Abstract
The purpose of the present study was to examine the immediate and prolonged immune response in circulating cytokine and adipocytokine concentrations after two different resistance exercise bouts: hypertrophic (HYP1, 5 × 10, 80% of 1RM) and maximal explosive (POW1, 10 × 5, 60% of 1RM) resistance exercise bouts and how 12 weeks of resistance training (RT) modifies these responses (HYP2, POW2). Eight men completed the study. RE-induced interleukin-6 (IL-6), interleukin-1β (IL-1β), interleukin-1 receptor antagonist (IL-1ra), monocyte-chemoattractant protein-1 (MCP-1), leptin, resistin, and adiponectin were measured before (PRE) and immediately (POST0), 24 (POST24) and 48 (POST48) hours after RE bouts before and after RT. In the untrained state, IL-6 increased immediately after RE in HYP1 (p = 0.002) and in POW1 (p = 0.003) whereas no changes were observed after RT. Similar results were observed in IL-1β, whereas conversely, IL-1ra increased only after RT in HYP2 and POW2 (p < 0.05). Resistin increased before RT in HYP1 and in POW1 (p = 0.011 and p = 0.003, respectively), but after RT, significant responses were not observed. Interestingly, in HYP2, MCP-1 increased significantly at POST24 (p = 0.009) and at POST48 (p = 0.032) only following RT. The present study shows that RT modifies RE-induced cytokine responses towards an anti-inflammatory direction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle is the largest organ in the human body, and ground-breaking work during the last decade has demonstrated that skeletal muscle is an active endocrine organ releasing a host of cytokines [1]. In recent years, studies reporting the acute effects of exercise on cytokines have been published quite extensively, whereas the specific time course of the effect of different resistance exercise protocols on cytokines remains unclear [2]. Cytokines are glycoproteins involved in the regulation and modulation of the immune response, and they are produced by a broad range of cells, including immune cells, skeletal muscle, connective tissue, and adipose tissue cells [3]. Resistance exercise (RE) is a potent activator of the immune system demonstrated by the changes in circulating pro-inflammatory and anti-inflammatory cytokine concentrations after exercise bouts [4]. For instance, a single bout of resistance exercise of moderate to high intensity has been shown to promote a transient increase in pro-inflammatory interleukins IL-6 and IL-1β as well as in the circulating levels of C-reactive protein (CRP), which is associated with a later increase in the levels of the anti-inflammatory IL-1 receptor antagonist (IL-1ra) [4, 5].
Far less is known about the effects of resistance exercise bout on so-called adipocytokines, e.g., leptin, adiponectin, and resistin. Adipocytokines are hormones that were first discovered to be secreted by adipose tissue and to regulate both energy metabolism and appetite. More recent findings on the ubiquitous expression of their receptors and on their cellular effects have revealed that adipocytokines also are involved in the regulation of a variety of biological functions related to immune responses and inflammatory diseases [6]. Previous studies have demonstrated that a single heavy resistance exercise bout exerts a specific acute effect on circulating adipocytokine concentrations, and the immediate response appears to be dependent on the duration and intensity of the exercise, as well as on training status and background [7,8,9].
Previous studies have suggested that exercise training can significantly alter the acute inflammatory responses to high intensity resistance exercise and recovery processes after the resistance exercise bout. Murton et al. [10] highlighted that the response to the first RE bout in participants with no background in resistance training (RT) is significantly different and has more inter-subject variability compared to the second resistance exercise. Izquierdo and colleagues [4] reported a significantly greater inflammation-responsive cytokine IL-6 response followed by a significantly enhanced response in the anti-inflammatory IL-1ra after a heavy resistance training intervention compared to the response before training. Cross-sectional studies have also suggested that training background affects the acute cytokine responses to a RE bout [9]. Thus, the overall effects of long-term RT appears to attenuate the acute inflammation response, but there are mixed findings on the effect of RT on specific markers [11].
It has been proposed that the acute anti-inflammatory (immunosuppressive) effect following a bout of resistance exercise could be beneficial for patients with autoimmunity disease, for senior citizens, and for obese individuals [3]. It is important to identify the specific resistance exercise-induced changes in circulating cytokines as well as the longitudinal effect of resistance training on immediate and prolonged responses after specific resistance exercise bouts in order to better understand possible health benefits, but also the possible health hazards, related to resistance training [12]. Pedersen and Febbraio [13] have linked skeletal muscle contraction to cytokine production. The extent of inflammation response to resistance exercise is affected by the physiological demands of RE, depending on the mode (eccentric and/or concentric muscular contractions), volume (total work of the session), load (weight lifted), and intensity (extent of neuromuscular and metabolic fatigue) of resistance exercise [14]. Many types of resistance exercises can be effectively used to improve muscular fitness and overall health [15]. Muscle strength and the ability of the muscles to develop force rapidly are important performance characteristics, which have also been shown to contribute to health and several tasks of daily life [16]. Since the cytokine responses appear to be related to the intensity of the exercise protocol, it is of interest to examine the cytokine responses of exercise protocols that aim for gains in muscle mass and strength [17], as well as in rapid force development [18, 19].
