for Windows was used for all analyses. The chi-squared test was used to examine possible correlations between Rab25 overexpression and clinicopathologic factors. Student’s t test was used to compare other data. p < 0.05 was considered to be statistically significant.
Results
The clinical significance of Rab25 in hepatocellular carcinoma
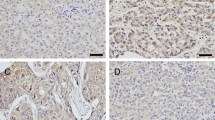
We examined Rab25 protein in 92 HCC specimens and corresponding normal tissues by immunohistochemistry. Rab25 expression was weak in normal tissues (Fig. 1a) and overexpressed in HCC tissues, which was localized in the cytoplasmic compartment of tumor cells (Fig. 1b–d). Overexpression of Rab25 was found in 50 out of 92 (54.3 %) HCC specimens. We analyzed the relationship between the Rab25 overexpression and clinicopathological parameters. As shown in Table 1, Rab25 overexpression significantly correlated with advanced TNM stage (I + II vs III + IV, p = 0.0261), T stage (T1-T2 vs T3-T4, p = 0.0243), and positive nodal status (p = 0.0285).
Expression of Rab25 in hepatocellular carcinoma. a Negative Rab25 expression in normal liver tissue. b Negative Rab25 expression in a case of well-differentiated, stage II hepatocelluar carcinoma. c Positive cytoplasmic Rab25 expression in a case of moderate-differentiated, stage III hepatocelluar carcinoma. d Strong Rab25 expression in a case of well-differentiated, stage III hepatocelluar carcinoma (magnification ×400)
Rab25 depletion in HCC cells inhibits proliferation and invasion
We examined Rab25 protein expression in three HCC cell lines (SK-Hep-1, Bel7402, HepG2) and normal liver cell line LO2 by western blot. Relatively high Rab25 protein was found in SK-Hep-1 and Bel7402 cell lines, and low Rab25 expression was observed in HepG2 and LO2 cell lines (Fig. 2a). Then, we employed Rab25 siRNA in the SK-Hep-1 and Bel7402 cell lines. Rab25 knockdown efficiency was determined by western blot analysis (Fig. 2b). We examined the effect of Rab25 depletion on HCC cell proliferation and colony formation by CCK-8 assay and colony formation assay (Fig. 3a, b). Rab25 depletion by siRNA inhibited cell growth rate and colony formation ability in both the Bel7402 and SK-Hep-1 cell lines.
Rab25 regulates HCC cell proliferation, colony formation and invasion. a CCK-8 assay showed that Rab25 siRNA inhibited cell proliferation rate in both Bel7402 and SK-Hep-1 cell lines. b Colony formation assay showed that Rab25 siRNA inhibited colony formation number in both Bel7402 and SK-Hep-1 cell lines. c Matrigel invasion assay showed that Rab25 siRNA inhibited the invading ability in both cell lines. * p < 0.05
We also performed Matrigel invasion assay to find out if Rab25 regulates HCC invading ability (Fig. 3c). The results showed Rab25 depletion inhibited cell invasion in both HCC cell lines.
Rab25 upregulates AKT and Wnt signaling in HCC cells
To investigate the possible mechanism of Rab25 on cell proliferation and invasion, we examined the effects of Rab25 depletion on several signaling pathways (Fig. 4a). Western blot revealed that knockdown of Rab25 reduced the expression of p-AKT (ser473) in both Bel7402 and SK-Hep-1 cell lines, indicating the involvement of AKT signaling in the biological function of Rab25. Wnt signaling plays an important role in carcinogensis of HCC. We examined the level of Wnt activation in Rab25-depleted cells using luciferase reporter assay (TOP-Flash) and found that Rab25 depletion could downregulate Wnt/TOP-Flash activity in both cell lines (Fig. 4b). In addition, western blot was carried out to examine Wnt target genes including cyclin D1, c-myc, and MMP7. As shown in Fig. 4a, Rab25 depletion decreased the levels of cyclin D1, c-myc, and MMP7 in both cell lines, suggesting Rab25 regulates biological behaviors of HCC cells possibly through the Wnt signaling pathway.
