Abstract
Ischemia/reperfusion (I/R) injury is associated with a strong inflammatory and oxidative stress response to hypoxia and reperfusion that impair organ function. We aimed to investigate the role of oxidative stress, renal inflammation, and apoptosis in the injury of the kidney tissue after ischemic reperfusion, and the protective effect of dose-dependent boric acid administration. For this purpose, 35 Sprague Dawley albino rats were divided into five groups of seven animals in each group: Sham, I/R and I/R + boric acid (BA) (i.p at doses of 50, 100, and 200 mg/kg). All animals underwent nephrectomy (the right kidney was removed) and were expected to recover for 15 days. After recovery, each animal received 45 min of ischemia. BA was injected intraperitoneally 10 min before reperfusion and a 24-h reperfusion procedure was performed. Sham group only underwent surgical stress procedure. In order to investigate the oxidative stress induced by I/R injury and antioxidant effects of different BA doses in the kidney tissue, TAS, TOS, MDA, SOD, CAT, and GSH levels were measured. DNA fragmentation, cytochrome C levels, caspase 3 activity were measured to determine apoptotic index in tissue. IL-6 and TNF-α levels were measured in the evaluation of inflammation. Hematoxylin-eosin and TUNEL staining was performed for histopathological examinations. As a result, increased oxidative stress, inflammation, and apoptosis after I/R were decreased with different doses of BA treatment. The application of high-dose BA was found to be lower in anti-apoptotic, anti-inflammatory, and antioxidant effects than in the low-dose groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal ischemia/reperfusion injury (I/R) causes acute kidney injury (AKI) and leads to high mortality rates in clinical and experimental studies [1,2,3]. Ischemia is a condition in which the blood supply to an organ for a variety of reasons becomes insufficient or stops by a clot or a mechanical agent (especially during vascular surgery and organ transplantation). Reperfusion is the restoration of blood flow to the tissue [4]. Currently, there is no clear treatment options because the harmful effects of renal I/R are the result of unclear, interrelated, and complex events. Previous studies indicated that apoptosis, inflammation, and oxidative stress are associated with renal I/R [5,6,7]. Reactive oxygen species (ROS) are responsible for increased oxidative stress, pro-apoptotic mediator expression, and inflammatory cytokines when reperfusion is seen in ischemic tissues [8].
Trace element boron (B), which is the characteristic element of boric acid (BA), may have significant effects on various metabolic and physiological systems in organism [9]. The effects of BA on vitamin, hormone, enzyme, energy, and mineral metabolism have been shown in some studies [10]. However, the biochemical mechanism of B and BA is not fully known yet. The researchers explain the biological effects of boric acid by two different hypotheses. In the first hypothesis, it is argued that B and BA are negative regulators affecting the path of competitive inhibition in key enzyme reactions [11]. In the second hypothesis, it is argued that boron plays an important role in cell membrane function, structure, and stability [12]. Therefore, it has recently attracted the attention of researchers due to its multifaceted effects in the pathogenesis of many diseases. Studies investigating the effect of BA on renal I/R injury are limited. In addition, there are several studies showing the antioxidant, anti-inflammatory, and anti-apoptotic effects of BA in different experimental and clinical studies [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. However, inconsistency is observed in the results.
According to our hypothesis, as shown in Fig. 1, BA exhibits antioxidant properties against oxidant molecules by quenching and chain-breaking through protons in the hydroxyl groups. In this way, we think that it may be involved in apoptotic and inflammatory processes. However, harmful effects can occur as high concentrations of BA take the radical on itself and change the pH of the medium.
In the current study, we aimed to investigate the role of oxidative stress, inflammation, and apoptosis in the injury of the kidney tissue after I/R, and the protective effect of dose-dependent BA administration. The effects of BA on I/R injury were investigated by measuring some oxidative, apoptotic, and inflammation parameters including superoxide dismutase (SOD) activity, catalase (CAT) activity, glutathione (GSH) levels, malondialdehyde (MDA) levels, total oxidant status (TOS), total antioxidant status (TAS), caspase 3 (CASP3) activity, cytochrome C (CYCS) levels, DNA fragmentation, tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6) levels. In addition, morphological changes were investigated with hematoxylin eosin staining (H&E) and apoptosis was examined by TUNEL staining.
