Abstract
Classically, two types of adipose tissue have been described: the white adipose tissue (WAT) and the brown adipose tissue (BAT), mainly composed by white and brown adipocytes, respectively. These adipocytes present different characteristics, while white adipocytes plays a role in energy storage by containing lipids in a single large lipid droplet, brown adipocytes activate adaptive thermogenesis controlling energy expenditure. In addition, recently the so called “brite” or “beige” adipose tissue has been found within certain WAT depots, and appear functionally similar to classical brown adipocytes. In this chapter we describe the main characteristics of BAT, by highlighting that makes it unique and different from WAT, including its localization in humans, origin and differentiation, physiology and molecular regulation. Moreover, we show its role in obesity and associated pathologies and how we can harness the anti-obesity potential for future therapeutic strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Obesity
- Brown adipose tissue
- Beige adipose tissue
- UCP-1
- Transdifferentiation
- Thermogenesis
- Irisin
- Cell therapy
Introduction
Adipose tissue is a connective tissue predominantly composed by adipocytes and is considered as a major endocrine organ. Classically it has been described the existence of two types of adipose tissue, the white adipose tissue (WAT), formed mainly by white adipocytes, and the brown adipose tissue (BAT), commonly composed by brown adipocytes. However, recently the so called “brite” or “beige” adipose tissue has been found within certain WAT depots, and appear functionally similar to classical brown adipocytes. BAT and WAT have different structure, composition and function. Adipose tissue has two types of depots, subcutaneous and visceral, and their respective amounts vary in relation to strain, age, gender, environmental and nutritional conditions [1–3].
BAT uniquely exists in mammals and presents a thermogenic function dissipating energy as heat [1]. Initially, scientific community thought that BAT was present only in newborns and children, but later, presence of BAT was discovered in adult humans exposed to cold or in pheochromocytoma where there is a hyper-adrenergic stimulation [4]. The BAT tissue was found in adult humans trying to identify metastatic cancers with 18F-fluorodeoxyglucose (FDG), an intravenously administered radioactive glucose analogue, in combination with positron emission tomography (FDG-PET). Afterward, its composition was determined by combining FDG with computed tomography (FDG/CT) [5, 6].
During the neonatal period, BAT plays an important thermogenic function helping to counteract the cold stress of birth [7, 8]. In adult mammals, it has been observed that BAT not only maintains the temperature homeostasis of the body to acute or chronic cold exposure, but also in the heat production to maintain an equilibrium between the food intake and energy expenditure [1, 9]. This provides a protective mechanism against energy overload [10–12].
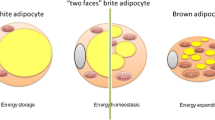
BAT characteristics are related with the functions performed: (i) it is highly vascularised and innervated in comparison with WAT, and (ii) it is composed of brown adipocytes which differ from white adipocytes in several features. White adipocytes present a compressed nucleus by lipids organized in a single large lipid droplet while brown adipocytes lipids have a roughly round nucleus organized as multiple small droplets. Moreover, mitochondria are large, numerous and are endowed with laminar cristae in brown adipocytes, whereas in white adipocytes mitochondrias are small and elongated, with randomly oriented cristae [3, 13, 14] (Fig. 2.1a).
Brown adipocytes express a specific mitochondrial protein, the uncoupling protein 1 (UCP1). This mitochondrial uncoupling protein transforms chemical energy into heat through uncoupling oxidative phosphorylation from ATP synthesis and it is considered as the molecular marker of BAT [1, 15]. The activation of brown adipocytes and subsequent activation of the UCP1 is through the control of the hypothalamus, which drives the release of norepinephrine (NE) by the sympathetic nervous system (SNS) that innervates BAT (Fig. 2.1b). This process leads to the hydrolysis of the triglycerides (TG) stored in the lipid droplets, and the released fatty acids activate UCP1 [9, 13].
Recently, it was discovered that white adipocytes can transdifferentiate into brown adipocytes, also known as brite (brown in white) or beige adipocytes. This process occurs in response to exposure of cold and β3-adrenergic receptors (AR) agonist stimulation and/or from the differentiation and maturation of white preadipocyte precursors [16]. When brite adipocytes are activated present many biochemical and morphological characteristics of BAT such as multilocular morphology and most notably the presence of UCP1 [2, 9]. Brite adipocytes could have a dual function, can acts as white adipocytes and store lipids, or can behave as brown adipocytes and dissipate energy when initiated by either cold exposure, stimulatory metabolic hormones, pharmacologic activator or sympathetic stimuli [17]. In fact, it has been reported that UCP1+ adipocytes could appear in WAT of mice in response to cold exposure, or different stimuli such as administration of PPARγ agonist, exposure to cardiac natriuretic peptides, FGF21, irisin or treatment with β-AR agonist [18–23].
BAT activity has an effect on metabolic disorders, reducing obesity and the associated risk of developing diabetes [13]. Anti-obesity effects of BAT have been demonstrated in experiments of genetic inactivation or upregulating UCP1 expression in murine models, and consequently these therapeutic effects were also evident in obesity related disorders, such as Type 2 diabetes [14]. Likewise, brite adipocytes have been shown to have anti-obesity and anti-diabetic activities in rodent models [17].
In this chapter, we present the main characteristics of BAT, by highlighting that makes it unique and different from WAT, including its localization in humans, origin and differentiation, physiology and molecular regulation. Moreover, we show its role in obesity and associated pathologies and how we can harness the anti-obesity potential for future therapeutic strategies.
