Abstract
Obesity is a leading health problem facing the modern world; however, no effective therapy for this health issue has yet been developed. A promising research direction to identify novel therapies to prevent obesity has emerged from discoveries on development and function of brown/brite adipocytes in mammals. Importantly, there is evidence for the presence and function of active thermogenic brown adipocytes in both infants and adult humans. Several new investigations have shown that thermogenic adipocytes are beneficial to maintain glucose homeostasis, insulin sensitivity, and a healthy body fat content. Such thermogenic adipocytes have been considered as targets to develop a therapy for preventing obesity. This short review seeks to highlight recent findings on the development and function of brown/brite adipocytes in humans and to discuss potential treatments based on these adipocytes to reduce obesity and its related disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is known that brown adipose tissues (BAT) are present in newborn infants and that they function to maintain normal body temperature during the early stages of life [2, 9, 15]. However, these adipose tissues regress following the development they may have only a modest impact on nonshivering thermogenesis in adults. Although these brown adipocytes were known to exist in adult humans since 1985 [4], they had not attracted serious attention from scientists. This neglect changed following the publication of discoveries on the presence of functional thermogenic adipocytes in adult humans during the past 7 years [6, 7, 19, 26, 27]. Active UCP1-expressing adipocytes were found in both patients and healthy people at adulthood under various physiological conditions by positron emission tomography (PET) and X-ray computed tomography (CT) [6, 7, 19, 26, 27]. These findings indicate possible roles of thermogenic adipocytes in thermogenesis of not only infants but also adult humans and this has been supported by several key investigations in the field [7, 11, 16, 19, 21, 26–28] with the latest one being released by Min et al. 6 months ago [16].
Types of thermogenic adipocyte in humans
The origin, development, and types of thermogenic adipocytes in humans remain unclear. The presence of Ucp1 expressing adipocytes in brown (BAT) not in white adipose tissues (WAT) in either normal or cold exposure condition [26] leads them to be considered as classical brown adipocytes. Another experiment also showed that thermogenic adipocytes differentiated from progenitors of human neck adipose tissues shared similarities in gene expression profiling and function with classical interscapular brown adipose tissues (IBAT) in rodents [7]. However, deeper assessments on gene and protein expression of specific markers for brown, brite/beige, and white adipocytes in human BAT showed that classical brown adipocytes and recruitable brite adipocytes coexist in adult human brown fats [11, 15, 28]. The presence of brite adipocytes in human BAT is strongly supported by RNA sequencing and unbiased genome-wide expression analyses of thermogenic adipocytes differentiated from adult BAT stromal vascular fraction (BAT SVF) [21]; these studies showed that UCP1-positive human adipocytes presented molecular signatures of brite adipocytes [21]. These findings suggest that thermogenic adipocytes in adult humans have at least two types of cells, including classical and inducible brown adipocytes (brite/beige adipocytes); these adipocytes may have different origins and have distinct developmental processes as they do in rodents [9].
Developmental origin of human thermogenic adipocytes
In rodent, there is strong evidence that the two types of thermogenic adipocytes arise from different populations of precursor cells [1, 9, 18, 20, 22]. Classical brown adipocytes are developed from myf5 positive (myf5+) myotomal precursors as muscle cells [1, 17, 22], but brite adipocytes can derive from both myf5+ and negative (myf5-) lineages [17, 18, 20]. The development and thermogenic function of brown and/or brite adipocytes are triggered and regulated by several factors such as cold exposure, natriuretic peptides, thiazolidinediones, thyroid hormones, bone morphogenetic protein 7 (Bmp7), bone morphogenetic protein 8b (Bmp8b), irisin, and orexin fibroblast growth factor 21 (Fgf21) [9, 14].
In human, the most recent studies on the developmental origin of brite adipocytes were reported in early 2016 by Min and coworkers [16]. They found that human brite adipocytes developed from capillary networks of adipose depots [16]. In an in vitro system, cells of microvessels developed from adipose tissue fragments differentiated into thermogenic adipocytes in a response to pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and human fibroblast growth factor B (hFGF-B) [16]. This investigation suggests that there is a population of progenitors, located in capillary networks of human adipose tissues, which has the potential to develop into brite adipocytes under suitable stimulations. It also supports the notion for the existence of different stem cells which can differentiate into classical brown or brite adipocytes in adult humans [11, 15, 28].
Function and potential application of human thermogenic adipocytes
In vivo, human thermogenic adipocytes can be activated by cold exposure [19, 26, 27] as indicated by a 15-fold increase in glucose uptake in BAT of healthy subjects by cold [27]. A mild cold exposure (16 °C) also activated BAT in 96% of volunteers in an experiment, and this activity was significantly higher in lean vs obese subjects [27]. Additionally, the amount and activity of BAT were negatively correlated with body mass index (BMI) [6, 26]. Some known drugs, such as mirabegron—a beta 3-adrenergic receptor agonist—were also found to activate BAT and benefit metabolic homeostasis in adult humans [5]. Cold exposure increases circulating irisin and Fgf21, which further activate the thermogenic function of BAT in humans [14].
Furthermore, some key publications showed that NAD+/sirtuin (SIRT) pathway could regulate adipogenesis and function of thermogenic adipocytes [3, 8, 10, 12, 13]. In 2012, Cantó C et al. found that nicotinamide riboside (NR) increased NAD+ levels and activated SIRT signaling resulting in improving BAT oxidative function, energy expenditure, and reducing diet induce obesity (DIO) in mice [3]. In a mouse model of type 2 diabetes (T2D), NR also improved glucose tolerance and reduced weight gain [25]. These were supported by a work done by Kahn NA et al. in 2014; they reported that NR facilitated thermogenesis by induction of mitochondrial function [13]. Another group found that suppression of SIRT by MicroRNA 34a in obesity inhibited the formation of thermogenic adipocytes [8]. In 2015, Traba J and coworkers proved that NR could reduce obesity-induced inflammation and the risk of inflammation-related diseases [23]. Then, in the early of this month, one article was released reporting the first human clinical trial for NR; it showed that NR increased levels of a cell metabolite critical for cellular energy production without any serious side effects [24]. Thus, current data justifies testing of NR in obese and/or T2D patients.