Materials and Methods
Animals and Experimental Design
The study was approved by the committee of Local Ethics Committee of Eskişehir Osmangazi University Animal Experiments (ESOGU HADYEK) (decision no. 657 of 14.03.2018). Thirty-five female Sprague Dawley albino rats weighing 180–220 g were divided into five groups of seven in each group. Sham, I/R, and I/R + BA (i.p at doses of 50, 100, and 200 mg/kg). The rats were housed in polycarbonate cages (3–4 rats per cage), under the conditions of conventional laboratory animal housekeeping conditions (controlled temperature (21 ± 2 °C), humidity (50 ± 5%), air change (cycle), and light (12 h light, 12 h dark). During the experiment, the animals were checked daily and their overall morphological appearance (hair loss, defecation disorders) was evaluated visually. The experimental animals were fed standard commercial rat pellets (purchased from Korkutelim Yem Gıda, Antalya, Turkey; 2.4% crude fat, 5.97% crude cellulose, 23.5% crude protein, 1–2% vitamins and minerals; 3% trace elements, iron, selenium, manganese, zinc, cobalt, iodide, 270 kcal 100 g−1) and allowed water ad libitum. Feed and water restriction has not been made to rats. All surgical procedures and euthanasia were performed under anesthesia.
The following methods were applied to groups: Nephrectomy was performed in all animals by intramuscular administration of 10 mg/kg xylazine and 80 mg/kg ketamine anesthesia. Right kidney nephrectomy was performed under sterile conditions. Sterilized physiological saline solution to the abdominal cavity to prevent the hypovolemic effects of the fluid lost after nephrectomy for each rat. Each animal was chemically sterilized, single-individual, transparent cages with polycarbonate composition were placed separately for 15 days. For the I/R procedure, rats underwent midline laparotomy under anesthesia. The left renal artery was isolated, and blood flow was stopped for 45 min with an anti-traumatic vascular clamp. Immediately after 45 min of ischemia, reperfusion was performed for 24 h. Sterilized physiological saline was given to the abdominal cavity to prevent hypovolemic effects of fluid lost during reperfusion. The BA solution applied to the experimental animals was freshly prepared immediately before the study, containing 50, 100, and 200 mg/kg BA in a 0.5-mL physiological saline.
After recovery, each animal received 45 min of ischemia. Sham group only underwent surgical stress procedure. Animals in the I/R group were injected into intraperitoneally only the 0.5-mL physiological saline 10 min prior to reperfusion. Animals in the I/R group + BA (50, 100, 200 mg/kg) were injected into intraperitoneally 50, 100, and 200 mg/kg (b.w.) separately with a 0.5-mL physiological saline 10 min prior to reperfusion. After 24 h of reperfusion, the animals in the groups were sacrificed by intracardiac blood to appropriate separator gel tubes under intramuscular anesthesia of ketamine (80 mg/kg) and xylazine (10 mg/kg) because of the experimental procedure.
Biochemical Measurements
After blood samples were centrifuged at 3500 rpm for 15 min (Jouan MR 22), some of the obtained serum were stored in the deep freezer (− 80 °C, Jouan VX350 series Thermo 26 Electron) until analyzed for ELISA by aliquot. The remaining fresh serum was also run in the auto-analyzer for measurement of some biochemical parameters. Kidney tissues were taken from the abdomen of the animals whose blood was taken. For histological examinations, some of the kidney samples were placed in 10% formaldehyde and the remaining tissues were stored for biochemical measurements.
Serum BUN and creatinine measurements for determination of renal function were performed with a Roche COBAS C501 auto-analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The results were expressed as mg/dl.
TAS and TOS levels (Rel Assay Diagnostics, Gaziantep, Turkey,) in per 100 mg tissue homogenates were measured by ELISA (PerkinElmer2030 Multilabel reader, VictorX3). The results were expressed as mmol Trolox Equiv/L and μmol H2O2 Equiv./L. MDA levels were measured using the method reported by Ohkawa et al. [28]. The results were expressed as nmol/mg protein. GSH levels were determined according to modified method of Srivastava and Beutler [29]. CAT activities were determined by the method of Aebi [30]. The reduction in optical density per minute was determined and the enzyme activity was expressed in U/mg protein. SOD activity was determined by the method of Winterbourn et al. [31]. One unit of SOD expressed in U/mg protein was designated as the amount of enzyme that inhibits the reduction of nitroblue tetrazolium reduction by 50%. Total protein level in tissue samples was performed according to the Bradford assay [32]. Manual measurement methods were performed using a spectrophotomer (Shimadzu UV-1601) device.