If the cytokine responses are related to the amount of muscle mass activated and respective metabolic changes, it is expected that the greatest responses will be observed after hypertrophic resistance exercise. However, in explosive RE, muscles are also activated maximally but with a shorter duration of each repetition, accompanied by a lower metabolic response. Thus, it is not clear whether this type of stimulus is large enough to cause considerable hormonal changes. We expected that the hypertrophic resistance exercise (HYP, 5 × 10, 80% of 1RM) would induce a significant response to the variables measured [4, 7]. The hormonal responses to a maximal explosive (POW, 10 × 5, 60% of 1RM) RE bout have been shown to be similar as in HYP but with a lower magnitude [18, 19]. Thus, we expected to observe a significant but lower response in the measured variables after POW RE bout. However, several studies have showed that hormonal responses to same hypertrophic [20] as well as explosive [21] RE are modified by 7–21 weeks of resistance training. Hence, we found it justified to assess whether the inflammatory response is modified after progressive resistance training. Therefore, the purpose of the present study was to examine the immediate and prolonged immune response, by measuring circulating cytokine and adipocytokine concentrations, induced by two different resistance exercise bouts: hypertrophic (HYP, 5 × 10, 80% of 1RM) and maximal explosive (POW, 10 × 5, 60% of 1RM) resistance exercise bouts. In addition, we measured how typically preiodized 12 weeks of RT possibly modified these responses.
Materials and methods
Subjects
This study was a part of a larger research project (TEKES Finland, Decision No. 70007/13). Eight healthy, slightly overweight, young men were selected for this study (age 31.0 ± 0.9 years, body weight 84.6 ± 1.9 kg, height 1.78 ± 0.04 m, fat percentage 25.3 ± 7.1%). All participants were physically active on a weekly basis, but none were competitive athletes or had a background in systematic strength training. The subjects’ physical activity was characterized by walking, cycling, or occasionally participating in team sports at light to moderate intensity and a frequency of 3 d week−1. Participants filled in a health questionnaire prior to participation in the study. All subjects reported that they were non-smokers, free from injury, and were not using any medications. Each subject was informed of the potential risks and discomforts associated with the measurements, and all the subjects gave their written informed consent to participate. The study was conducted according to the Declaration of Helsinki, and ethical approval for the study procedures were granted by the Ethical Committee at the University of Jyväskylä and by the Ethical Committee of the Central Hospital, Jyväskylä.
Study design and experimental resistance exercise bouts
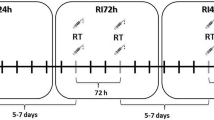
The study design (A) and experimental RE protocols (B) are presented in Fig. 1. The first phase of the study was a 4-week long preparatory RT period, during which the subjects were familiarized to RT and underwent pretesting. Subsequently, the cross-over study was started, and the subjects were randomly assigned to perform HYP1 or POW1 RE bout first and then after 10 days of recovery, POW1 or HYP1, respectively. Thereafter, they trained for 12 weeks according to supervised progressive RT protocol and did the same RE bouts with the same order as before training (HYP2/POW2). The POW RE bout included 10 sets of 5 repetitions of the concentric phase as fast as possible at 60% of 1 repetition maximum (RM) and the HYP RE bout was 5 sets of 10 repetitions at 80% of 1RM for the leg press (David D210 horizontal leg press device, David Health Solutions Ltd., Helsinki, Finland) exercise. The loads used during the first set were determined from the 1RM load measured during pretesting. The loads were adjusted during the sessions to enable completion of the required repetitions. The inter-set rest period was 3 min in POW and 2 min in HYP. The duration of POW was 32 min, whereas HYP was completed in 20 min. Total volume (load × repetitions) was 7160 ± 272 and 7550 ± 427 kg in POW and HYP, respectively. All experiments were conducted at the same time of day (± 1 h) for each subject.
