Abstract
Renal fibrosis is a major pathological event in the development of diabetic nephropathy (DN). Baicalin is a flavonoid glycoside that possesses multiple pharmacological properties including anti-fibrotic activity. In the present study, the effects of baicalin on renal fibrosis along with related molecular basis were investigated in streptozotocin (STZ)-induced DN mouse model and high glucose (HG)-treated HK-2 human proximal tubule epithelial cell model. Renal injury was evaluated through blood urea nitrogen (BUN) and serum creatinine (Scr) levels and urine albumin creatine ratio (ACR). Renal fibrosis was assessed by type IV collagen (COLIV) and fibronectin (FN) protein expression and histopathologic analysis via Masson trichrome staining. Protein levels of COLIV, FN, NF-κB inhibitor alpha (IκBα), phosphorylated IκBα (p-IκBα), p65, phosphorylated p65 (p-p65), and toll-like receptor 4 (TLR4) were measured by western blot assay. MicroRNA-124 (miR-124) and TLR4 mRNA levels were detected by RT-qPCR assay. The interaction of miR-124 and TLR4 was examined by bioinformatics analysis, luciferase reporter assay, and RIP assay. Baicalin or miR-124 attenuated renal injury and fibrosis in STZ-induced DN mice. Baicalin inhibited the increase of COLIV and FN expression induced by HG through upregulating miR-124 in HK-2 cells. TLR4 was a target of miR-124. MiR-124 inhibited TLR4/NF-κB pathway activation and the inactivation of the NF-κB pathway hindered COLIV and FN expression in HG-stimulated HK-2 cells. Baicalin prevented renal fibrosis by increasing miR-124 and inactivating downstream TLR4/NF-κB pathway in DN, hinting the pivotal values of baicalin and miR-124 in the management of DN and renal fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy (DN) is a common complication of diabetes and this renal injury usually occurs in about 30–40% of diabetic patients [31]. Renal fibrosis is a major pathological event in the development of multiple chronic renal diseases including DN [9, 22]. Renal fibrosis is usually characterized by the excessive accumulation of extracellular matrix (ECM) proteins (e.g., fibronectin (FN) and collagen) in the glomerulus and renal tubulointerstitium [9]. Inflammatory molecules and pathways have been identified as crucial players in the pathogenesis of DN and renal fibrosis [3, 25].
Recently, traditional Chinese medicines (TCMs) have attracted much attention from researchers due to their therapeutic effects on diabetes and its complications such as DN [30]. Baicalin, a flavone glycoside extracted from the root of Scutellaria baicalensis, possesses multiple potential pharmacological properties such as anti-inflammatory, anti-oxidant, and anti-fibrotic activities. Moreover, previous studies suggested that baicalin exerted potential protective effects on multiple renal diseases including renal fibrosis [27, 39]. In the present study, the effect of baicalin on renal fibrosis and related molecular regulatory mechanisms were further explored in DN.
MicroRNAs (miRNAs) are a group of small (∼ 21 nucleotides) non-coding RNAs that can regulate gene expression at posttranscriptional levels [2]. Moreover, accumulating miRNAs have been found to be crucial players in the development and progression of renal diseases including DN and renal fibrosis [6, 7, 13]. In addition, recent studies showed that active components of some TCMs might exert protective roles through miRNAs and miRNA-related signaling pathways in renal diseases including renal fibrosis [30]. For instance, total flavonoids from leaves of Carya Cathayensis alleviated renal fibrosis by downregulating miR-21 and upregulating miR-21 target Smad7 expression in unilateral ureteral obstruction (UUO) mouse model and transforming growth factor-β1 (TGF-β1)-induced mouse tubular epithelial cell model [34]. Triptolide ameliorated renal function and weakened renal epithelial-mesenchymal transition (EMT) induced by streptozotocin (STZ) in diabetic rats and inhibited high glucose (HG)-induced EMT in human proximal tubular epithelial cells via regulating miR-188-5p/PI3K/AKT pathway [36].
MiR-124 has been found to be closely linked with the pathogenesis of some renal diseases including DN and renal fibrosis [18, 19, 37]. Bioinformatics analysis by TargetScan database shows that TLR4 is a potential target of miR-124. In addition, a previous report pointed out that TLR4 was a target of miR-124 and miR-124 inhibited pro-inflammatory cytokine expression and promoted anti-inflammatory factor expression by regulating TLR4 signaling pathway in cocaine-exposed microglial cells [29].