Anatomical Locations of BAT in Humans
Human newborns and children have large deposits of BAT, whereas adults suffer an involution of this tissue, and its presence and activity are more restricted [4]. The major locations of subcutaneous BAT include depots in interscapular, paraspinal, supraclavicular and axillary sites [5, 13]. Also, we can find depots in the anterior abdominal wall and in the inguinal area [5]. In children, the interscapular, paraspinal and supraclavicular BAT accumulations are higher than in adults [15]. Furthermore, BAT has visceral localizations that include perivascular (aorta, common carotid artery, brachiocephalic artery, paracardial mediastinal fat, epicardial coronary artery and cardiac veins, internal mammary artery, and intercostal artery and vein), periviscus (heart, trachea, major bronchi at lung hilum, oesophagus, greater omentum and transverse mesocolon) and around solid organs (pancreas, kidney, adrenal, liver and hilum of spleen) [5].
The distribution of BAT depots is similar in men and women, but its mass and activity are higher in women. In fact, BAT was more prominent in cervical and supraclavicular zone in woman than in men at ratio 2:1 as detected by FDG-PET/CT [4]. Moreover, the reduction of BAT mass with age is more rapidly in males, while moderately declines in women [24]. Rodriguez-Cuenca et al. correlated the sexual dimorphism in rats with differences in lipolytic and thermogenic adrenergic pathway activation and suggest that these differences in adrenergic control could be responsible for the higher mitochondrial recruitment with a higher cristae density in female rats [25].
One external factor, the cold, appears to be associated with a higher mass of BAT. Studies of biopsy specimens in northern Finland revealed more BAT around the neck arteries in outdoor workers than in indoor workers [26]. This result is in concordance with the correlation observed between the prevalence of detectable BAT and outdoor temperature [4].
Regarding to relationship of age and BAT, BAT develops from the fifth gestational week, reaches maximum expression around birth, and in the past it has been thought that declines over the next 9 months of the birth [27]. However, recently it has been described the presence of brown adipocytes in classical subcutaneous localization and in WAT depots in non-obese children up to 10 years of age [28]. The involution of BAT with age could be related to heat production for the maintenance of body temperature, since the increase of body involves a decrease in surface/volume ratio and a decreased requirement of BAT for heat production [1, 9].
Origin and Differentiation
Despite the fact it was previously considered that brown adipocytes come from the same progenitor cell that white adipose cells, the lineage analysis revealed that their embryological origin is different. It has been determined that BAT precursor cells express myogenic factor 5, (Myf5+), which is also found in myoblasts, suggesting that BAT precursors develop from a progenitor close to skeletal muscle cells [29–31] (Fig. 2.2).
Origen of adipocytes: from mesenchymal stem cells up to adipocyte progenitors. Myf5 progenitor cells give rise to brown adipocytes and to skeletal muscle. Despite Myf5 negative progenitors are common precursors for both beige and white adipocytes, recent studies suggest that white adipocytes can also derive from Myf5 +. Beige adipocytes can derive from the transdifferentiation of white adipocyte or from beige preadipocyte. Like brown adipocytes, beige adipocytes can express UCP1
It is known that brown adipocytes initially arise in the fetus and form discrete depots in the interscapular and perirenal BAT and it is thought that they come from dermatomal precursors (Fig. 2.2) [8, 32]. In contrast, little is known about the developmental origin of “beige” adipocytes. The mRNA levels of general adipocyte markers as well as typical brown markers were very similar in classical brown and brite adipocytes populations [33]. However, it has been reported that there are different gene expression signatures to distinguish classical brown from brite adipocytes suggesting a different cell lineage from classical brown cells [17, 20]. Genes related with the presence of brown adipocytes include Myf5, PRDM16, BMP7, BMP4, and Zic1, while transmembrane protein 26 (Tmem26), CD137, and T-box 1 (Tbx1) are considered unique markers expressed by beige cells [17].
During the fetal life, brown adipocyte differentiation involves a cascade of transcriptional factor interactions similar to white adipocytes. The transcription factors peroxisome proliferator-activated receptor gamma (PPARγ) and the CCAAT/enhancer-binding proteins (C/EBP) family members (i.e. C/EBPα, C/EBPβ, and C/EBPδ) are the main key players which form part of the transcriptional cascade and direct differentiation of both brown and white adipocytes. However, during the development of brown adipose tissue an increase in expression of C/EBPβ and C/EBPδ comes before C/EBPα activation, then, PPARγ and C/EBPα coordinate the expression of many adipocyte genes to induce adipocyte differentiation [34].
It has been described that depending on the expression of the transcriptional positive regulatory domain containing 16 (PRDM16) cell fate switch between skeletal myoblasts and brown adipocytes. In fact, in the myogenic precursors the expression of PPARγ is induced by the PRDM16-C/EBP-β complex resulting in the activation of the brown adipogenic gene program [8]. Other transcriptional regulator of brown adipocytes is the master regulator of mitochondrial biogenesis, PPARγ coactivator-1α (PGC-1α) which is essential in brown adipogenesis since it stimulates UCP1 expression. In fact, UCP1 is responsible for the rapid generation of large amounts of heat at birth [35]. In addition, concentration gradients of certain morphogens and other secreted signals are implicated in the formation of brown adipocytes and may regulate their developmental patterning during embryogenesis. The morphogenic signals implicated includes Wnt- (named after the Wingless and INT proteins in Drosophila), the bone morphogenetic protein- (BMP), the fibroblast growth factor (FGF), and the Hedgehog-signaling pathways [36]. For instance, recently it has been shown that BMP7 and BMP4 induce the formation of brown adipocytes [37]. Similarly to BMPs, β-AR signaling is considered important for the development of brown and beige cells. Thus, adrenergic stimulation of β1-AR induces preadipocyte proliferation [38].