Pretesting
All subjects participated in a pre-test session, which included anthropometrics and body composition measurement, as well as the 1RM test performed in the leg press device. This bilateral 1RM test was used to determine the loads used in each acute RE bout. Three warm-up sets (5 × 70–75, 3 × 80–85, and 2 × 90–95% of estimated 1RM) with 1 min of rest between sets were performed before the 1RM trials. Upon verbal instruction, subjects performed a full leg extension (knee angle 180°) from a starting knee angle of below 60°. After each successful completion, the load was increased. Subjects were allowed a maximum of 5 trials. The trial with the highest completed load was accepted as the 1RM.
Training
Subjects were asked to maintain individual habitual physical activity (e.g., light walking, cycling, and occasional team sports) throughout the study period. All prescribed training in the study was consistently supervised by qualified instructors. The training was designed to reflect a program designed for physically active populations according to recommendations outlined by the American College of Sports Medicine [15]. The detailed training program (hypertrophic-strength-group) has been previously reported by Hulmi et al. [22]. Briefly, in the preparatory 4-week RT period before POW1/HYP1, see above, the subjects exercised using whole-body workouts two times per week to standardize training status, to minimize the effects of stressors related to unaccustomed exercise, and to overcome strong neural and learning adaptations known to occur within the first few weeks of RT. Training loads were 50–80% of one repetition maximum (1 RM) increasing throughout the preparatory RT period.
The veritable 12-week progressive RT program was started after POW1/HYP1 and divided into three different blocks. Each block consisted of 4 weeks of RT. The first block consisted only of hypertrophic RE sessions. In the second block, 75% of the sessions were hypertrophic and 25% were maximal-strength sessions, and in the last block, 25% were hypertrophic and 75% maximal-strength RE sessions. The subjects did on average nine exercises in each session, 2–3 sets per exercise. Bilateral leg press, bilateral knee extension, and bilateral knee flexion exercises were performed during each RE session.
Body composition
Whole body composition was estimated before and after RT by dual X-ray absorptiometry (DXA, LUNAR Prodigy, GE Medical Systems) after an overnight fast and 48 h without training. Abdominal fat was calculated manually defining a range of interest confined cranially by the upper end plate of the first lumbar vertebra, laterally by the ribs and caudally by the iliac crest [23] at PRE. This customized range was then copied to the DXA scans at week 4 and week 16, respectively.
Nutrition
Dietary intake was recorded over three weekdays and one weekend day during the training period. The subjects were instructed to follow the same diet before all the acute exercise bouts. The breakfast before RE bouts was standardized and served at the laboratory.
Subjective muscle soreness
Muscle soreness was rated on a visual analogic scale (VAS) of 0 (= no pain) to 100 (= maximum pain) in millimeters for the overall muscle soreness of the quadriceps muscles at PRE, POST24, and POST48.
Blood samples and analyses
Blood lactate was measured to determine the metabolic effect of work performed in RE bouts. Blood samples were obtained from the fingertip and collected into capillary tubes (20 μL), which were placed in a 1-mL hemolyzing solution and analyzed automatically after the completion of testing according to the manufacturer’s instructions (EKF diagnostic, C-line system, Biosen, Germany). To assess the immediate and prolonged (up to 48 h) impact from exercise protocols, blood samples were collected pre-exercise (PRE) and during recovery as follows: immediately (POST0), 24 h (POST24), and 48 h (POST48) after the exercises.
Venous blood samples were drawn from the antecubital vein into EDTA tubes (Venosafe, Terumo, Belgium). Hemoglobin and hematocrit were determined with Sysmex KX-21N (TOA Medical Electronics Co., Ltd., Kobe, Japan), and plasma volume change was determined after exercise at POST0 from hemoglobin and hematocrit concentrations using the equation by Dill and Costill [24]. Plasma glucose (GLU) was measured using the KONELAB 20XTi analyzer (Thermo Fisher Scientific Inc., Waltham, MA, USA).
The serum samples were held for 15 min at room temperature before being centrifuged for 10 min at 2000×g (Megafuge 1.0 R, Heraeus, Germany). The serum was kept at − 80 °C until analyzed. High-sensitivity C-reactive protein (hsCRP), creatine kinase (CK), cortisol (COR), and interleukin-1 beta (IL-β) in serum samples were analyzed from using the Immulite 1000 and immunoassay kits (Immulite, Siemens, IL, USA). The detection limits and inter-assay coefficients of variation, respectively, were 0.1 mg L−1 and 10% for hsCRP, 3.9 pg mL−1 and 5.9% for CK, 5.5 nmol L−1 and 7.9% for COR, and 1.5 pg mL−1 and 2.8% for IL-1β.