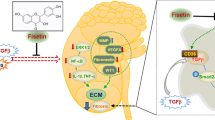
Considering the close link of baicalin, miR-124, TLR4, inflammation, and renal fibrosis, we further investigated the association of baicalin and miR-124/TLR4/NF-κB axis in STZ-induced DN mouse model and HG-induced human proximal tubular epithelial cell model. In the present study, we demonstrated that baicalin or miR-124 overexpression could weaken renal damage and alleviate renal fibrosis in STZ-induced DN mouse model. Baicalin inhibited the expression of fibrotic markers by regulating the miR-124/TLR4/NF-κB pathway in the HG-induced human proximal tubular epithelial cell model.
Materials and methods
Experimental animals, biochemical parameter determination, and histological analysis
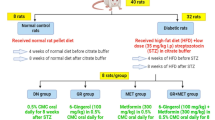
All animal experiments were carried out with the approval of the Animal Ethics Committee of The First Affiliated Hospital of Henan University of Chinese Medicine and the standard for the Care and Use of Laboratory Animals by the National Institutes of Health Guide. C57BL/6 male healthy mice (n = 42, 8 weeks old, 20‑25 g, Laboratory Animal Center of Henan Province) with good life status were randomly divided into 7 groups after 1 week of feeding (Normal, DN, DN + LC, DN + MC, DN + HC, DN + miR-NC, DN + miR-124) with 6 mice in each group. Next, mice were fasted for 6 h and intraperitoneally injected with STZ (125 mg/day/kg body weight, Sigma-Aldrich, St. Louis, MO, USA) in 0.02 M citrate buffer (pH 4.5) for 2 consecutive days to induce DN model. Also, an equal volume of citrate buffer was intraperitoneally injected into normal group mice. Blood glucose concentration was monitored daily after the last STZ injection using a Medisense glucometer (Abbott Laboratories, Bedford, MA, USA) through tail vein sampling at specified sites and time. Urine (24 h) was collected in metabolic cages, followed by the treatment of centrifugation to remove debris. Urinary albumin content or urinary creatinine content was determined by albumin ELISA kit (Bethyl Laboratories, Montgomery, TX, USA) or Creatinine Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), respectively. Urine albumin creatine ratio (ACR) = urinary albumin (μg)/urinary creatine (mg). DN mice were successfully established when their morning blood glucose level and urine ACR is higher than 16 mM and 300 μg/mg, respectively (3 consecutive times). However, diabetic mice need to be treated with insulin (0.4 U) every second day when blood glucose level > 30 mM to prevent weight loss and maintain blood glucose level at the range of 16–30 mM.
At 12 weeks after STZ stimulation, DN mice daily received different doses of baicalin (LC, low concentration 15 mg/day/kg; MC, middle concentration 30 mg/day/kg; HC, high concentration 45 mg/day/kg) by food orally for a total of 8 weeks. Also, at 12 weeks after STZ stimulation, miR-124 mimic or its negative control miR-NC (Thermo Scientific, Waltham, MA, USA) was intravenously injected into the tail vein of DN mice at a dose of 2 mg/kg body weight at 3-day intervals for a total of 8 weeks.
Finally, blood/urine samples and renal tissues were collected to measure corresponding biochemical parameters and renal pathological changes at 20 weeks after the onset of diabetes. And, serum was isolated from whole blood samples through centrifugation (3000 rpm for 10 min).
Serum creatinine (Scr) or blood urea nitrogen (BUN) level was respectively detected using Creatinine Colorimetric Assay Kit (Cayman Chemical) or BUN detection kit (StressMarq Biosciences, Victoria, British Columbia) following the instructions of the manufacturer.
Histological analysis was conducted using Masson’s Trichrome Stain Kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) to assess the degree of renal fibrosis, which was further quantified using the Image Pro Plus v5.1 software (Media Cybernetics, Sliver Spring, MD, USA) by measuring the percentage of blue stained area in 10 random fields under high magnification (× 200). Renal fibrosis was observed in both cortex and medulla.
Cell culture
Human proximal tubule epithelial cells (HK-2) were ordered from American Type Culture Collection (ATCC, Manassas, VA, USA). HK-2 cells were cultured in Keratinocyte serum-free medium (K-SFM, Thermo Scientific) supplemented with human recombinant epidermal growth factor (5 ng/ml, EGF, Sigma-Aldrich) and bovine pituitary extract (0.05 mg/ml, BPE, Sigma-Aldrich).
Reagents and transfection
Pyrrolidinedithio-carbamate ammonium (PDTC), an inhibitor of NF-κB signaling, was ordered from Sigma-Aldrich, Inc. MiR-124 inhibitor (anti-miR-124) and its negative control anti-miR-124 were ordered from Thermo Scientific Co., Ltd. Small interference RNA-specific targeting TLR4 (si-TLR4) and its negative control si-NC were obtained from GenePharma Co. Ltd. (Shanghai, China). The full-length sequences of TLR4 coding region were constructed into pcDNA3.1 vector by Sangon Biotech Co., Ltd. (Shanghai, China). Cell transfection was conducted using Lipofectamine 3000 reagent (Thermo Scientific).