Concerning the developmental origin of beige cells several hypotheses have emerged: (i) One of them postulates that beige adipocytes came from the direct transformation or transdifferentiation of existing white adipocytes (Fig. 2.2). This approach is based on the observation of an increase in brown adipocytes but not in preadipocytes upon cold acclimatization [33, 39]. In addition, these cells also had a mixed mitochondrioma with classic brown and white mitochondria, suggesting an intermediate state between mature white adipocytes and brown adipocytes [39]. However, as only a subset of apparently white adipocytes is capable of turning into brite adipocytes upon cold adaptation, it is not well understood whether it is due to different white adipocyte populations or due to different microenvironments [40]. (ii) Others studies found that Myf5 precursors are not the exclusive source of brown adipocytes and contribute more to the mature white and brite adipocyte populations than previously thought [41, 42]. Recently, Sanchez-Gurmaches and Guertin quantified the Myf5 lineage contribution to the mature brown and white adipocyte population, proposing that brown, white, and brite adipocytes originate from multiple developmental lineages that are distributed heterogeneously in depot-specific patterns. They found that Myf5 lineage distribution in adipose tissue changes in response to modifiable and non-modifiable factors suggesting that adipocyte lineages may selectively expand in response to certain factors. They also observed that adipocyte lineages can compensate for each other indicating that lineage plasticity exists and that the degree of plasticity varies depending upon the depot [42]. For example, it has been described that the epididymal depot remains purely white fat in comparison to inguinal adipose tissue capable of transdifferentiation [40]. These results show a high degree of heterogeneity of adipogenic cells suggesting the relevance of physiological local signals that regulate their fate and that need to be determined.
Regulation of BAT Thermogenesis
BAT possesses a number of specialized features enabling it to function as a thermoregulatory organ. Apart from lipids stores in multilocular lipid droplets, brown adipocytes display more abundant mitochondria enriched in UCP1, which is located in the inner mitochondrial membrane. This UCP1 uncouples substrate oxidation from ATP production and as result heat is produced [1]. High vascularisation of BAT allows sufficient substrate and oxygen supply as well as the efficient distribution of heat within the body.
The thermogenic activity is primarily regulated by the SNS, reflected by the strong innervation of BAT by sympathetic nerve fibers and the high density of β-AR that are responsible for its activation. In the case of cold stimulus, input from the skin sensitive nerves are received and transmitted to a center for the integration of the thermal information. Likewise, the energy status is provided by inputs from the arterial chemoreceptor in sensory neurons about a variety of metabolic signals such as fuel substrate and oxygen [10–12, 43, 44]. Both cold stimulus and energy status regulate BAT activation through sympathetic neurons. Although this is the main way to control BAT activity, hormones (thyroid hormone triiodothyronine), cytokines, and other circulating factors also are involved.
Cold sensation triggers to stimulation of the hypothalamus which activates sympathetic nerve that highly innervates BAT. This activation causes the release of NE neurotransmissor that binds to β-ARs, which couple to Gα G-proteins activating downstream cAMP-PKA signalling that ends by increasing thermoregulatory gene expression, like UCP, and his activation, mitochondrial biogenesis and lipolysis (Fig. 2.3). This metabolic process is accomplished by adipose triglyceride lipase (ATGL) activation that hydrolyzes TG stored in the lipid droplet to free fatty acids (FFAs), which undergo β-oxidation [1, 45]. Thereby, respiratory chain proteins from the mitochondrial internal membrane generate a proton electrochemical gradient between the mitochondrial matrix and the intermembrane space, but the presence of UCP1 mediates the re-entry of protons into the mitochondria and dissipates energy as heat instead of producing adenosine triphosphate (ADP) (Fig. 2.3) [46, 47]. In addition, apart from cAMP-PKA signalling, cGMP–PKG pathway shares the same final target. This pathway is activated by released cardiac natriuretic peptides in response to physical exercise or cold exporure, which can induce lipolysis and the expression of thermogenic genes [48, 49].
Molecular machinery of brown adipocytes activation. The sympathetic nervous system triggers intracellular signalling events that lead to increased expression of UCP1 and other thermogenic genes, mitochondrial biogenesis and lipolysis. FFAs released by this process is followed by his oxidation that finally produces heat
BAT thermogenesis requires the consumption of energy stores, initially those present in the BAT lipid droplets and, with extended BAT activation, those derived from catabolism of WAT, which provide FFAs to locally activate UCP-1 and to serve as substrates for oxidation [1, 50]. The substrate uptake machinery is also upregulated allowing to lipids released by WAT to be taken up by BAT [1]. Whether there is enough activation that allows high TG metabolization, it would have an effect on body weight [51]. Apart from this cascade of intracellular signalling activation by central nervous system, chronic cold exposure will also induces proliferation and differentiation of brown adipocyte precursors, thus increasing thermogenic capacity [1].
BAT, Obesity and Obesity-Related Diseases
Obesity no longer refers only to being overweight. The World Health Organization (WHO) has officially recognized obesity as a chronic disease and it is defined as an accumulation of adipose tissue that is of sufficient magnitude to impair health (WHO, 2014).
Obesity is linked to health risks and can lead to various metabolic disorders, such as Type II diabetes, cardiovascular disease, hypertension and certain cancers. The fundamental cause of obesity is an energy imbalance between energy input and output. Generally, this may be due to the increased intake of energy-dense foods and decreased physical activity [52].
Obesity, well known to be associated with a number of co-morbidities, including insulin resistance and Type 2 diabetes, has become a major public health problem in recent decades reaching epidemic proportions, not only in high-income countries, but also in most middle-income societies. Excess weight is usually defined by the body mass index or BMI. The normal BMI range is 18.5–25 kg/m2, although the range may vary for different countries. Individuals with a BMI above 30 kg/m2 are classified as obese; those with a BMI between 25 and 30 kg/m2 are considered to be overweight. In general, the term obesity applies to both the obese and the overweight subjects.