The EDTA-treated samples were centrifuged for 10 min at + 4 °C with 2000×g (Megafuge 1.0 R, Heraeus, Germany). The plasma was kept at − 80 °C until analyzed. Concentrations of interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), interleukin-1 receptor antagonist (IL-1ra), adiponectin, leptin, and resistin were determined by enzyme-linked immunosorbent assay (ELISA) with commercial reagents (R&D Systems, Europe Ltd., Abingdon, UK). The detection limits and inter-assay coefficients of variation, respectively, were 0.2 pg mL−1 and 1.8% for IL-6, 3.9 pg mL−1 and 5.0% for MCP-1, 31.3 pg mL−1 and 2.0% for IL-1ra, and 0.78 ng mL−1 and 2.2% for adiponectin, 15.6 pg mL−1 and 4.0% for resistin, and 15.6 pg mL−1 and 5.1% for leptin.
Statistical analyses
Conventional statistical methods were used for the calculation of means and standard deviations and standard errors. Before applying further statistical methods, the data was checked for sphericity and normality. If a specific variable violated the assumptions of parametric tests, then log-transformation was used. For IL-6, adiponectin, and leptin, the log-transformation provided sufficient remedy for normality or homogeneity of variance. Absolute changes were analyzed via two-way repeated analysis of variance for time (PRE, POST0, POST24, POST48), training (before, after), and interaction (time × training) effects. This was followed by one-way repeated measures ANOVA on each RE bout to examine a main effect of time. If a main or interaction effect was observed at p ≤ 0.05, the change from pre-values to POST0, POST24, and POST48 was compared between type or time using paired t tests. Effect sizes (ES) are given as Cohen’s d with an effect size of 0.20–0.50 being considered small, 0.50–0.80 medium, and > 0.80 large. Data was analyzed using PASW statistic 18.0 (SPSS, Chicago, IL, USA). The level of statistical significance was set at p ≤ 0.05.
Results
Descriptive statistics of the anthropometric characteristics and 1RM are presented in Table 1. After RT, whole body fat-free mass was increased significantly (p < 0.05), whereas body weight, whole body fat mass, and abdominal fat mass stayed unaltered after RT. 1RM increased significantly (+ 13%, p = 0.032). Table 2 shows that RT significantly (p < 0.05) suppressed the immediate increases in lactate and glucose and prolonged those of creatine kinase and muscle soreness induced by HYP. A significant increase in cortisol was observed before RT in HYP (p < 0.001), whereas after RT, this was not observed. POW reduced average circulating concentrations before and after RT, but the decrease was statistically significant only after RT (p < 0.05).
Effects of HYP and POW RE on the circulating levels of inflammatory markers before and after RT intervention are shown in Table 3. The most notable changes were seen in MCP-1 and resistin (Fig. 1). A significant time × training effect was observed in circulating MCP-1 concentration in HYP (p = 0.002, ES = 0.924) (Fig. 1a): after RT in HYP2, a significant increase in MCP-1 was observed during recovery at POST24 (p = 0.009) and at POST48 (p = 0.032). An increasing trend in circulating MCP-1 concentration was seen also at POST24 and POST48 in POW2 but that effect did not reach statistical significance.
There was a significant main effect of time (p = 0.005, ES = 0.560) and time × training (p = 0.028, ES = 0.732) in circulating resistin levels in POW (Fig. 1b). A significant increase in resistin concentration from PRE to POST0 was observed before RT in POW1 (p = 0.003, ES = 0.997); however, after RT in POW2, such significant response was not observed (p = 0.102). In HYP, a similar effect, although smaller in quantity, in circulating resistin concentration was found: there was an increase in HYP1 from PRE to POST0 before RT (p = 0.011, ES = 0.789) whereas resistin stayed statistically unaltered by HYP2 after RT (p = 0.248) (Fig. 1b).
Neither of the RE bouts, before or after RT, elicited significant changes in circulating CRP concentrations (Table 3). IL-6 response was significantly affected by RT in HYP (time × training interaction, p = 0.048, ES = 0.534) and in POW (time × training interaction, p = 0.013, ES = 0.808). A significant increase in IL-6 was observed at POST0 in HYP1 (p = 0.002, ES = 0.719) and in POW1 (p = 0.003, ES = 0.878) before training whereas no effect was observed after training (Table 3).
A significant main effect of time in circulating IL-1β was observed in HYP (p = 0.022, ES = 0.775) and in POW (p = 0.043, ES = 0.496) (Table 3). Significant increases from PRE to POST0 were observed in IL-1β in HYP1 before RT (p = 0.019, ES = 0.795) whereas after RT, statistically significant changes were not observed. Similarly in POW1 before RT, a significant increase was observed in circulating IL-1β concentration from PRE to POST0 (p = 0.048), whereas after RT, significant changes were not observed (Fig. 2).