RNA isolation and reverse transcription-quantitative PCR assay
Total RNA was isolated from mouse renal tissues and HK-2 cells through Trizol reagent (Thermo Scientific) referring to the protocols of the manufacturer. The MiR-124 level was determined using TaqMan MicroRNA Reverse Transcription Kit and TaqMan MicroRNA Assay Kit (Thermo Scientific) with U6 small nuclear RNA (U6 snRNA) as the internal control. For TLR4 expression analysis, RNA was reversely transcribed into cDNA using M-MLV Reverse Transcriptase (Thermo Scientific) and then a quantitative PCR reaction was conducted using SYBR™ Green PCR Master Mix (Thermo Scientific) with β-actin as the endogenous inference. The primer sequences for TLR4 and β-actin were as follows: 5′-ATCTCAGCAAAATCCCTCAT-3′ (sense) and 5′-AATCCAGCCACTGAAGTTGT-3′ (antisense) for TLR4 and 5′-GTGGGCCGCCCTAGGCACCA-3′ (sense) and 5′-CGGTTGGCCTTAGGGTTCAGAGGG-3′ (antisense) for β-actin.
Luciferase reporter assay
Partial sequences of TLR4 3′UTR containing putative miR-124 binding sites were subcloned into psiCHECK™-2 luciferase vector by Hanbio Biotechnology Co., Ltd. (Shanghai, China) to generate TLR4-WT reporter. Also, TLR4-MUT reporter with mutant miR-124 binding sites was constructed by Hanbio Biotechnology Co., Ltd. Then, TLR4-WT or TLR4-MUT reporter was transfected into HK-2 cells along with miR-124 mimic or miR-NC, followed by the determination of luciferase activities through dual-luciferase reporter assay kit (Promega, Madison, WI, USA).
Western blot assay
Proteins were extracted from renal homogenates and HK-2 cells using RIPA lysis buffer (Beyotime, Shanghai, China) supplemented with Protease and Phosphatase Inhibitor Cocktail (Thermo Scientific) and quantified through Pierce™ BCA Protein Assay Kit (Thermo Scientific). Then, an equal amount of proteins (35 μg/sample) were separated by SDS-PAGE and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA). Next, the membranes were blocked in 5% skim milk prior to the incubation with primary antibodies against type IV collagen (COLIV), FN, TLR4, NF-κB inhibitor alpha (IκBα), phosphorylated IκBα (p-IκBα), p65, phosphorylated p65 (p-p65), and β-actin. Subsequently, the membranes were incubated with secondary antibody conjugated with horseradish peroxidase (HRP). Finally, the immunoreactive bands were visualized using Clarity Western ECL Substrate (Bio-Rad Laboratories, Hercules, CA, USA). Protein relative expression levels were estimated by Quantity One Software (Bio-Rad Laboratories) via densitometry analysis. β-actin served as the house-keeping gene to normalize the expression of other proteins. Primary antibodies aganist COL IV, FN, TLR4, and β-actin and secondary antibodies were purchased from Abcam Co. Ltd. (Cambridge, UK). Primary antibodies aganist p-IκBα, p-p65, IκBα, and p65 were purchased from Cell Signaling Technology Co. Ltd. (Danvers, MA, USA).
RNA immunoprecipitation assay
HK-2 cells were transfected with miR-124 mimic or miR-NC. Forty-eight hours later, RNA immunoprecipitation (RIP) assay was conducted using EZ-Magna RIP kit (Millipore, Billerica, MA, USA) and IgG (Sigma-Aldrich) or argonaute-2 (Ago 2, Sigma-Aldrich) antibody referring to the instructions of the manufacturer. Then, RNA was isolated and purified from the immunoprecipitation complex or input samples. Finally, TLR4 level was detected by reverse transcription-quantitative PCR (RT-qPCR) assay.
Statistical analysis
Outcomes were presented as mean ± standard deviation. In vitro data were obtained from more than 3 times independent experiments. Data difference was analyzed through Student’s t test (two group data) or one-way analysis of variance (ANOVA, more than two group data) with P < 0.05 as statistically significant.