WAT is the dominant type of adipose tissue distributed throughout the human body. It functions primarily to store excess energy in the form of TGs. As a person’s weight increases, WAT is expanded by both increased adipocyte size (hypertrophy) and increased adipocyte numbers (hyperplasia) [52]. More than the total body weight, the distribution of the stored fat is of importance for the development of obesity and its co-morbidities. Thus, central or visceral obesity, in which fat accumulates in the trunk and in the abdominal cavity (in the mesentery and around the viscera), is associated with a much higher risk for several diseases than excess subcutaneous fat accumulation. Obesity has profound effects on tissue insulin sensitivity, and therefore on systemic glucose homeostasis [53].
Distribution areas of fat depots in the body play contrasting healthy and pathological metabolic roles. Thus, BAT has beneficial effects, subcutaneous WAT (SAT) especially gluteofemoral, appears metabolically protective, and visceral WAT, together with intra-abdominal tissue lipid, are considered potentially harmful. Variation in these different lipid depots may therefore impact significantly on metabolic health. In fact, it has been demonstrated that humans who remain insulin-sensitive despite being obese have lower amounts of intra-abdominal fat compared to insulin-resistant obese humans. There are several suggestions as to the means by which intra-abdominal fat creates its adverse effects: (i) lability of lipolysis with direct drainage of fatty acids to the liver via the portal vein; (ii) the excessive production of inflammatory molecules from immune cells whose numbers may be higher in visceral fat than other fat depots in lean and obese humans; (iii) as possible mediator of inflammation in liver and kidney [54, 55]. Also, it is possible that visceral fat is the source of an as-yet-undiscovered adipokine(s) with adverse systemic effects, including inhibition of adiponectin secretion, which is strongly linked to visceral fat volume [56].
Because BAT was recently discovered in adult humans and is correlated inversely with obesity, it has gained a considerable amount of attention with regard to efforts to overcome obesity by burning excess energy. Recent studies suggest an inverse correlation between BAT activity and BMI as well as between BAT activity and percentage of body fat [4]. Moreover, increased BAT metabolism may significantly contribute to energy expenditure during acute cold exposure in humans [57]. Therefore, adults also have metabolically active BAT that may play an important role in energy homeostasis, and it can be induced to increase glucose uptake [58]. In fact, characteristic genes of human BAT have been shown to negatively correlate with obesity and insulin sensitivity [59].
Metabolic syndrome is a disorder that includes numerous diseases or risk factors such as impaired glucose (diabetes), low HDL-cholesterol, increased production of TGs, high blood pressure and abdominal obesity, factors that all are associated with obesity. Thereby, dysfunctional adipose tissue with low-grade, chronic and systemic inflammation links the metabolic and vascular pathogenesis including dyslipidemia, low-grade inflammation and insulin resistance. This dysfunctional adipose tissue is a hallmark of disorders such as Type 2 diabetes and cardiovascular disease. In addition, factors such as lifestyle and genetic predisposition are also implicated [59].
Often BAT is associated with improving benefits in obesity in adult humans, but there are some diseases or conditions where BAT plays an antagonist role and might not be beneficial to health (Fig. 2.4). Some of these cases are:
Cancer Cachexia Syndrome (CCS)
It is a progressive metabolic syndrome clinically characterized by profound weight loss, fat depletion, skeletal muscle wasting, and asthenia that are not solely attributable to inadequate nutritional intake. Until the present, there is not evidences about the molecular mechanisms implicated in the clinical manifestations of the disease. Inasmuch as regions of BAT are not only lipid depots but has a significant role in regulating energy balance and fat accumulation in rodents and humans, its involvement in hypermetabolic diseases such as cancer cachexia has been suggested [60]. The activation of BAT results in a hypermetabolic state that is partially responsible for weight loss in cancer patients.
Animal studies of cancer cachexia models show some degree of BAT activation, but they do not establish the quantitative impact on the amount of hypermetabolism that is relevant to the development of cachexia [61]. Energy homeostasis in metabolic organs is controlled by central and peripheral circadian clocks through tight regulation of expression and activity of enzymes involved in metabolic pathways. In fact, aberrant circadian rhythms provoke hyperphagia, obesity and metabolic syndrome [62]. Recently, Tsoli et al. showed BAT activation in cachectic mice bearing tumours that cannot be attributed to the effects of reduced food intake or inability to maintain core body temperature. Moreover, they demonstrated a disruption of diurnal regulation of the transcription factor that control lipid homeostasis and thermogenesis in BAT. Finally, they described the role of cytokine signalling in BAT for tumour-induced systemic inflammation [60].
In human, autopsy samples have shown increased BAT in periadrenal tissues of cachectic cancer patients. BAT was observed in 80 % of the cancer patients compared with 13 % of the age-matched patients who died from other illnesses without cancer or cachexia [63]). Moreover, in some type of tumors it has been shown a potential correlation between BAT hyperactivity and body weight loss in cachexia in humans. For example, in hibernoma, a rare soft tissue benign tumor composed of brown fat cells, has been described a massive weight loss as a primary symptom [64]. Also, in pheochromocytoma an abundant BAT hyperactivity can also be seen on F-FDG PET scanning as a result of chronic stimulation of the SNS by high levels of circulating catecholamines [18]. In one case, after removing the pheochromocytoma, and thus the excess of NE, the intense uptake of FFDG in BAT was no longer seen, presumably reflecting an involution of BAT [65, 66]. Other studies addressed a possible relationship of BAT F-FDG activity with cancer; however, none of these observed a higher incidence of BAT activity in active and/or PET-positive cancer patients [18]. BAT activity was generally found in about 50 % of cases, either with or without active malignancy [63].
Thus, animal and human data in cancer cachexia indicate that BAT activation occurs, but its quantitative contribution to any alteration in energy expenditure and, thus, on the degree of cachexia remains controversial. Careful consideration of factors co-acting on BAT recruitment and activity, such as diet, cold exposure, physical activity, insulin levels and BMI are necessaries to understand the real role of BAT in cachectic cancer patients. Therefore, further studies using PET should focus in the quantitative significance of BAT activity for increased energy expenditure and body weight loss in humans.