MCP-1 (a, b) and resistin (c, d) responses to hypertrophic (HYP, left) and explosive (POW, right) resistance exercises before (HYP1/POW1) and after (HYP2/POW2) RT (mean ± SD). A significant time × training interaction was observed in MCP-1 in HYP (p = 0.002) and in resistin in POW (p = 0.028). *p < 0.05, **p < 0.01, ***p < 0.001 when compared to pre value in the corresponding RE bout
Circulating IL-1ra remained unaltered before RT in HYP1 and in POW1, but after RT, the circulating IL-1ra concentration increased significantly from PRE to POST0 in HYP2 (p = 0.048) and in POW2 (p = 0.024), see Table 3.
Following RT, the circulating leptin concentrations were significantly higher at PRE before both loadings. In HYP1, a significant reduction in circulating leptin concentration was observed at POST24 (p < 0.05) and in HYP2 at POST0 (p < 0.05). Pre-exercise circulating adiponectin concentration was significantly higher after RT in HYP2 (p = 0.048) and in POW2 (p = 0.026) and stayed unaltered after RE bouts.
Discussion
The purpose of the present study was to examine the immediate and prolonged immune response, by measuring circulating cytokine and adipocytokine concentrations, induced by two different resistance exercise bouts: hypertrophic (HYP, 5 × 10, 80% of 1RM) and maximal explosive (POW, 10 × 5, 60% of 1RM) resistance exercise bouts, and how 12 weeks of RT may modify these responses. We expected HYP RE to elicit greater responses in the selected cytokines. However, the differences between the loadings were cytokine-specific. Nevertheless, the pro- and anti-inflammatory responses were modified differently: the pro-inflammatory IL-6, IL-1β, and resistin response was blunted, whereas anti-inflammatory IL-1ra response was enhanced in both HYP and POW as a consequence of training. Interestingly, the prolonged MCP-1 response was enhanced after RT in HYP, possibly as a marker of muscle regeneration. The present data also demonstrated that progressive RT increases muscle strength and alters immediate and prolonged response of cytokine and adipocytokine concentrations to HYP and POW similarly. Interestingly, RT affected responses to POW similarly even though the training did not include explosive type of training. This could indicate that the effect of training on immediate prolonged cytokine response is more dependent on overall training status than on specific RE training background.
Regular exercise reduces the risk of chronic metabolic and cardiorespiratory diseases, and this reduction has been linked to the anti-inflammatory effect of exercise [25]. It has been suggested that this anti-inflammatory effect of exercise is mediated via the introduction of an anti-inflammatory environment following each bout of endurance or resistance exercise [25]. The present study showed increased IL-6 and IL-1β levels immediately post-exercise before RT but not after RT, whereas circulating IL-1ra increased only after RT in both resistance exercise bouts. This modified IL-6 response is in line with the study by Izquierdo et al. [4], which showed exercise-induced IL-6 response only in the initial phase of resistance training. Contracting skeletal muscle has been proposed to be the main source of increased IL-6 in circulation during and following exercise but also connective tissue, brain, and adipose tissue contribute to the exercise-induced increased IL-6 levels [1]. IL-6 production in the muscle cells is increased when glycogen is compromised suggesting that IL-6 has a role as an energy sensor in the exercising muscle, and IL-6 has been shown to enhance basal and insulin-stimulated glucose uptake in muscle cells and to its favorable effects on energy metabolism, and its anti-inflammatory effects, IL-6 has also pro-inflammatory effects in inflammatory diseases and in connection to obesity and metabolic syndrome. Therefore, the observed adaptation of the IL-6 response to heavy exercise induced by resistance training as observed in the present study can be regarded as a beneficial form of acclimatization considering that exercise training has also been found to increase IL-6 receptor expression and IL-6 sensitivity in skeletal muscle [26]. The present study also demonstrated a significant increase in IL-1β concentrations before RT in HYP and POW, whereas after RT, the response was blunted. IL-1 has been reported to enhance the secretion of hypothalamic corticotropin-releasing factor, which further stimulates glucocorticoid release [20]. In the present study, the increase in IL-1β was accompanied by an increase in cortisol before RT in HYP, and neither were increased following RT supporting a possible link between those two. Nevertheless, in POW, we did not observe a significant cortisol response before RT; however, a significant increase in IL-1 β was observed. To summarize, in the present study after RT, a blunted IL-6 response, increased IL-1ra concentration, and suppressed IL-1β response were observed regardless of the type of RE bout. These observations lead to a hypothesis that RT enhances anti-inflammatory effects and that might suppress the immediate pro-inflammatory response at the cellular level. Such an anti-inflammatory response within the circulation may provide positive metabolic changes through increased fat oxidation and glucose uptake [13, 27]. In line with the observed responses in HYP, the present study observed a significant increase in blood glucose before RT, whereas after RT, blood glucose response was suppressed and a similar blunted response was observed in cortisol.