Results
Baicalin inhibited renal fibrosis in STZ-induced DN mouse model
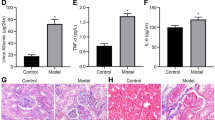
Our data revealed that BUN and Scr levels were markedly increased in STZ-induced DN mice than that in control mice (Fig. 1a, b). Proteinuria is commonly studied as a proxy of diabetic injury and cause of interstitial fibrosis. Hence, ACR value was measured to assess proteinuria patterns of normal and DN mice. Results showed that ACR value was markedly increased in DN mice (ACR > 300 μg/mg) compared with that in normal mice (Fig. 1c). These data suggested that the kidneys of DN mice were seriously damaged by STZ. In addition, western blot assay revealed that the protein levels of COLIV and FN (two renal fibrotic markers) were strikingly upregulated in the renal tissues of DN mice compared with those in C57BL/6 control mice (Fig. 1d), indicating the renal fibrosis in DN mice. Masson staining analysis further disclosed that DN mice had a serious renal fibrosis performance in contrast to C57BL/6 control mice (Fig. 1e). Moreover, the introduction of baicalin led to a dose-dependent reduction of BUN level, Scr level, and ACR value in DN mice (Fig. 1a‑c), suggesting that baicalin eased renal injury in STZ-induced DN mice. Additionally, baicalin inhibited COLIV and FN protein expression in a concentration-dependent fashion (Fig. 1d). Furthermore, the Masson staining analysis showed that the degree of renal fibrosis was gradually reduced in DN mice treated with increasing baicalin (Fig. 1e). In a word, these outcomes showed that baicalin ameliorated renal fibrosis in STZ-induced DN mouse model.
Baicalin inhibited renal fibrosis in STZ-induced DN mouse model. a‑c BUN and Scr concentrations and ACR ratio were determined in C57BL/6 control mice, DN mice, DN mice treated with different doses of baicalin (DN + LC, DN + MC, DN + HC). d Protein levels of COLIV and FN were detected by western blot assay in renal tissues of C57BL/6 control mice, DN mice, DN mice treated with different doses of baicalin. e Representational Masson staining images of renal tissues in C57BL/6 control mice, DN mice, and DN mice treated with different doses of baicalin. *P < 0.05
MiR-124 overexpression inhibited renal fibrosis in STZ-induced DN mouse model
RT-qPCR assay showed that miR-124 level was strikingly downregulated in renal tissues of DN mice relative to normal mice (Fig. 2a). The introduction of baicalin induced a dose-dependent upregulation of miR-124 level in renal tissues of DN mice (Fig. 2a), suggesting that baicalin-mediated protective effect against DN was linked with miR-124 expression. Also, RT-qPCR assay confirmed that miR-124 level was dramatically upregulated in renal tissues of DN mice injected with miR-124 mimic compared with the DN + miR-NC group (Fig. 2b). Functional analysis revealed that miR-124 overexpression led to the obvious reduction of Scr level, BUN level, and ACR value in DN mice (Fig. 2c‑e), manifesting that miR-124 could relieve STZ-induced renal injury in DN mice. In addition, enforced expression of miR-124 hampered renal fibrosis in STZ-induced DN mice, as evidenced by the remarkable reduction of COLIV and FN protein levels (Fig. 2f) and fibrosis extent (Fig. 2g) in renal tissues of miR-124-stimulated DN mice compared with miR-NC-treated DN mice.
MiR-124 alleviated renal fibrosis in STZ-induced DN mouse model. a MiR-124 level was measured by RT-qPCR assay in renal tissues of normal control mice, DN mice, and DN mice treated with different doses of baicalin (DN + LC, DN + MC, DN + HC). b‑f MiR-124, BUN, and Scr levels; ACR value; and COLIV and FN protein expression were examined in blood/urine samples or kidneys of normal control mice, DN mice, DN mice with miR-NC injection, or DN mice with miR-124 treatment. g Representational Masson staining images of renal tissues in normal mice, DN mice, and DN mice injected with miR-124 mimic or miR-NC. *P < 0.05
Baicalin suppressed the increase of fibrotic marker expression induced by HG by upregulating miR-124 in HK-2 cells
Next, our data further unveiled that miR-124 level was significantly downregulated in HG-cultured HK-2 cells than that in NG-cultured cells (Fig. 3a). Also, a noticeable upregulation of COLIV and FN protein levels was detected in HG-treated HK-2 cells versus NG-cultured cells (Fig. 3b). Moreover, the addition of baicalin triggered the noticeable increase of miR-124 expression and striking reduction of COLIV and FN expression in HK-2 cells under the HG condition, while these effects of baicalin were partly abrogated by miR-124 inhibitor. That is to say, baicalin curbed the expression of fibrotic markers by upregulating miR-124 in HG-stimulated HK-2 cells.