DNA fragmentation was evaluated according to the method applied by Wyllie. DNA fragmentation in the kidney tissue was expressed as a percentage of total DNA in the supernatant fraction [33]. CYCS levels and CASP3 activities were measured using a commercial kit (Cloud-Clone Corp., USA, cat no. SEA594Ra and SEA626Ra, respectively). The concentrations of CASP3 and CYCS in per 100 mg tissue homogenates were shown as ng/mL in comparison with the optical density of the standard curve.
TNF-α and IL-6 levels were measured using a commercial kit (Cloud-Clone Corp., USA, cat no. SEA133Ra and SEA079Ra). The concentrations of TNF-α and IL-6 in serum samples were shown as pg/mL in comparison with the optical density of the standard curve.
Histopathological Assessments
Each of the middle kidney tissues was left in a 10% buffered neutral formaldehyde solution for 24 h for histological examination. Chemical fixation was achieved, dehydrated, and embedded in paraffin for H&E staining. In total, 4–5 μm thick sections were taken using a microtome (Leica RM 2025) and paraffin was opened in water bath and closed on poly-l-lysine–coated slides. Sections were kept in the oven at 37 °C for one night and deparaffinized in xylol. H&E staining was carried out by decreasing the degree of ethyl alcohol in the alcohol series. H&E-stained kidney sections were examined by light microscopy. All tissue sections were examined by an Olympus brand, a CH40 light microscope, and photographed by a Spot Insight 3.2.0. model digital camera and a Spot advanced, with the help of the 4.0.6 version program. The degree of the kidney damage in the sections was evaluated as absent (−), less (+), moderate (++), and severe (+++). Sections were scored by two blind observers (Donmez, D and Senturk, H).
Apoptotic cells in the kidney sections were detected with transferase-mediated dUTP nick-end labeling (TUNEL) assay by an observer. The TUNEL staining was conducted using an assay kit according to the manufacturer’s instructions (Millipore, USA, cat no. S7101).
Statistical Analysis
SPSS Version 21.0 package program was used for statistical analysis of biochemical measurements and histopathological assessments. The results were evaluated using the Kolmogorov-Smirnov and Shapiro-Wilk normality tests. The Kruskal-Wallis test was applied for data not showing normal distribution. One-way analysis of variance (ANOVA) tests for normal distribution data. The Tukey HSD test was used for differences between groups. Results were expressed as the mean ± standard deviation. The statistical significance was considered as p < 0.05.
Results
Biochemical Results
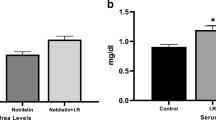
The comparison of serum BUN and creatinine levels among the groups in the evaluation of renal function were shown in our previous study [34]. Serum creatinine and BUN levels in the I/R group were found to be statistically higher compared with the sham group (p < 0.05), while creatinine and BUN values were lower in the 50 mg/kg BA group (p < 0.05). There was no statistically significant difference in BUN and creatinine levels between the other treatment groups (100 and 200 mg/kg BA) (p > 0.05).
In order to investigate the oxidative stress induced by I/R injury in the kidney tissue and antioxidant effects of different BA doses, TAS, TOS, MDA, SOD, CAT, and GSH levels are shown in Table 1. MDA levels were significantly higher in the renal tissue of the I/R group compared with the sham group (p < 0.001). MDA levels showed a significant decrease compared with the I/R group in the BA-treated groups. In addition, SOD, CAT, and GSH levels increased significantly compared with I/R groups in BA-treated groups. The highest average TOS values were detected in the I/R group. TOS levels were statistically lower in the 50 and 100 mg/kg BA groups than the I/R group (p < 0.001). BA applications showed a dose-dependent increase in TAS levels compared with the I/R group (p < 0.001). However, no statistically significant difference was observed in both TOS and TAS levels in 200 mg/kg BA application. According to these results, BA applications were found to suppress the oxidative stress by inducing the antioxidant system and it was revealed that BA should be used in appropriate doses.
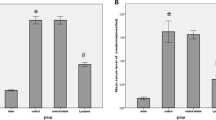
Detection of DNA fragmentation is the evaluation of mono or oligonucleotides fragmented at the end of the apoptotic process and dispersed in the cytoplasm. DNA inhibition in all experimental groups is shown in Fig. 2 as percent inhibition. The percentage of DNA fragmentation due to kidney damage was found to be statistically higher in the I/R group than the control group (p < 0.001). DNA fragmentation levels were found to be statistically lower in 50, 100, and 200 mg/kg BA treatment groups when compared with the I/R group (p < 0.001). There was no statistically significant difference in DNA fragmentation levels between groups with different doses of BA (p > 0.05).