MCP-1 is a potent chemotactic and activating factor for macrophages, inflammation, and skeletal muscle regeneration [28]. MCP-1 response has been studied mostly after eccentric exercise [29, 30]. To our knowledge, only two other studies have examined changes in plasma MCP-1 after acute traditional RE. Our previous study found a significant decrease 30 min after hypertrophic RE [7] whereas Wells and colleagues [31] reported a significant increase in circulating MCP-1 immediately following damaging RE. The present study added longer tracking into recovery with two measurement points to the existing data; namely 24 and 48 h after the present RE. Interestingly, MCP-1 significantly increased after resistance training in HYP 24 h after the RE. Hubal et al. [30] showed that MCP-1 mRNA levels were significantly elevated after muscle-lengthening lower body exercise and the response was enhanced in repeated bouts. Furthermore, the immunohistochemistry analysis in their study showed that MCP-1 was localized with resident macrophages and satellite cell populations, which link MCP-1 to muscle regeneration. Skeletal muscle has an intrinsic protective mechanism to adapt after muscle damaging exercise to resist future muscle damage [32]. Deyhle et al. [32] measured intracellular MCP-1 after initial lengthening contraction and 27 days after the first bout and suggested that the muscle or the immune system becomes sensitized to the initial bout of damaging exercise such that inflammatory cell infiltration into the muscle is enhanced upon a repeated bout of damaging exercise. In the present study, a significant level of muscle damage, as assessed by CK increase, was observed after all RE bouts, which could be one of the mechanisms that lead to increased MCP-1, however, the mechanisms behind the enhanced MCP-1 response after RT remains unclear. The present study demonstrates that not only the initial response to unfamiliar resistance exercise differs from the later responses but also that RT significantly affects the MCP-1 response.
Adipocytokines are released from the adipose tissue and have been associated strongly not only with metabolism but also with inflammation [33]. Previous studies have reported no effect [34] or acute decreases [7, 9] on circulating resistin concentration following RE. Interestingly, regardless of the lack of total or abdominal fat loss, there was a beneficial significant increase in pre-exercise leptin and adiponectin concentrations. The present study observed a significant immediate increase in circulating resistin concentration immediately after both HYP and POW bouts before but not after RT. This is in line with the observation by Varady and colleagues [9] that resistance training background modifies the resistin response. However, they reported a significant reduction after RE bout in resistin in the subjects with RT background whereas no significant response was observed in participants with sedentary and running background. The mechanism of the resistin response has been hypothesized to be related to metabolic demand of exercise, which could explain the blunted response in HYP. However, our data does not fully support this hypothesis as the lactate after RT immediately after HYP was an average of 10.1 ± 3.4 mmol L−1 and no significant response in resistin levels was observed whereas in POW before RT with the average lactate of 3.0 mmol L−1, a significant increase in resistin levels was observed. Hence, in addition to the metabolic stress, other mechanisms have to be involved. Skeletal muscle cells have been shown to release resistin [35]. In addition, mechanical stress has been shown to enhance the expression of resistin in cardiomyocytes [36]. Thus, one origin of increased resistin concentration could be the skeletal muscle as it has been shown with IL-6 [1]. Another mechanism could be related to the loading of joints. It has been shown that adipokines are produced also in joints and have a role in joint diseases such as osteoarthritis [37]. Vuolteenaho et al. [38] reported a significant effect of marathon running on circulating resistin concentrations, and one may hypothesize that it could be related to the cartilage degradation or strenuous muscle exercise. Interestingly, in the present study, a significant immediate increase in resistin concentration was observed only before training in both resistance exercise bouts. Especially, maximal explosive resistance exercise bout characterized by explosive muscle contraction produces stress on tendons and joints [39]. While speculative, the blunted resistin response after RT may suggest that RT could elicit protective mechanisms in cartilage, which could be observed as a reduced immediate resistin response to resistance exercise bout.