Baicalin suppressed the increase of fibrotic marker expression induced by HG by upregulating miR-124 in HK-2 cells. a, b HG (30 mM)-cultured HK-2 cells were transfected with anti-miR-NC or anti-miR-124 in the presence of baicalin (100 μmol/l). At 48 h after transfection, miR-124 level was examined by RT-qPCR assay, and COLIV and FN protein levels were determined by western blot assay in NG (5 mM)-cultured HK-2 cells, HG-cultured HK-2 cells, DMSO- or baicalin-treated HK-2 cells under HG conditions, and HK-2 cells treated with baicalin and HG and transfected with anti-miR-NC or anti-miR-124. *P < 0.05
TLR4 was a target of miR-124
Next, prediction analysis by the TargetScan website reveals that miR-124 has a possibility to interact with TLR4. To further validate this prediction, TLR4-WT or TLR4-MUT reporter was transfected into HK-2 cells along with miR-124 mimic or miR-NC. Subsequent luciferase reporter assay showed that ectopic expression of miR-124 could remarkably reduce the luciferase activity of TLR4-WT reporter, but had no much impact on luciferase activity of TLR4-MUT reporter (Fig. 4b), hinting the interaction between miR-124 and TLR4 3′UTR via putative binding sites. Moreover, RIP assay revealed that miR-124 upregulation led to the copious enrichment of TLR4 in Ago2 immunoprecipitation complex in HK-2 cells (Fig. 4c), further elucidating the interaction of miR-124 and TLR4. In a word, these data disclosed that TLR4 was a target of miR-124 in HK-2 cells.
TLR4 was a target of miR-124. a Putative binding sites between miR-124 and TLR4 3′UTR and mutant sites in TLR4-MUT reporter. b HK-2 cells were co-transfected with miR-NC or miR-124 and TLR4-WT or TLR4-MUT reporter. Forty-eight hours later, luciferase activities were measured by luciferase reporter assay. c HK-2 cells were transfected with miR-NC or miR-124. At 48 h after transfection, RIP assay and RT-qPCR assay were sequentially conducted to measure TLR4 enrichment level in Ago2 or IgG immunoprecipitation complexes. *P < 0.05
MiR-124 inhibited the activation of NF-κB signaling pathway through downregulating TLR4
Next, western blot assay presented that ectopic expression of miR-124 led to the conspicuous downregulation of p-IκBα, p-p65, and TLR4 protein levels in HG-cultured HK-2 cells (Fig. 5a), suggesting that miR-124 suppressed TLR4 expression and NF-κB signaling activation. Moreover, we further demonstrated that the introduction of TLR4 overexpression plasmid abrogated the inhibitory effect of miR-124 on NF-κB signaling and TLR4 expression in HG-stimulated HK-2 cells (Fig. 5a). These data suggested that miR-124 inactivated TLR4/NF-κB signaling pathway. Conversely, the depletion of miR-124 induced the activation of the TLR4/NF-κB pathway in HG-treated HK-2 cells (Fig. 5B). TLR4 knockdown inhibited the activation of the TLR4/NF-κB pathway triggered by miR-124 loss in HG-cultured HK-2 cells (Fig. 5b).
MiR-124 inhibited the activation of NF-κB signaling pathway through TLR4. a, b HG-cultured HK-2 cells were transfected with miR-NC, miR-124, miR-124 + pcDNA, miR-124 + pcDNA-TLR4, anti-miR-NC, anti-miR-124, anti-miR-124 + si-NC, or miR-124 + si-TLR4. Forty-eight hours later, protein levels of IκBα, p-IκBα, p65, p-p65, and TLR4 were determined by western blot assay. *P < 0.05
The inhibition of NF-κB signaling pathway triggered the reduction of COLIV and FN expression in HG-treated HK-2 cells
Next, we further demonstrated that the introduction of PDTC resulted in the reduction of COLIV, FN, p-IκBα, and p-p65 protein expression in HG-exposed HK-2 cells (Fig. 6a, b). That was to say, the inhibition of the NF-κB signaling pathway by PDTC reduced the expression of fibrotic markers in HG-exposed HK-2 cells.
The inhibition of NF-κB signaling pathway triggered the reduction of COLIV and FN expression in HG-treated HK-2 cells. a, b HG-exposed HK-2 cells were treated with PDTC (20 μM) for 24 h, followed by the detection of COLIV, FN, IκBα, p-IκBα, p65, and p-p65 protein levels via western blot assay. *P < 0.05
Discussion
DN and renal fibrosis is a serious threat to human health and life that can ultimately result in end-stage renal failure, a condition that demands dialysis or renal transplantation to maintain patients’ life [1, 24]. TCMs alone or in combination with Western medicines have been demonstrated to be effective in the treatment of multiple diseases including renal fibrosis in some Asian countries including China [35, 41].