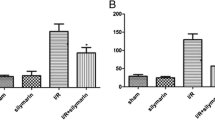
A statistically significant difference was found between the Sham and I/R groups in terms of CYCS levels and CASP3 activity (p < 0.001). These data indicate that the levels of CYCS and CASP3 activity increase in the damaged tissue following I/R injury in Table 2. The administration of 50 mg/kg BA showed a statistically significant decrease in tissue CASP3 activity compared with the I/R group (p < 0.001). Statistically significant decrease in tissue CYCS and CASP3 levels was observed in 100 mg/kg BA group compared with I/R group. BA application inhibits apoptotic activity after renal I/R. However, the effect of 200 mg/kg BA on apoptotic activity was mild and there was no statistically significant difference.
TNF-α and IL-6 levels in the I/R group were significantly higher in the Sham (p < 0.001). TNF-α and IL-6 levels in 50 mg/kg BA and 100 mg/kg BA treatment groups were significantly lower than in the I/R group (p < 0.001). In the 200 mg/kg BA group, there was a decrease in the levels of TNF-α and IL-6 of approximately 2–3% compared with I/R group but not statistically significant.
Histopathological Results
In our study, histopathological examination of the kidney tissues and kidney damage for each rat were calculated. These calculated values are shown in Table 3. The light microscopic image of all experimental groups is shown in Fig. 3. As the DNAs of apoptotic cells are rapidly degrading, the chromatin network in the cell suddenly loses its integrity and the number of DNA particles containing 3′-OH is very high. In the cell, the terminal deoxynucleotidyl transferase (TdT) enzyme transfers the added biotin dUTP to the free 3′-OH ends of the fragmented DNA particles. Biotin-labeled DNA fragments become visible on the fluorescence microscope when light streptavidin is added to the microscope or a fluorescent agent. This method is a very sensitive test in cell cultures and tissue sections as it can show in situ apoptosis in individual cells. TUNEL images of all experimental groups are shown in Fig. 4.
The cortex and the medulla structure (A1, B1) were normal in the Sham group. The kidney body (►), proximal tubule (pt), and distal tubule (dt) structures were seen in normal histological structure. In the I/R group, glomerular damage (thick arrow), tubular damage (→), and dilatation (d) were observed in the cortex (A2) and medulla (B2). Tubular hyaline cast (c) structures were seen in the medulla (B2) in I/R groups. Kidney bodies (►), proximal tubule (pt), and distal tubule (dt) structures were seen near normal histological structure. In addition, cortex (A3) and medulla (B3) drew attention to reduced damage in I/R + 50 mg/kg BA groups. Kidney bodies (►), proximal tubule (pt), and distal tubule (dt) histological structures were similar to the sham group. Reduced damage was observed in the cortex (A4) and medulla (B4) in I/R + 100 mg/kg BA groups. Glomerular damage (thick arrow), tubular damage (→) and dilatation (d) were observed similar to the I/R group in the cortex (A5) and medulla (B5) of I/R + 200 mg/kg BA groups.
When the scoring results of the experimental groups were examined, it was observed that I/R + 50 mg/kg BA treatment decreased the glomerular damage rate in the I/R group but not statistically significant (p = 0.121). There was a statistically significant decrease in tubular damage and dilatation compared with I/R group, respectively (p = 0.023, p = 0.006). There was no statistically significant difference in the 50 mg/kg BA group compared with the other treatment groups (p > 0.05). In the application of 100 mg/kg BA, there was a statistically significant decrease in glomerular damage and tubular dilatation compared with 50 mg/kg BA, respectively (p = 0.027, p = 0.006). In addition, there was no statistically significant difference in the degree of kidney damage compared with the Sham group (p > 0.05).
The kidney sections from all of the groups were stained by TUNEL staining to investigate apoptosis in Fig. 4. We observed that low doses of BA prevented apoptosis due to I/R injury.
TUNEL positive stained cells in several tubule epithelial cells in the kidney cortex drew attention (→) (A1–A2) in sham groups. Numerous TUNEL positive stained cells in the cortex of the rat kidneys of the I/R group, especially in the distal tubule epithelial cells (→) (B1–B2). A small number of TUNEL positive stained cells in the cortex of the rat kidneys of the I/R + 50 mg/kg BA, especially in the distal tubule epithelial cells compared with the I/R group (→) (C1–C2). In the rat kidneys of I/R + 100 mg/kg BA, a minimal number of TUNEL positive stained cells in the cortical epithelial cells of the distal tubule epithelial cells, especially in the distal tubule cells, drew attention to a minimal number of TUNEL-stained cells (→) (D1-D2). In the rat kidneys of I/R + 200 mg/kg BA, especially in the distal tubule epithelial cells in the cortex, especially in the distal tubule epithelial cells in the middle TUNEL positive cells in the epithelial cells attracted attention (→) (E1–E2).