Multiple Symmetric Lipomatosis (Madelung’s Disease/Syndrome or Launois Bensaude Syndrome) (MSL)
It is a rare syndrome originally described in 1846, characterized by the painless and symmetrical accumulation of abnormal tumour-like SAT, mostly affecting heavy drinking men. Individuals with MSL have increased SAT, either as discrete non-encapsulated lipomas or as a confluent increase in SAT in a symmetrical distribution on the neck, the back, mediastinum, upper arms or on the thighs. MSL usually spares the distal limbs but not in many women with MSL where the altered fat may be global. Moreover, this syndrome is accompanied by the presence of a somatic and autonomic neuropathies and alcohol-induced liver disease [67]. There is not inherited demonstrated about MSL but is thought that mitochondrial mutations would be related. In addition, the phenotype of MSL may require a combined effect of alcohol and a currently unknown genetic mutation.
The localization of lipomatous masses suggests that MSL lipomas could originate from BAT. It is believed that MSL SAT is derived from BAT or WAT that transdifferentiates into BAT. Adipocytes in MSL SAT are monovacuolar or multivacuolar. Moreover, stem and immune polymorphic cells, present in the stroma vascular fraction, contain thin microfilaments suggestive of elevated metabolic activity, are multivacuolar, and with large mitochondria packed with cristae suggesting a more BAT phenotype. The ultrastructure of these cells can be described as similar to that recently reported for the pauci-locular adipocytes, considered intermediate stage of transdifferentiating adipocytes, i.e. cells with intermediated morphology between white and brown adipose fat cells. The bidirectional switch between brown fat cells (and not white fat cells) and skeletal myoblasts controlled by PRMD16-C/EBP-β transcriptional complex suggest a transdifferentiation process rather than a lipomatous adipocytes infiltration.
The hypothesis that brown fat cells of MSL could arise from a common skeletal muscle and brown adipose cell precursors has been proposed. An alternative explanation is that adipocyte precursors residing within the muscle or de novo differentiation could be the sources of the adipocyte infiltration [39]. In addition, SAT cells from subjects with MSL express UCP-1 suggesting its origin as BAT [67].
The increase in MSL fat is extensive and deforming, compressing tissue structures and vessels. Early, MSL SAT is watery but later becomes fibrotic and scars easily. Similarly to obesity, fat excess physically impedes collection and flow lymph. Therefore, protein-rich lymphatic fluid collects in SAT, resulting in lymphedema and tissue hypoxia. Further accumulation of fluid in the setting of decreased oxygen tension leads to fibrosis. Congestion of lymph nodes by other means, such as lymphoma in the neck, induces fat growth similar to MSL [67]. All data indicate that the pathogenesis of MSL lipomatous fat deposits may originate from functionally defective BAT, accumulating an excess of lip. In fact, these findings are consistent with the hypothesis that MSL is a neoplastic disease originating from brown adipose cells as the result of a disorder in the proliferation and differentiation of human BAT cells.
Huntington’s Disease (HD)
It is an adult-onset dominantly heritable neurodegenerative disorder with a prominent energy deficit phenotype. It is caused by the expansion of a CAG repeat in the gene encoding the protein huntingtin, leading to expression of mutant huntingtin with expanded polyglutamine repeats. The huntingtin protein was recently shown to play a role linking the glycolytic enzyme GAPDH to vesicles, to supply energy from glycolysis for fast axonal transport. Both, a gain-of-function (for mutant huntingtin) and a loss-of-function (for normal huntingtin) hypothesis have been put forward to explain HD pathogenesis. HD is characterized by progressive motor impairment, personality changes, psychiatric illness and gradual intellectual decline [68].
Transcriptional deregulation, protein aggregation, mitochondrial dysfunction and enhanced oxidative stress have been implicated in the disease pathogenesis. A key feature of HD patients is pronounced weight loss, despite sustained caloric intake. Deficits in energy expenditure have been linked with mitochondrial dysfunction in HD [69]. PGC-1 family of co-activators is an extensively regulated group of proteins that are highly responsive to a variety of environmental cues, from temperature to nutritional status, to physical activity. Impaired PGC-1α expression and/or function has emerged as a common underlying cause of mitochondrial dysfunction in HD [70]. Involvement of PGC-1α in HD was first suggested by the findings that PGC-1α knockout mice exhibit mitochondrial dysfunction, defective bioenergetics, a hyperkinetic movement disorder and striatal degeneration, which are features also observed in HD. PGC-1α, which was initially identified as a PPARγ-interacting protein from brown fat, plays an important role in induction of UCP1 [69]. Two studies using transgenic mouse models of HD demonstrated that mice had hypothermia and significant reductions in body temperature during cold challenge and found that UCP-1 mRNA up-regulation was severely blunted. Moreover, BAT from these transgenic mice showed abnormal lipid-containing vacuoles [9, 71].
Role of Bat in Obesity Treatment
The epidemic of obesity is widely recognized as a major public health problem, given the worldwide increasing prevalence over the last decades. Generally, obesity develops upon chronic imbalance between energy intake and energy expenditure. Changes in lifestyle, basically the reduction of food intake and increased physical activity, are considered to be key elements of obesity treatment. However, most people fail to substantially and sustainably reduce body weight by basic behavioral changes, in particular due to potent compensatory mechanisms promoting re-gain of body weight [1].
One exciting prospect in this area is to increase the amount and/or activity of brown or beige fat. Animals with high BAT and/or beige/brite abundance are protected against obesity, diabetes, hepatic steatosis, and hyperlipidemia. Therefore, BAT and/or beige/brite fat expanding strategies would have good therapeutic results in the fight against obesity and related disorders in humans [55].