Prior experimental pieces of evidence showed that baicalin exerted a potential anti-fibrotic activity in multiple organs including hepatic [28] and lung [21]. Additionally, Zhang et al. pointed out that baicalin alleviated renal fibrosis induced by UUO through inactivating the TGF-β/Smad pathway [40]. Baicalin exerted a protective effect on kidneys in rats with severe acute pancreatitis [38]. Here, we aimed to further investigate the effects of baicalin on renal fibrosis and underlying molecular mechanisms in DN models. Our data disclosed that the introduction of baicalin significantly alleviated renal injury and hampered renal fibrosis progression in STZ-induced DN mouse model. Cell model experiments showed that baicalin suppressed the production of fibrotic markers (COLIV and FN) in HG-treated HK-2 cells.
Also, previous studies demonstrated that miR-124 played vital roles in the development and progression of some renal diseases such as clear cell renal cell carcinoma [4], diabetic nephropathy [18], and acute renal injury [20]. For example, miR-124 overexpression ameliorated mouse survival outcomes, suppressed the expression of monocyte chemoattractant protein-1 (MCP-1) and pro-inflammatory cytokines (tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) and IL-6), and alleviated acute renal injury in candidiasis-induced septic mice [20]. The depletion of miR-124 relieved podocytic adhesive ability injury in STZ-induced uninephrectomized diabetic rats [18, 19]. Moreover, miR-124 was lower expressed in hypoxia-cultured renal proximal tubular epithelial cells (RPTEC) (an in vitro model of renal fibrosis) relative to normoxia-cultured cells and ectopic expression of miR-124 antagonized hypoxia-induced RPTEC migration [37]. A recent document further pointed out that miR-124 expression was strikingly downregulated in UUO rat model and TGF-β1-induced HK-2 cell model of renal fibrosis, and miR-124 depletion could promote renal fibrosis [42]. Our present study unveiled that miR-124 level was dramatically downregulated in kidneys of STZ-induced DN mice and HG-stimulated HK-2 cells. Baicalin induced miR-124 expression in the kidneys of STZ-induced DN mice and HG-stimulated HK-2 cells. Moreover, miR-124 overexpression produced similar outcomes as baicalin in STZ-induced DN mouse model. The depletion of miR-124 weakened the inhibitory effects of baicalin on COLIV and FN expression in HG-treated HK-2 cells.
Subsequent bioinformatics analysis, luciferase reporter assay, and RIP assay further demonstrated that TLR4 was a target of miR-124. Toll-like receptors (TLRs) are a group of pattern-recognition receptors that can recognize a wide range of structural components unique to different microbial pathogens [5, 32]. TLRs have been well documented as key players in immunological and inflammatory responses [11, 15]. TLR4, a member of TLR family, is closely linked with the pathophysiology of multiple diseases such as cancers, Alzheimer’s disease, and diabetes [12, 26]. Moreover, TLR4 has been recognized as pathogenic signaling in DN and renal fibrosis [8, 33]. Additionally, it is well known that TLRs including TLR4 can exert their function by activating multiple downstream regulatory pathways or molecules such as NF-κB and interferon regulatory factors [16, 17]. For example, TLR4 expression and NF-κB promoter activity were significantly upregulated in the kidneys of STZ-induced diabetic mice, and TLR4 loss mitigated renal injury, tubulointerstitial fibrosis, and inflammation induced by diabetes [14]. The depletion of TLR4 lessened renal hypertrophy, alleviated renal injury, and inhibited inflammatory and fibrotic responses in STZ-induced DN mice [23]. Furthermore, HG stimulation induced the activation of TLR4 signaling in podocytes and tubular epithelial cells, giving rise to the activation of NF-κB signaling and upregulation of inflammatory and fibrogenic factors [23]. Also, TLR4 loss alleviated tubular injury and hampered the development of renal fibrosis through inactivating some pro-inflammatory signals including NF-κB in cyclosporine nephrotoxicity [10]. In this text, we demonstrated that miR-124 inhibited the activation of the TLR4/NF-κB signaling pathway in HG-treated HK-2 cells. Moreover, the inactivation of the NF-κB signaling pathway by its inhibitor PDTC led to the reduction of COLIV and FN expression in HG-treated HK-2 cells.
In summary, these outcomes revealed that baicalin alleviated renal fibrosis through upregulating miR-124 and inhibiting TLR4/NF-κB pathway activation in STZ-induced DN mice and HG-treated HK-2 cells, providing a novel insight into the molecular basis of baicalin and some candidate biomarkers or targets in the management of DN and renal fibrosis. However, it is imperative to further verify our conclusion by in vivo knockdown or overexpression experiments.