The present study demonstrated that the I/R injury increased levels of some renal function parameters, oxidative stress, apoptosis, and inflammation. Application of low doses of BA prevented this increased levels.
Discussion
AKI or renal failure is defined as a rapid dysfunction of the kidney and I/R damage is the most important cause of acute renal failure [35]. I/R has been reported to cause serious histopathological and biochemical damage in both patients and experimental animals [36,37,38]. Experimental studies have shown that BA has protective effects against oxidative stress, hepatotoxicity, pancreatic damage, genotoxicity, and cardiotoxicity. We have demonstrated the distant tissue damage and dose-dependent BA effect caused by I/R in our studies on the pancreas, brain, heart, and liver [34, 39,40,41,42]. When the literature is evaluated, dose-dependent BA application on renal I/R damage was first shown with this study.
Significantly, increased BUN and creatinine levels are typical signs of acute kidney injury [43,44,45,46]. There are many studies in the literature in which ischemia and reperfusion were performed at different times and increased levels of damage markers such as serum creatinine, urea, uric acid, and BUN were observed and in these studies [47,48,49,50,51]. Mousavi et al. applied renal ischemia period as 60 min [49]. In the study of Chen et al., the rats were treated with ischemia for 45 min and then reperfusion [50]. Wang et al. performed 45 min ischemia and 6 h reperfusion [48]. In our study, ischemia period was performed for 45 min and reperfusion was achieved for 24 h. Our previous results show that the increase in BUN and creatinine levels, which are indicative of glomerular filtration disorder, are consistent with the results of renal I/R studies previously performed with different ischemia-reperfusion times. Increased creatinine and BUN values were decreased in BA-treated groups. This decrease shows the beneficial effect of BA on kidney function. In the study of Başbuğ et al., 200 mg/kg BA application (i.p.) decreased creatinine levels compared with the I/R group but could not detect statistical difference [15]. However, we found that a dose of 200 mg/kg BA caused an increase in creatinine levels in our previous study [34].
Re-oxygenation of tissue, especially after hypoxia, causes the accumulation of free radicals known as oxidative stress. Ischemia increases the production of reactive oxygen species (ROS) [16]. Since the level of tissue TOS is significantly increased in the I/R group, we think that I/R induces oxidative stress. In our study, BA doses decreased TOS level and increased TAS level. It is estimated that BA is effective in reducing oxidative stress and damage induced by I/R in ischemic rats as the BA dose decreases TOS level and increases the level of TAS. However, we observed that the level of TOS increased and the level of TAS decreased in the 200 mg/kg BA application compared with the other dose groups. In the study of Geyikoglu et al., the rats were administered with a 14 mg/kg BA gavage 1 h before ischemia. At the end of the study, MDA levels were statistically decreased in the BA-treated groups compared with the I/R group, whereas the SOD and GSH levels were increased statistically [17]. Although these results are considered to be in parallel with our study, the differences between the doses come to the fore. Some applied BA doses share inconsistent results in different studies. In a study of 200 mg/kg BA supplementation, it was reported that MDA levels was decreased by increasing antioxidant activity such as GSH, SOD, and CAT, and no toxic effect was observed in the 200 mg/kg treated group [18]. Söğüt et al. found that a significant decrease in MDA levels in the 100 mg/kg BA group with alcohol, while they observed an increase in GSH, CAT, and GPx levels [19]. İnce et al. showed that the addition of BA to rats reduced intracellular ROS produced by cyclophosphamide and that cellular components were protected against DNA damage and membrane lipid peroxidation [20]. Türkez et al. reported that low doses of BA (5–50 mg/L) did not alter the MDA concentration, but increased MDA concentration in human peripheral blood cultures exposed to BA compounds at high doses (5–500 mg/L) [21]. Mohora et al. found that 80 mg/kg BA supplementation in the diet increased MDA levels in rat liver tissue [22].