Together, the above findings suggest that the activators of human BAT formation and activity are promising to be used as therapies to prevent obesity and overweight in humans.
In vitro, brown and brite adipocytes can be differentiated from SVF of human brown and white adipose tissues, and these primary thermogenic adipocytes are activated and function to burn lipid ex vivo in several experiments [7, 11, 16, 21]. Supplementation of basic adipogenic media with reagents such as dibutyryl–cyclic AMP (cAMP), forskolin, or norepinephrine induces and activates thermogenic adipocytes in vitro from precursor cells of adult human fat depots [7, 11, 21]. The activation and thermogenic function of these primary adipocytes indicate their possible use as a therapy to treat obesity and its related disorders in vivo. This goal has been further developed through the work of Min and colleagues [16] who found that activated brite adipocytes in vitro from SVF of human tissues maintain their thermogenic function in vivo. Importantly, the implantation of these human primary brite fat cells improves metabolic homeostasis of normal or diet-induced obesity immunodeficient mice [16].
Conclusion
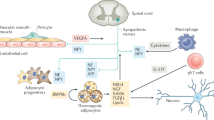
In conclusion, recent accumulating evidence clearly indicates that there are two types of thermogenic adipocytes in human beings; they are classical brown adipocytes and brite/beige adipocytes (Fig. 1). They are distinct adipocytes and may have different developmental origins, as described in rodents. In their current work Min et al. have pointed that brite adipocytes develop from progenitors of capillary networks (Fig. 1). Human brown and brite adipocytes are functional and can be activated by cold exposure or adrenergic receptor agonists. The thermogenic function of BAT is also triggered and increased by nicotinamide riboside, a pyridine-nucleoside form of vitamin B3 (Fig. 1). The pre-activated brite adipocytes in vitro show therapeutic results in vivo after being transplanted into mouse models of obesity (Fig. 1); this predicts a promising use of thermogenic adipocytes to prevent obesity and it related disorders in human.
The development, types, and therapeutic effects of human thermogenic adipocytes. Human thermogenic fat cells include classical brown and brite adipocytes which may develop from different progenitors. These brown and brite adipocytes contribute to the brown adipose content (BAT) of the human body. Under stimulation by cold, selective drugs or vitamins such as nicotinamide riboside (NR) human BAT is activated and burns lipid to improve metabolism, increase glucose homeostasis, and insulin sensitivity. Progenitors of human brite adipocytes come from capillary networks of adipose tissues. Pre-activated primary brite adipocytes in vitro could improve systemic glucose tolerance of normal or obese mice after being transplanted
References
Atit R et al (2006) β-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol 296(1):164–176
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84(1):277–359
Cantó C et al (2012) The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15(6):838–847
Cunningham S et al (1985) The characterization and energetic potential of brown adipose tissue in man. Clin Sci 69(3):343–348
Cypess AM, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21(1):33–38
Cypess AM et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360(15):1509–1517
Cypess AM et al (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19(5):635–639
Fu T et al (2014) MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol Cell Biol 34(22):4130–4142
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19(10):1252–1263
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13(4):225–238
Jespersen NZ et al (2013) A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 17(5):798–805
Jukarainen S et al (2015) Obesity is associated with low NAD+/SIRT pathway expression in adipose tissue of BMI-discordant monozygotic twins. The Journal of Clinical Endocrinology & Metabolism 101(1):275–283
Khan NA et al (2014) Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Molecular Medicine 6(6):721–731
Lee P et al (2014) Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 19(2):302–309
Lidell ME et al (2013) Evidence for two types of brown adipose tissue in humans. Nat Med 19(5):631–634
Min SY et al (2016) Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med 22(3):312–318
Peirce V, Carobbio S, Vidal-Puig A (2014) The different shades of fat. Nature 510(7503):76–83
Petrovic N et al (2010) Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285(10):7153–7164
Saito M et al (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58(7):1526–1531
Seale P et al (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454(7207):961–967
Shinoda K et al (2015) Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 21(4):389–394
Timmons JA et al (2007) Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci 104(11):4401–4406
Traba J et al Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J Clin Invest 125(12):4592–4600
Trammell SAJ et al (2016) Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7:12948
Trammell SAJ et al (2016) Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Scientific Reports 6:26933
Van Marken Lichtenbelt WD et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360(15):1500–1508
Virtanen KA et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360(15):1518–1525
Wu J et al (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150(2):366–376
Acknowledgments
We would like to thank Dr. Leslie P. Kozak (Maine Medical Center Research Institute, USA) for critical reading and helpful comments to improve this manuscript before submitting.
Dinh-Toi Chu is a current Marie Skłodowska-Curie postdoc under the Scientia Fellows programme cofunded by Faculty of Medicine, University of Oslo and the EU Seventh Framework Programme (FP7) under Marie S. Curie scheme—People: Cofunding of Regional, National and International Programmes (COFUND), grant agreement no. 609020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, DT., Tao, Y. Human thermogenic adipocytes: a reflection on types of adipocyte, developmental origin, and potential application. J Physiol Biochem 73, 1–4 (2017). https://doi.org/10.1007/s13105-016-0536-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0536-y