References
Ahmad J (2015) Management of diabetic nephropathy: recent progress and future perspective. Diabetes Metab Syndr 9:343–358
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Barutta F, Bruno G, Grimaldi S, Gruden G (2015) Inflammation in diabetic nephropathy: moving toward clinical biomarkers and targets for treatment. Endocrine 48:730–742
Butz H, Szabo PM, Khella HW, Nofech-Mozes R, Patocs A, Yousef GM (2015) miRNA-target network reveals miR-124as a key miRNA contributing to clear cell renal cell carcinoma aggressive behaviour by targeting CAV1 and FLOT1. Oncotarget 6:12543–12557
Chen JQ, Szodoray P, Zeher M (2016) Toll-like receptor pathways in autoimmune diseases. Clin Rev Allergy Immunol 50:1–17
Chung AC-K, Lan HY (2015) MicroRNAs in renal fibrosis. Front Physiol 6:50
Dewanjee S, Bhattacharjee N (2018) MicroRNA: a new generation therapeutic target in diabetic nephropathy. Biochem Pharmacol 155:32–47
Garibotto G, Carta A, Picciotto D, Viazzi F, Verzola D (2017) Toll-like receptor-4 signaling mediates inflammation and tissue injury in diabetic nephropathy. J Nephrol 30:719–727
Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P (2014) The extracellular matrix in the kidney: a source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 7:4
González-Guerrero C, Cannata-Ortiz P, Guerri C, Egido J, Ortiz A, Ramos AM (2017) TLR4-mediated inflammation is a key pathogenic event leading to kidney damage and fibrosis in cyclosporine nephrotoxicity. Arch Toxicol 91:1925–1939
Gülden E, Wen L (2014) Toll-like receptor activation in immunity vs. tolerance in autoimmune diabetes. Front Immunol 5:119
Huang NQ, Jin H, Zhou SY, Shi JS, Jin F (2017) TLR4 is a link between diabetes and Alzheimer’s disease. Behav Brain Res 316:234–244
Ichii O, Horino T (2018) MicroRNAs associated with the development of kidney diseases in humans and animals. J Toxicol Pathol 31:23–34
Jheng HF, Tsai PJ, Chuang YL, Shen YT, Tai TA, Chen WC, Chou CK, Ho LC, Tang MJ, Lai KT, Sung JM, Tsai YS (2015) Albumin stimulates renal tubular inflammation through an HSP70-TLR4 axis in mice with early diabetic nephropathy. Dis Model Mech 8:1311–1321
Jiménez-Dalmaroni MJ, Gerswhin ME, Adamopoulos IE (2016) The critical role of toll-like receptors—from microbial recognition to autoimmunity: a comprehensive review. Autoimmun Rev 15:1–8
Kawai T, Akira S (2007) Signaling to NF-κB by toll-like receptors. Trends Mol Med 13:460–469
Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathways. Front Immunol 5:461
Li D, Lu Z, Jia J, Zheng Z, Lin S (2013a) Curcumin ameliorates Podocytic adhesive capacity damage under mechanical stress by inhibiting miR-124 expression. Kidney Blood Press Res 38:61–71
Li D, Lu Z, Jia J, Zheng Z, Lin S (2013b) MiR-124 is related to podocytic adhesive capacity damage in STZ-induced uninephrectomized diabetic rats. Kidney Blood Press Res 37:422–431
Li XY, Zhang YQ, Xu G, Li SH, Li H (2018) miR-124/MCP-1 signaling pathway modulates the protective effect of itraconazole on acute kidney injury in a mouse model of disseminated candidiasis. Int J Mol Med 41:3468–3476
Liu T, Dai W, Li C, Liu F, Chen Y, Weng D, Chen J (2015) Baicalin alleviates silica-induced lung inflammation and fibrosis by inhibiting the Th17 response in C57BL/6 mice. J Nat Prod 78:3049–3057
Loeffler I, Wolf G (2015) Epithelial-to-mesenchymal transition in diabetic nephropathy: fact or fiction? Cells 4:631–652
Ma J, Chadban SJ, Zhao CY, Chen X, Kwan T, Panchapakesan U, Pollock CA, Wu H (2014) TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS One 9:e97985
Magee C, Grieve DJ, Watson CJ, Brazil DP (2017) Diabetic nephropathy: a tangled web to unweave. Cardiovasc Drugs Ther 31:579–592
Meng XM, Nikolic-Paterson DJ, Lan HY (2014) Inflammatory processes in renal fibrosis. Nat Rev Nephrol 10:493–503
Mishra V, Pathak C (2018) Human toll-like receptor 4 (hTLR4): structural and functional dynamics in cancer. Int J Biol Macromol 122:425–451
Nam J, Kim JH, Park K, Lee SB, Nam JS, Park JS, Park SJ, Kim YS, Ahn CW. 2019. 548-P: Baicalin attenuates fibrosis process in human renal proximal tubular cells in hyperglycemic Conditioneditor^editors: am diabetes Assoc.