Pharmacological Strategies
There are some obesity therapies ranging from weight loss-promoting drugs to surgical interventions, in cases of extreme obesity after failure of conventional treatments, but only have limited success. Since it has been determined that, in addition to thermogenic function, BAT acts as a crucial regulator in energy metabolism due to its high oxidative capacity, the idea of inducing weight loss by pharmacological targeting of thermogenic adipose tissues has been revived. In rodent models, pharmacological induction of BAT function has been shown to be beneficial in counteracting obesity using indirect sympathomimetics such as β3-AR agonists (for example, ephedrine and sibutramine). However, in humans are ineffective not only because β3-AR are expressed at low levels in adipocytes but also they produces adverse effects due to the broad and nonspecific action of adrenergic stimulation, particularly the heart increasing cardiovascular complications and stroke events. In this regard, non-canonical thermogenic stimulators, which work independently of adrenergic receptors, could help increasing BAT activity without causing adverse outcomes or patient discomfort [72].
Irisin and BMP7
Non-SNS therapeutics based on newly discovered fat browning and/or BAT-activating cytokines have strong potential. FGF21 and irisin are particularly relevant as they are potent endocrine human BAT activators that are stimulated by cold exposure in adults. It is important to point out that in all of these studies it is not possible to exclude non-cell autonomous effects for all of these perturbations including a central effect to repress food intake [55].
The hormone-like myokine termed irisin has recently been described that induces the browning of adipose tissue and BAT activation. As this molecule was originally reported to be released after physical activity, it gained huge interest as a potential mediator of the health-promoting effects of physical exercise. Irisin is a 112 amino acid peptide cleaved from fibronectin type III domain containing protein 5 (FNDC5), a type I membrane protein which was claimed to be upregulated by exercise training in both mice and humans [73]. In addition, a moderate increase in circulating irisin levels by three folds augmented energy expenditure, reduced the body weight gain under high-fat diet, and improved diet- induced insulin resistance. These results suggested a potential protective role of irisin in the development of Type 2 diabetes, one of the major obesity- associated metabolic diseases [74].
As irisin has initially been described to protect against diet-induced weight gain, mediated by browning of WAT and thus increased energy expenditure, many studies have investigated the correlation of circulating irisin with obesity in humans. In line with the suggested protective role of the myokine irisin against obesity, negative correlations of circulating irisin levels with the BMI have been reported in humans [75]. However, controversy exists regarding the relation between irisin levels and the BMI. Several studies reported a positive correlation of serum irisin levels with BMI while others could not detect a change in circulating irisin in obesity [76, 77]. This could be related to different populations analyzed in the different studies, as some include obese subjects without metabolic disorders whereas others enclose obese patients with metabolic diseases such as type 2 diabetes [74].
Since current data obtained from human reveal that FNDC5/irisin has no a real effect on browning of WAT depots, other alternative therapeutic strategies could be the use of inducers of BAT differentiation such as BMP7. A recent study demonstrated that in high-fat diet fed lean mice BMP7 mediated recruitment and sympathetic activation of BAT at subthermoneutral temperature. BMP7-treated mice diminished WAT mass, increased genes related to intracellular lipolysis and browning of WAT [16].
Cold-Induced Energy Expenditure
On the other hand, an increase in BAT activity and cold-induced energy expenditure was also observed in response to acute cold exposure in subjects with low BAT activity, demonstrating the possible occurrence of BAT recruitment in humans. Very recently, chronic cold acclimation in human subjects was reported to increase the volume of metabolically active BAT, increasing its oxidative capacity and therefore, promoting cold-induced thermogenesis. Last studies are in keeping with data showing a physiological role of BAT in whole-body energy expenditure, glucose homeostasis and insulin sensitivity in humans during prolonged cold exposure [53].
Melatonin
The manipulation of photoperiods could be used to induce BAT formation. Melatonin (MEL) is naturally produced in the body in response to the perception of light and may play a valuable therapeutic role in the differentiation of adult stem cells (ASCs) into brown adipocytes. MEL is mostly secreted by the pineal gland, reaching the highest physiological levels at night, and its secretion is substantially greater in winter than in summer. MEL biosynthesis in the pineal gland declines with age and this decline has been correlated with increased visceral adipose tissue in small mammals [13]. The effects of MEL on obesity and metabolic disorders are encouraging given that it has been demonstrated that MEL restrains body weight gain without changing food intake [78]. We recently have shown the beneficial effects of melatonin on metabolic disorders using the Zücker diabetic fatty (ZDF) rat model. This active component has shown amelioration of inflammation and oxidative stress related to an excess of WAT, an improvement of glucose homeostasis and beneficial effects on the lipid profile [79–81]. Finally, the potential of melatonin to promote BAT development in WAT has been demonstrated. Chronic oral administration induced browning of inguinal WAT and induced measurable amounts of UCP1 and stimulated ~2-folds the levels of PGC-1α in ZDF animals [82].
Cell-Based Therapies
BAT transplantation or cell-based therapies could be used to expand BAT. Human adipose-derived stem cells (hASCs) and inducible pluripotent stem (iPS) cells have been used to investigate and understand the signaling that determines progenitor fate and activity during BAT expansion and WAT browning. Extracellular environment parameters such as tissue vascularization, angiogenesis and innervation levels must be considered when decoding the integration of the signals that direct the behavior of progenitor [72].
Cell-therapy strategies to improve metabolic disorders are based on the implantation of brown adipocyte cells in the interscapular areas to regenerate BAT in humans. Injection of ASCs and preadipocytes from WAT formed BAT pads when implanted into the interscapular regions of mice [83, 84]. Moreover, adult progenitor cells can be induced to differentiate into BAT and are easily expanded in the laboratory for transplantation. Thereby, the transplantation of genetically manipulated hASCs with specific BAT transcription factors has been proposed to promote adipogenesis by inhibiting differentiation into unwanted cell lineages. The use of adenoviral vectors has been tested in animals for this purpose, although liposomes would be more suitable in humans because they may trigger a lower inflammatory response when releasing the RNA into the cell [13].