DNA damage caused by ROS is very important because it initiates and increases carcinogenesis [23]. In the determination of apoptotic processes in renal I/R injury, apart from several investigations, the appropriate dose range of BA was not determined. BA application may reduce I/R induced DNA damage. In one study, it was shown that DNA damage decreased in animals given 200 mg/kg (i.p.) before induction of the IR in BA group compared with control group [15]. TNF-α and other cytokines activate many proteases such as caspase-3 and 8 during reperfusion. A subsequent sequence of events ultimately leads to the destruction of DNA [24]. In a study demonstrated by elevated TNF-α and IL-6 resulting in DNA damage after I/R, they concluded that the damage was reduced by administration of 200 mg/kg BA [15]. Apoptotic cells, which are too rare to be detected in DNA studies, can be observed using TUNEL staining. In another study, 200 mg/kg BA administration against cisplatin toxicity was shown to suppress caspase-3 mRNA levels. Similarly, when the TUNEL scores of the groups treated with BA after cisplatin toxicity were examined, apoptosis rates decreased [25]. The effective doses determined from toxicity induced kidney damage were found at higher concentrations than our study. In our study, the application of BA in all dose groups after I/R decreased the degree of DNA fragmentation by preventing partially oxidative damage. This finding is supported by a decrease in apoptotic cell index determined by TUNEL staining. However, no significant change was observed in CASP3 activities and CYCS levels in 200 mg/kg BA.
Geyikoglu et al. observed a decrease in the number of histopathological changes when propolis and BA (14 mg/kg intragastric administration 1 h before ischemia) were applied on the rat kidney with I/R. In our study, it was shown that severe histopathological damage was improved with BA application in glomerular and tubular damage, hyaline cast and tubular dilatation image examination in renal tissue taken after I/R. In Bahadoran’s study on rats and Naghii’s study on humans, low-dose boron (3 and 10 mg/day) supplementation was observed to prevent kidney stone formation [26]. However, there was no protective effect against nephrolithiasis and oxidative stress in a high dose boron treatment [27]. Although our biochemical and histological findings were consistent with the above-mentioned studies, statistically significant differences were found in our 200 mg/kg BA application.
Conclusions
We conclude that oxidative stress, inflammation, and apoptosis may be reduced after renal ischemia/reperfusion injury with different doses of BA treatments. The obtained data indicate that the anti-apoptotic, anti-inflammatory, and antioxidant effects of 200 mg/kg BA may be less than the other dose groups. In our subsequent studies, we planned to investigate the effective dose of new substance synthesis by the formation of boric acid and derivative compounds on different diseases. In this way, we think that this trace element mechanism will emerge better.
References
Jochmans I, Meurisse N, Neyrinck A, Verhaegen M, MonbaliuD PJ (2017) Hepatic ischemia/reperfusion injury associates with acute kidney injury in liver transplantation: prospective cohort study. Liver Transpl 23:634–644. https://doi.org/10.1002/lt.24728
Neri M, Riezzo I, Pascale N, Pomara C, Turillazzi E (2017) Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediat Inflamm 2017:1–14. https://doi.org/10.1155/2017/7018393
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW (2018) Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem 46:1650–1667. https://doi.org/10.1159/000489241
Uyanoğlu M, Canbek M, Şentürk H, Bayramoğlu G, Gündüz Ö, Özen A ve Turgak Ö (2011) Preventing organ injury with carvacrol after renal ischemia/reperfusion. 5:2–80
Kelly KJ, Plotkin Z, Dagher PC (2001) Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Investig 108:1291–1298. https://doi.org/10.1172/JCI13018
Marko L, Vigolo E, Hinze C, Park JK, Roel G, Balogh A, Choi M, Wubken A, Cording J, Blasig IE, Luft FC, Scheidereit C, Schmidt-Ott KM, Schmidt-Ullrich R, Muller DN (2016) Tubular epithelial NF-kappaB activity regulates ischemic AKI. J Am Soc Nephrol 27:2658–2669. https://doi.org/10.1681/ASN.2015070748