Rab25 regulates Wnt and AKT signaling. a siRNA knockdown of Rab25 reduced protein expression of Wnt target genes cyclin D1, c-Myc, and MMP7. Rab25 depletion also reduced AKT phosphorylation in both Bel7402 and SK-Hep-1 cell lines. b Rab25 siRNA treatment inhibited TOP-Flash activity in both cell lines
Discussion
Accumulating evidence has shown that Rab25 is involved in human cancer progression. Thus, we aimed to explore its function in human HCC. For the first time, we found that Rab25 expression was elevated in HCC, which was associated with advanced TNM stage. In addition, our study showed that Rab25 depletion inhibited proliferation, invasion, and suppressed the AKT and Wnt signaling pathway, suggesting that Rab25 is an oncoprotein in HCC.
In this study, we examined Rab25 protein in HCC samples by immunohistochemistry and found that the positive staining of Rab25 protein, which is located in the cyctoplasm of tumor cells, was upregulated in 54.3 % HCC tissues and correlated with advanced TNM stage. These data was in accord with former reports, which provide evidence that Rab25 overexpression is an important step during HCC development and facilitate malignant phenotype of HCC cells.
To explore the biological roles of Rab25 in HCC cell lines, we examined the effect of Rab25 depletion on the biological behavior of HCC and we demonstrated that Rab25 depletion inhibited cancer cell proliferation and invasion. These results were consistent with the immunohistochemical results and previous reports, implying Rab25 might serve as a biomarker for malignant biological behavior. Activation of Wnt signaling cascade plays an important role during HCC progression [19, 20]. In this study, we reported, for the first time, that Rab25 was able to regulate Wnt signaling and its downstream target genes such as cyclin D1, c-myc, and MMP7. cyclin D1 and c-myc are two important regulators of HCC malignant proliferation, which were reported to correlate with poor patient prognosis [21–23]. Thus, our results showed that Rab25 regulates HCC cell proliferation possibly through regulation of Wnt signaling cascade. Rab25 was reported to play an important role in vesicular traffic between the cytoplasm and membrane. Vesicular trafficking also influences Wnt signaling in both ligand-producing and ligand-receiving cells [24–26]. Thus, it is possible that Rab25 depletion impaired vesicular trafficking ability, which in turn reduced canonical Wnt activity. The precise mechanism needs further investigation. In addition, we found that the level of AKT and phosphorylation were also inhibited after Rab25 depletion, which was reported in several other cancers [27, 28].
The role of Rab25 as a tumor suppressor has been reported in colorectal cancer, triple-negative breast cancer, and esophageal cancer [15, 17, 18]. Since Rab25 mainly regulates intracellular trafficking, the characteristic of intracellular vesicle and signaling could determine the effect of Rab25. Thus, we believe that the biological role of Rab25 on human cancers may be tissue specific.
In conclusion, this study demonstrated that Rab25 was overexpressed in human hepatocellular carcinoma and correlated with advanced stage. Rab25 promotes cell proliferation and invasion, possibly through the AKT/Wnt signaling pathway.
References
Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48(4):1312–27.
Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S206–14.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–82.
Yamamoto J, Okada S, Shimada K, Okusaka T, Yamasaki S, Ueno H, et al. Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology. 2001;34(4 Pt 1):707–13.
Qin YR, Tang H, Xie F, Liu H, Zhu Y, Ai J, et al. Characterization of tumor-suppressive function of SOX6 in human esophageal squamous cell carcinoma. Clin Cancer Res. 2011;17(1):46–55.
Pereira-Leal JB, Seabra MC. Evolution of the Rab family of small GTP-binding proteins. J Mol Biol. 2001;313(4):889–901.
Stenmark H, Olkkonen VM. The Rab GTPase family. Genome Biol. 2001;2(5):REVIEWS3007.
Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem. 1993;268(25):18419–22.
Chia WJ, Tang BL. Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta. 2009;1795(2):110–6.