Limitations of this study should be noted. The most notable limitation of the present study was the small sample size. In addition, the mechanisms by which resistance training alters the inflammatory response to an acute resistance exercise bouts remain to be explored. Serial blood samples and additional muscle biopsies are needed to investigate the series of events initiated by resistance exercise bouts on the cytokine kinetics after traditional RE bouts. Hence, the present study cannot go further than to state that the acute pro- and anti-inflammatory response is altered by resistance training towards an anti-inflammatory direction. It is also possible that the nutritional status of the participants had an effect on the acute cytokine responses as the nutrition on the day before the RE bout was not strictly controlled [13]. It is notable that the exercise protocols in this study were intense, which must be taken into account when evaluating the present results. The present study showed that the pro-inflammatory response in novice trainers is blunted by the resistance training. Generally, a repetition range between 8 and 12 RM is used for novice training and 1–12 RM in a periodized fashion for individuals with resistance training experience are recommended [15]. Our findings support the recommendation. However, to ensure optimal health and fitness gains, resistance training should be undertaken with proper preparation, guidance, and surveillance.
Conclusion
Twelve weeks of resistance training blunted IL-6, IL-1β, and resistin responses in the circulation in both HYP and POW, whereas the response of the IL-1ra was enhanced. In addition, enhanced MCP-1 response in HYP was observed only after the RT intervention. This study emphasizes the importance of reporting training background when investigating immediate cytokine and adipocytokine responses after resistance exercise. The improvement in the anti-inflammatory and the blunted pro-inflammatory response achieved in the present study by resistance training may be an effective means for reducing systemic low-grade inflammation and thus improve the future health trajectory of young men.
References
Pedersen BK (2011) Muscles and their myokines. J Exp Biol 214:337–346. https://doi.org/10.1242/jeb.048074
Brown WM, Davison GW, McClean CM, Murphy MH (2015) A systematic review of the acute effects of exercise on immune and inflammatory indices in untrained adults. Sports Medicine-Open 1:1–10
Görgens SW, Eckardt K, Jensen J, Drevon CA, Eckel J (2015) Chapter thirteen-exercise and regulation of adipokine and myokine production. Prog Mol Biol Transl Sci 135:313–336
Izquierdo M, Ibañez J, Calbet JA et al (2009) Cytokine and hormone responses to resistance training. Eur J Appl Physiol 107:397–409
Steensberg A, Fischer CP, Sacchetti M et al (2003) Acute interleukin- 6 administration does not impair muscle glucose uptake or whole-body glucose disposal in healthy humans. J Physiol Lond 548:631–638
Ouchi N, Parker JL, Lugus JJ, Walsh K (2011) Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11:85–97
Ihalainen J, Walker S, Paulsen G et al (2014) Acute leukocyte, cytokine and adipocytokine responses to maximal and hypertrophic resistance exercise bouts. Eur J Appl Physiol 114:2607–2616
Bouassida A, Chamari K, Zaouali M, Feki Y, Zbidi A, Tabka Z (2010) Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br J Sports Med 44:620–630. https://doi.org/10.1136/bjsm.2008.046151
Varady KA, Bhutani S, Church EC, Phillips SA (2010) Adipokine responses to acute resistance exercise in trained and untrained men. Med Sci Sports Exerc 42:456–462
Murton AJ, Billeter R, Stephens FB et al (2014) Transient transcriptional events in human skeletal muscle at the outset of concentric resistance exercise training. J Appl Physiol (1985), https://doi.org/10.1152/japplphysiol.00426.2013 116:113–125
Vanhees L, Geladas N, Hansen D et al (2012) Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: recommendations from the EACPR. Part II. Eur J Prev Cardiol 19:1005–1033
Cooper DM, Radom-Aizik S, Schwindt C, Zaldivar F (1985) Jr (2007) dangerous exercise: lessons learned from dysregulated inflammatory responses to physical activity. J Appl Physiol 103:700–709
Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88:1379–1406. https://doi.org/10.1152/physrev.90100.2007
Peake JM, Neubauer O, Della Gatta PA, Nosaka K (2017) Muscle damage and inflammation during recovery from exercise. J Appl Physiol (1985) 122:559–570. https://doi.org/10.1152/japplphysiol.00971.2016
STAND P (2009) Progression models in resistance training for healthy adults. Med Sci Sports Exerc 41:687–708
Bassey EJ, Fiatarone MA, O'Neill EF, Kelly M, Evans WJ, Lipsitz LA (1992) Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 82:321–327
Hulmi JJ, Walker S, Ahtiainen JP, Nyman K, Kraemer WJ, Häkkinen K (2010) Muscle hypertrophy and metabolic signaling after two different resistance exercises in young men. FASEB J 24:1046.6–1046.6