Zhao L, Xu L, Tao X, Han X, Yin L, Qi Y, Peng J (2016) Protective effect of the total flavonoids from Rosa laevigata Michx fruit on renal ischemia-reperfusion injury through suppression of oxidative stress and inflammation. Molecules 21:952. https://doi.org/10.3390/molecules21070952
Weng XF, Li ST, Song Q, Zhu Q, Song DD, Qin ZH, Xie Y (2018) Protective effect of nicotinamide adenine dinucleotide phosphate on renal ischemia-reperfusion injury. Kidney Blood Press Res 43:651–663. https://doi.org/10.1159/000489620
Khaliq H, Juming Z, Ke-Mei P (2018) The physiological role of boron on health. Biol Trace Elem Res 186:31–51. https://doi.org/10.1007/s12011-018-1284-3
Ince S, Kucukkurt I, Cigerci IH, Fidan AF, Eryavuz A (2010) The effects of dietary boric acid and borax supplementation on lipid peroxidation, antioxidant activity, and DNA damage in rats. J Trace Elem Med Biol 24:161–164. https://doi.org/10.1016/j.jtemb.2010.01.003
Hunt CD (1994) The biochemical effects of physiologic amount of dietary boron in animal nutrition models. Environ Health Perspect 102:35–43. https://doi.org/10.1289/ehp.94102s735
Nielsen FH (1991) Nutritional requirements for boron, silicon, vanadium, nickel and arsenic: current knowledge and speculation. FASEB J 5:2661–2667. https://doi.org/10.1096/fasebj.5.12.1916090
Hunt CD (1998) Regulation of enzymatic activity: one possible role of dietary boron in higher animals and humans. Biol Trace Elem Res 66:205–225
Cao J, Jiang L, Zhang X, Yao X, Geng C, Xue X, Zhong L (2008) Boric acid inhibits LPS-induced TNF-alpha formation through a thiol-dependent mechanism in THP-1 cells. J Trace Elem Med Biol 22:189–195
Basbug M, Yildar M, Yaman I, Ozkan OF, Aksit H, Cavdar F et al (2015) Effects of boric acid in an experimental rat model of hepatic ischemia-reperfusion injury. Acta Medica Mediterr 31:1067–1073
Lévigne D, Tobalem M, Modarressi A, Pittet-Cuénod B (2013) Hyperglycemia increases susceptibility to ischemic necrosis. Biomed Res Int 2013:1–5.31
Geyikoglu F, Koc K, Colak S, Erol HS, Cerig S, Yardimci BK et al (2019) Propolis and its combination with boric acid protect against ischemia/reperfusion-induced acute kidney injury by inhibiting oxidative stress, inflammation, DNA damage, and apoptosis in rats. Biol Trace Elem Res 1–8
Ince S, Keles H, Erdogan M, Hazman O, Kucukkurt I (2012) Protective effect of boric acid against carbon tetrachloride-induced hepatotoxicity in mice. Drug Chem Toxicol 35(3):285–292
Sogut I, Oglakci A, Kartkaya K, Ol KK, Sogut MS, Kanbak G, Inal ME (2015) Effect of boric acid on oxidative stress in rats with fetal alcohol syndrome. Exp Ther Med 9(3):1023–1027
Ince S, Kücükkurt I, Demirel HH, Acaröz DA, Akbel E, Ciğerci IH (2014) Protective effects of boron on cyclophosphamide induced lipid peroxidation and genotoxicity in rats. Chemosphere 108:197–204
Turkez H, Geyikoglu F, Tatar A, Keles S, Ozkanc A (2007) Effects of some boron compounds on peripheral human blood. Z Naturforsch 62:889–896
Mohora M, Boghianu L, Muscurel C, Duta C, Dumitrache C (2002) Effects of boric acid on redox status in the rat liver. Romanian J Biophys 12:77–82
Saravan R, Pugalendi KV (2005) Assessment of the pharmacological effect of silymarin on ethanol induced DNA damage by sindle cell gelelectrophoresis. Indian J Pharmacol 37:261–262
Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM (2004) The role of the complement system in ischemia-reperfusion injury. Shock 21(5):401–409
Hazman Ö, Bozkurt MF, Fidan AF, Uysal FE, Çelik S (2018) The effect of boric acid and borax on oxidative stress, inflammation, ER stress and apoptosis in cisplatin toxication and nephrotoxicity developing as a result of toxication. Inflammation 41(3):1032–1048
Bahadoran H, Naghii MR, Mofid M, Asadi MH, Ahmadi K, Sarveazad A (2016) Protective effects of boron and vitamin E on ethylene glycol-induced renal crystal calcium deposition in rat. Endocr Regul 50:194–206
Ergul AB, Kara M, Karakukcu C, Tasdemir A, Aslaner H, Ergul MA, Muhtaroglu S, Zararsiz GE, Torun YA (2018) High doses of boron have no protective effect against nephrolithiasis or oxidative stress in a rat model. Biol Trace Elem Res 186(1):218–225
Okhawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–890