Cheng KW, Lahad JP, Gray JW, Mills GB. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65(7):2516–9.
Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10(11):1251–6.
Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13(4):496–510.
Dozynkiewicz MA, Jamieson NB, Macpherson I, Grindlay J, van den Berghe PV, von Thun A, et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev Cell. 2012;22(1):131–45.
Cheng JM, Ding M, Aribi A, Shah P, Rao K. Loss of RAB25 expression in breast cancer. Int J Cancer. 2006;118(12):2957–64.
Nam KT, Lee HJ, Smith JJ, Lapierre LA, Kamath VP, Chen X, et al. Loss of Rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J Clin Invest. 2010;120(3):840–9.
Goldenring JR, Nam KT. Rab25 as a tumour suppressor in colon carcinogenesis. Br J Cancer. 2011;104(1):33–6.
Tong M, Chan KW, Bao JY, Wong KY, Chen JN, Kwan PS, et al. Rab25 is a tumor suppressor gene with antiangiogenic and anti-invasive activities in esophageal squamous cell carcinoma. Cancer Res. 2012;72(22):6024–35.
Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, et al. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48(5):780–91.
Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901–15.
Che Y, Ye F, Xu R, Qing H, Wang X, Yin F, et al. Co-expression of XIAP and cyclin D1 complex correlates with a poor prognosis in patients with hepatocellular carcinoma. Am J Pathol. 2012;180(5):1798–807.
Peng SY, Chou SP, Hsu HC. Association of downregulation of cyclin D1 and of overexpression of cyclin E with p53 mutation, high tumor grade and poor prognosis in hepatocellular carcinoma. J Hepatol. 1998;29(2):281–9.
Abou-Elella A, Gramlich T, Fritsch C, Gansler T. c-myc amplification in hepatocellular carcinoma predicts unfavorable prognosis. Mod Pathol. 1996;9(2):95–8.
Feng Q, Gao N. Keeping Wnt signalosome in check by vesicular traffic. J Cell Physiol. 2015;230(6):1170–80.
Ibarrola-Villava M, Kumar R, Nagore E, Benfodda M, Guedj M, Gazal S, et al. Genes involved in the WNT and vesicular trafficking pathways are associated with melanoma predisposition. Int J Cancer. 2015;136(9):2109–19.
Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139(2):393–404.
Zhang J, Wei J, Lu J, Tong Z, Liao B, Yu B, et al. Overexpression of Rab25 contributes to metastasis of bladder cancer through induction of epithelial-mesenchymal transition and activation of Akt/GSK-3beta/Snail signaling. Carcinogenesis. 2013;34(10):2401–8.
Fan Y, Wang L, Han X, Liu X, Ma H. Rab25 is responsible for phosphoinositide 3-kinase/AKT-mediated cisplatin resistance in human epithelial ovarian cancer cells. Mol Med Rep. 2015;11(3):2173–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
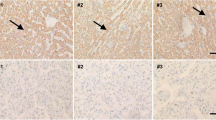
Expression of cyclin D1, c-myc, p-AKT and MMP7 in hepatocellular carcinoma cell lines and LO2 cell line. Western blot analysis showed that cyclin D1, c-myc, p-AKT and MMP7 expression was higher in Bel7402 and SK-Hep-1 cell lines than LO2 cell line. (GIF 9 kb)
Supplementary Figure 2
AKT activator IGF-1 rescues HCC cells from growth and migration inhibition caused by Rab25 knockdown. A. Matrigel invasion assay showed that IGF-1 upregulated cell invasion in Rab25 siRNA treated HCC cells. B. CCK-8 assay showed that IGF-1 upregulated cell growth rate in Rab25 depleted Bel7402 and SK-Hep-1 cells. (GIF 72 kb)
Rights and permissions
About this article
Cite this article
Geng, D., Zhao, W., Feng, Y. et al. Overexpression of Rab25 promotes hepatocellular carcinoma cell proliferation and invasion. Tumor Biol. 37, 7713–7718 (2016). https://doi.org/10.1007/s13277-015-4606-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4606-5