Peng XD, Dai LL, Huang CQ, He CM, Chen LJ (2009) Correlation between anti-fibrotic effect of baicalin and serum cytokines in rat hepatic fibrosis. World J Gastroenterol: WJG 15:4720–4725
Periyasamy P, Liao K, Kook YH, Niu F, Callen SE, Guo M-L, Buch S (2018) Cocaine-mediated downregulation of miR-124 activates microglia by targeting KLF4 and TLR4 signaling. Mol Neurobiol 55:3196–3210
Sun G-d, Li C-y, Cui W-p, Guo Q-y, Dong C-q, Zou H-b, Liu S-j, Dong W-p, L-n M (2016) Review of herbal traditional Chinese medicine for the treatment of diabetic nephropathy. J Diabetes Res 2016:5749857
Tesch GH (2017) Diabetic nephropathy - is this an immune disorder? Clin Sci (Lond) 131:2183–2199
Vierbuchen T, Stein K, Heine H (2018) RNA is taking its toll - impact of RNA-specific toll-like receptors on health and disease. Allergy 74:223–235
Wada J, Makino H (2016) Innate immunity in diabetes and diabetic nephropathy. Nat Rev Nephrol 12:13–26
Wu X, Ding X, Ding Z, Jia P (2018) Total flavonoids from leaves of Carya Cathayensis ameliorate renal fibrosis via the miR-21/Smad7 signaling pathway. Cell Physiol Biochem 49:1551–1563
Xia J, He LQ, Su X (2016) Interventional mechanisms of herbs or herbal extracts on renal interstitial fibrosis. J Integr Med 14:165–173
Xue M, Cheng Y, Han F, Chang Y, Yang Y, Li X, Chen L, Lu Y, Sun B, Chen L (2018) Triptolide attenuates renal tubular epithelial-mesenchymal transition via the MiR-188-5p-mediated PI3K/AKT pathway in diabetic kidney disease. Int J Biol Sci 14:1545–1557
Zell S, Schmitt R, Witting S, Kreipe HH, Hussein K, Becker JU (2013) Hypoxia induces mesenchymal gene expression in renal tubular epithelial cells: an in vitro model of kidney transplant fibrosis. Nephron Extra 3:50–58
Zhang XP, Tian H, Lai YH, Chen L, Zhang L, Cheng QH, Yan W, Li Y, Li QY, He Q (2007) Protective effects and mechanisms of Baicalin and octreotide on renal injury of rats with severe acute pancreatitis. World J Gastroenterol: WJG 13:5079–5089
Zhang XT, Wang G, Ye LF, Pu Y, Li RT, Liang J, Wang L, Ka Ho Lee K, Yang X 2018. Protective effects of baicalin on diabetes mellitus-induced renal fibrosis in mice Available at SSRN 3309387
Zheng L, Zhang C, Li L, Hu C, Hu M, Sidikejiang N, Wang X, Lin M, Rong R (2017) Baicalin ameliorates renal fibrosis via inhibition of transforming growth factor β1 production and downstream signal transduction. Mol Med Rep 15:1702–1712
Zhou X, Seto SW, Chang D, Kiat H, Razmovski-Naumovski V, Chan K, Bensoussan A (2016) Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front Pharmacol 7:201
Zhou H, Gao L, Yu ZH, Hong SJ, Zhang ZW, Qiu ZZ (2018) LncRNA HOTAIR promotes renal interstitial fibrosis by regulating Notch1 pathway via the modulation of miR-124. Nephrology (Carlton)
Funding
This research was supported by the Henan Research Institute of Chinese Medicine Pre-research Fund (No. 1704570).
Author information
Authors and Affiliations
Contributions
Shefeng Zhang designed and performed the experiments, wrote the manuscript. Li Xu and Ruifeng Liang contributed to experimental work and data analysis. Chenhua Yang and Peiren Wang conducted the experiments and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
All animal experiments were carried out with the approval of Animal Ethics Committee of The First Affiliated Hospital of Henan University of Chinese Medicine and the standard for the Care and Use of Laboratory Animals by the National Institutes of Health Guide.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Baicalin or miR-124 overexpression attenuated renal fibrosis in STZ-induced DN mice.
• Baicalin inhibited the expression of fibrosis markers by upregulating miR-124 in HG-exposed HK-2 cells.
• MiR-124 inhibited the activation of the NF-κB pathway by targeting TLR4.
• Baicalin prevented renal fibrosis by regulating the miR-124/TLR4/NF-κB pathway in DN.
Rights and permissions
About this article
Cite this article
Zhang, S., Xu, L., Liang, R. et al. Baicalin suppresses renal fibrosis through microRNA-124/TLR4/NF-κB axis in streptozotocin-induced diabetic nephropathy mice and high glucose-treated human proximal tubule epithelial cells. J Physiol Biochem 76, 407–416 (2020). https://doi.org/10.1007/s13105-020-00747-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-020-00747-z