Linnamo V, Pakarinen A, Komi PV, Kraemer WJ, Häkkinen K (2005) Acute hormonal responses to submaximal and maximal heavy resistance and explosive exercises in men and women. J Strength Condition Res 19:566
McCaulley GO, McBride JM, Cormie P et al (2009) Acute hormonal and neuromuscular responses to hypertrophy, strength and power type resistance exercise. Eur J Appl Physiol 105:695–704
Ahtiainen JP, Pakarinen A, Alen M, Kraemer WJ, Häkkinen K (2003) Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur J Appl Physiol 89:555–563
Walker S, Ahtiainen J, Häkkinen K (2010) Acute neuromuscular and hormonal responses during contrast loading: effect of 11 weeks of contrast training. Scand J Med Sci Sports 20:226–234
Hulmi JJ, Laakso M, Mero AA, Häkkinen K, Ahtiainen JP, Peltonen H (2015) The effects of whey protein with or without carbohydrates on resistance training adaptations. J Int Soc Sports Nutr 12:1–13
Tallroth K, Kettunen JA, Kujala UM (2013) Reproducibility of regional DEXA examinations of abdominal fat and lean tissue. Obes Facts 6:203–210. https://doi.org/10.1159/000348238
Dill D, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA (2011) The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11:607–615
Keller P, Penkowa M, Keller C et al (2005) Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. FASEB J 19:1181–1183
Walsh NP, Gleeson M, Shephard RJ et al (2011) Position statement part one: Immune function and exercise
Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM (2007) MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 81:775–785
Paulsen G, Benestad HB, Strom-Gundersen I, Morkrid L, Lappegard KT, Raastad T (2005) Delayed leukocytosis and cytokine response to high-force eccentric exercise. Med Sci Sports Exerc 37:1877–1883
Hubal MJ, Chen TC, Thompson PD, Clarkson PM (2008) Inflammatory gene changes associated with the repeated-bout effect. Am J Physiol Regul Integr Comp Physiol 294:R1628–R1637. https://doi.org/10.1152/ajpregu.00853.2007
Wells AJ, Hoffman JR, Jatner AR, Varanoske AN, Church DD, Mangine GT (2016) Monocyte recruitment following high-intensity and high-volume resistance exercise. DigitalCommons@ Kennesaw State University
Deyhle MR, Gier AM, Evans KC et al (2015) Skeletal muscle inflammation following repeated bouts of lengthening contractions in humans Frontiers in Physiology 6
Klöting N, Blüher M (2014) Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord 15:277–287
Jamurtas AZ, Theocharis V, Koukoulis G et al (2006) The effects of acute exercise on serum adiponectin and resistin levels and their relation to insulin sensitivity in overweight males. Eur J Appl Physiol 97:122–126
Dietze D, Koenen M, Rohrig K, Horikoshi H, Hauner H, Eckel J (2002) Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes 51:2369–2376
Wang BW, Hung HF, Chang H, Kuan P, Shyu KG (2007) Mechanical stretch enhances the expression of resistin gene in cultured cardiomyocytes via tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol 293:H2305–H2312
Scotece M, Conde J, Vuolteenaho K et al (2014) Adipokines as drug targets in joint and bone disease. Drug Discov Today 19:241–258
Vuolteenaho K, Leppänen T, Kekkonen R, Korpela R, Moilanen E (2014) Running a marathon induces changes in adipokine levels and in markers of cartilage degradation–novel role for resistin. PLoS One 9:e110481
Stengel SV, Kemmler W, Pintag R et al (1985) (2005) Power training is more effective than strength training for maintaining bone mineral density in postmenopausal women. J Appl Physiol 99:181–188
Acknowledgements
This work was supported by Tekes-National Technology Agency of Finland with University of Jyväskylä (Decision No. 70007/13), Juho Vainion Foundation, Jenny and Antti Wihuri Foundation, Yrjö Jahnsson foundation, the Department of Biology of Physical Activity, University of Jyväskylä, and the competitive research funding of Pirkanmaa Hospital District. The authors would like to thank the subjects and research assistants. For ELISA analyses, the excellent technical assistance of Terhi Salonen and Salla Hietakangas is warmly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was conducted according to the Declaration of Helsinki, and ethical approval for the study procedures was granted by the Ethical Committee at the University of Jyväskylä and by the Ethical Committee of the Central Hospital, Jyväskylä.
ᅟ
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the manuscript.
Rights and permissions
About this article
Cite this article
Ihalainen, J.K., Ahtiainen, J.P., Walker, S. et al. Resistance training status modifies inflammatory response to explosive and hypertrophic resistance exercise bouts. J Physiol Biochem 73, 595–604 (2017). https://doi.org/10.1007/s13105-017-0590-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-017-0590-0