In humans, ASCs isolated from skeletal muscle have also been differentiated into brown adipocytes expressing UCP1 [85]. A recent study showed that local administration of BMP2 leads to the expansion, migration, and differentiation of progenitor cells from the peripheral nerve perineurium to brown adipose-like cells [86]. Furthermore, as already described in this review, MEL, a physiological molecule, can stimulate ASCs differentiation into BAT precursor cells, and it may also have the potential to recruit BAT and activate nonshivering thermogenesis. Taking together, these properties make ASCs an attractive cell candidate to be investigated for the regeneration of BAT.
Conclusion
Until recently, it was thought that the presence of BAT was restricted to newborns and child. Considerable amounts of BAT are present in adult humans and each time it becomes clearer its morphology, physiology, molecular regulation and origin. The involution of BAT with age could be related to heat production to maintenance body temperature, and the distribution in adults along the vasculature and around critical organs are related with the maintenance of vital functions in cold environments. This fact is supported by the BAT increase in people exposed to cold environments, as well as BAT deposits in WAT, and the process which permits transform white adipocytes in brown adipocytes.
BAT and obesity are closely related based in the anti-obesity effect of this tissue. In fact, the increased presence and activity of BAT is limited to thin people. However, there are some diseases or conditions where BAT plays an antagonist role causing certain pathologies. Novel therapeutic strategies based in BAT regeneration, activation or white-to-brown adipocyte trandifferentiation are being studied to treat obesity and related disorders. Potential therapies are based on pharmacological strategies, hormones and transcription factors, cold-induced energy expenditure, the manipulation of photoperiods and cell-based therapies. The development of new treatments focused in BAT properties aimed at increasing energy expenditure and, therefore, to reduce WAT mass and restore body energy balance, suppose a challenge for researchers since a huge number of patients affected by obesity and associated metabolic disorders will be benefitted.
References
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359.
Lo KA, Sun L. Turning WAT into BAT: a review on regulators controlling the browning of white adipocytes. Biosci Rep. 2013;33(5):711–9.
Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis. 2006;16(8):569–74.
Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17.
Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62(6):1783–90.
Zafrir B. Brown adipose tissue: research milestones of a potential player in human energy balance and obesity. Horm Metab Res. 2013;45(11):774–85.
Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–63.
Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–7.
Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11(4):268–72.
Madden CJ. Glucoprivation in the ventrolateral medulla decreases brown adipose tissue sympathetic nerve activity by decreasing the activity of neurons in raphe pallidus. Am J Physiol Regul Integr Comp Physiol. 2012;302(2):R224–32.
Buchanan TA, Cane P, Eng CC, et al. Hypothermia is critical for survival during prolonged insulin-induced hypoglycemia in rats. Metabolism. 1991;40(3):330–4.
Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond). 1983;64(1):19–23.
Roman S, Agil A, Peran M, et al. Brown adipose tissue and novel therapeutic approaches to treat metabolic disorders. Transl Res. 2015;165(4):464–79.
Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11(4):253–6.
Enerbäck S. Brown adipose tissue in humans. Int J Obes. 2010;34:S43–6.
Boon MR, van den Berg SAA, Wang Y, et al. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS One. 2013;8(9):e74083.
Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27(3):234–50.
Cousin B, Cinti S, Morroni M, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103(Pt 4):931–42.
Guerra C, Koza RA, Yamashita H, et al. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102(2):412–20.
Petrovic N, Walden TB, Shabalina IG, et al. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocyt. J Biol Chem. 2010;285(10):7153–64.
Vernochet C, Peres SB, Davis KE, et al. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29(17):4714–28.
Collins S, Daniel KW, Petro AE, et al. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology. 1997;138(1):405–13.
Granneman JG, Li P, Zhu Z, et al. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289(4):E608–16.
Pfannenberg C, Werner MK, Ripkens S, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59(7):1789–93.
Rodriguez-Cuenca S, Pujol E, Justo R, et al. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem. 2002;277(45):42958–63.
Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46(4):339–45.
Symonds ME, Pope M, Sharkey D, et al. Adipose tissue and fetal programming. Diabetologia. 2012;55(6):1597–606.
Rockstroh D, Landgraf K, Wagner IV, et al. Direct evidence of brown adipocytes in different fat depots in children. PLoS One. 2015;10(2):e0117841.
Frühbeck G, Sesma P, Burrell MA. PRDM16: the interconvertible adipo-myocyte switch. Trends Cell Biol. 2009;19(4):141–6.
Gesta S, Tseng Y-H, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–56.
Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104(11):4401–6.
Ohno H, Shinoda K, Ohyama K. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504(7478):163–7.
Rosenwald M, Perdikari A, Rülicke T, et al. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15(6):659–67.
Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–96.
Manchado C, Yubero P, Viñas O, et al. CCAAT/enhancer-binding proteins alpha and beta in brown adipose tissue: evidence for a tissue-specific pattern of expression during development. Biochem J. 1994;302(Pt 3):695–700.
Schulz TJ, Tseng Y-H. Brown adipose tissue: development, metabolism and beyond. Biochem J. 2013;453(2):167–78.
Xue R, Wan Y, Zhang S, et al. Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. Am J Physiol Endocrinol Metab. 2014;306(4):E363–72.
Bronnikov G, Houstĕk J, Nedergaard J. Beta-adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via beta 1 but not via beta 3 adrenoceptors. J Biol Chem. 1992;267(3):2006–13.
Barbatelli G, Murano I, Madsen L, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298(6):E1244–53.
Rosenwald M, Wolfrum C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte. 2014;3(1):4–9.