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Winterbourn CC, Hawkins RE, Brian M, Carrell RW (1975) The estimation of red cell superoxide dismutase activity. J Lab Clin Med 85:337–341
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284(5756):555–556
Şentürk H, Kar F, Hacıoğlu C, Kanbak G Renal İskemi Reperfüzyon ile İndüklenmiş Oksidatif Stres Hasarının Pankreas Üzerine Etkisi Doza Bağımlı Borik Asidin Rolü. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Dergisi 21:944–949. https://doi.org/10.18016/ksutarimdoga.vi.430369
Friedewald JJ, Rabb H (2004) Inflammatory cells in ischemic acute renal failure. Kidney Int 66:486–491
Holderied A, Andersen NH (2014) Animal models of kidney inflammation in translational medicine. Drug Discov Today Dis Model 11:19–27.24
Bonventre JV, Zuk A (2004) Ischemic acute renal failure: an inflammatory disease? Kidney Int 66:480–485
Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121:4210–21.26
Kar F, Hacıoğlu C, Kar E, Kara Y, Şentürk H, Kanbak G (2018) Renal ischemia reperfusion effect on spleen asremote tissue damage and role of boric acid. Presented At The Tbs International Biochemistry Congress
Hacıoğlu C, Kar F, Kar E, Kara Y, Şentürk H, Kanbak G (2018) Neuroprotectıve effects of boric acid on brain against renal ischemia reperfusion injury. Presented At The Tbs International Biochemistry Congress
Kara Y, Hacıoğlu C, Kar F, Kar E, Şentürk H, Kanbak G (2018) Effects of boric acid on heart tissue damage caused by renal ischemia/reperfusion. Presented At The Tbs International Biochemistry Congress
Hacıoğlu C, Kar F, Şentürk H, Kanbak G (2018) Effects of boric acid on electrolyte balance and lipid profile against renal ischemia reperfusion injury. Biol Divers Conserv 11:76–81 ISSN 1308-8084
Star RA (1998) Treatment of acute renal failure. Kidney Int 54:1817–31.27
Molina A, Ubeda M, Escribese MM, García-Bermejo L, Sancho D, Pérez de Lema G et al (2005) Renal ischemia/reperfusion injury: functional tissue preservation by anti-activated 1 integrin therapy. J Am Soc Nephrol 16:374–82.28
Visnagri A, Kandhare AD, Bodhankar SL (2015) Renoprotective effect of berberine via intonation on apoptosis and mitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Ren Fail 7:482–93.29
Kaya C, Karabulut R, Turkyilmaz Z, Sonmez K, Kulduk G, Gülbahar Ö, Köse F, Basaklar AC (2015) Lycopene has reduced renal damage histopathologically and biochemically in experimental renal ischemia–reperfusion injury. Ren Fail 37:1390–1395
Hoffmann D, Bijol V, Krishnamoorthy A, Gonzalez VR, Frendl G, Zhang Q et al (2012) Fibrinogen excretion in the urine and immunoreactivity in the kidney serves as a translational biomarker for acute kidney injury. AJP 181(3):818–828
Wang P, Zhu Q, Wu N, Siow YL, Aukema H, O K. (2013) Tyrosol attenuates ischemia-reperfusion-induced kidney injury via inhibition of induceble nitric oxide synthase. J Agric Food Chem 61:3669–3675
Mousavi G (2015) Study on the effect of black cumin (Nigella sativa Linn) on experimental renal ischemia-reperfusion injury in rats. Acta Cir Bras 30(18):542–550
Chen H, Xing B, Lui X, Zhan B, Zhou J, Zhu H et al (2008) Ozone oxidative preconditioning protects the rat kidney from reperfusion injury: the role of nitric oxide. J Surg Res 149:287–295
Guan Z, Gobè G, Willgoss D, Endre ZH (2006) Renal endothelial dysfunction and impaired autoregulation after ischemia-reperfusion injury result from excess nitric oxide. Am J Physiol Renal Physiol 291(3):F619–F628
Funding
This study was supported by the Eskişehir Osmangazi University Scientific Research Projects Commission (project no. 2018-2085).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Committee of Local Ethics Committee of Eskişehir Osmangazi University Animal Experiments (ESOGU HADYEK) (decision no: 657 of 14.03.2018).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kar, F., Hacioglu, C., Senturk, H. et al. The Role of Oxidative Stress, Renal Inflammation, and Apoptosis in Post Ischemic Reperfusion Injury of Kidney Tissue: the Protective Effect of Dose-Dependent Boric Acid Administration. Biol Trace Elem Res 195, 150–158 (2020). https://doi.org/10.1007/s12011-019-01824-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01824-1