Long JZ, Svensson KJ, Tsai L, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19(5):810–20.
Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099.
Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16(3):296–309.
Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432(2):197–216.
Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp Physiol. 2003;88(1):141–8.
Rousset S, Alves-Guerra M-C, Mozo J, et al. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53 Suppl 1:S130–5.
Klingenberg M. Uncoupling protein – a useful energy dissipator. J Bioenerg Biomembr. 1999;31(5):419–30.
Lafontan M, Moro C, Berlan M, et al. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19(4):130–7.
Bordicchia M, Liu D, Amri E-Z, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022–36.
Collins S, Surwit RS. The beta-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog Horm Res. 2001;56:309–28.
Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200–5.
Kim EY, Kim WK, Oh K-J, et al. Recent advances in proteomic studies of adipose tissues and adipocytes. Int J Mol Sci. 2015;16(3):4581–99.
Poher A-L, Altirriba J, Veyrat-Durebex C, et al. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol. 2015;6:4.
Hocking S, Samocha-Bonet D, Milner K-L, et al. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34(4):463–500.
Ma X, Lee P, Chisholm DJ, et al. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol (Lausanne). 2015;6:1.
Yatagai T, Nagasaka S, Taniguchi A, et al. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52(10):1274–8.
Ouellet V, Labbé SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122(2):545–52.
Saely CH, Geiger K, Drexel H. Brown versus white adipose tissue: a mini-review. Gerontology. 2012;58(1):15–23.
Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17(4):332–41.
Tsoli M, Moore M, Burg D, et al. Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res. 2012;72(17):4372–82.
Beijer E, Schoenmakers J, Vijgen G, et al. A role of active brown adipose tissue in cancer cachexia? Oncol Rev. 2012;6(1):88–94.
Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106(3):447–62.
De Vos-Geelen J, Fearon KCH, Schols AMW. The energy balance in cancer cachexia revisited. Curr Opin Clin Nutr Metab Care. 2014;17(6):509–14.
Essadel A, Bensaid Alaoui S, Mssrouri R, et al. Hibernoma: a rare case of massive weight loss. Ann Chir. 2002;127(3):215–7.
Wang Q, Zhang M, Ning G, et al. Brown adipose tissue in humans is activated by elevated plasma catecholamines levels and is inversely related to central obesity. PLoS One. 2011;6(6):e21006.
Yamaga LYI, Thom AF, Wagner J, et al. The effect of catecholamines on the glucose uptake in brown adipose tissue demonstrated by (18)F-FDG PET/CT in a patient with adrenal pheochromocytoma. Eur J Nucl Med Mol Imaging. 2008;35(2):446–7.
Herbst KL. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol Sin. 2012;33(2):155–72.
Johri A, Chandra A, Beal MF. PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radic Biol Med. 2013;62:37–46.
Johri A, Calingasan NY, Hennessey TM, et al. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Hum Mol Genet. 2012;21(5):1124–37.
Leone TC, Lehman JJ, Finck BN, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3(4):672–87.
Weydt P, Pineda VV, Torrence AE, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1α in Huntington’s disease neurodegeneration. Cell Metab. 2006;4(5):349–62.
Lee Y-H, Jung Y-S, Choi D. Recent advance in brown adipose physiology and its therapeutic potential. Exp Mol Med. Nature Publishing Group. 2014;46(2):e78.
Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–8.
Elsen M, Raschke S, Eckel J. Browning of white fat: does irisin play a role in humans? J Endocrinol. 2014;222(1):R25–38.
Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769–78.
Crujeiras AB, Pardo M, Arturo R-R, et al. Longitudinal variation of circulating irisin after an energy restriction-induced weight loss and following weight regain in obese men and women. Am J Hum Biol. 2014;26(2):198–207.
Kurdiova T, Balaz M, Vician M, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592(Pt 5):1091–107.
Wolden-Hanson T, Mitton DR, McCants RL, et al. Daily melatonin administration to middle-aged male rats suppresses body weight, intraabdominal adiposity, and plasma leptin and insulin independent of food intake and total body fat. Endocrinology. 2000;141(2):487–97.
Agil A, Reiter RJ, Jiménez-Aranda A, et al. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J Pineal Res. 2013;54(4):381–8.
Agil A, Rosado I, Ruiz R, et al. Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J Pineal Res. 2012;52(2):203–10.
Agil A, Navarro-Alarcón M, Ruiz R, et al. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J Pineal Res. 2011;50(2):207–12.
Jiménez-Aranda A, Fernández-Vázquez G, Campos D, et al. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J Pineal Res. 2013;55(4):416–23.
Rieck B, Schlaak S. In vivo tracking of rat preadipocytes after autologous transplantation. Ann Plast Surg. 2003;51(3):294–300.
Yagi K, Kondo D, Okazaki Y, et al. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321(4):967–74.
Joe AWB, Yi L, Even Y, et al. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27(10):2563–70.
Salisbury EA, Lazard ZW, Ubogu EE, et al. Transient brown adipocyte-like cells derive from peripheral nerve progenitors in response to bone morphogenetic protein 2. Stem Cells Transl Med. 2012;1(12):874–85.
Acknowledgement
This work was supported by the Consejería de Economía, Innovación y Ciencia (Junta de Andalucía, excellence project number CTS-6568). We acknowledge the Junta de Andalucía for providing a fellowship granted to GJ and a post-doctoral fellowship to ELR.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jiménez, G., López-Ruiz, E., Griñán-Lisón, C., Antich, C., Marchal, J.A. (2016). Brown Adipose Tissue and Obesity. In: Ahmad, S., Imam, S. (eds) Obesity. Springer, Cham. https://doi.org/10.1007/978-3-319-19821-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-19821-7_2
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-19820-0
Online ISBN: 978-3-319-19821-7
eBook Packages: MedicineMedicine (R0)