Abstract
Hypertension and aging are leading risk factors for stroke and vascular contributions to cognitive impairment and dementia (VCID). Most animal models fail to capture the complex interplay between these pathophysiological processes. In the current study, we examined the development of cognitive impairment in 18-month-old spontaneously hypertensive rats (SHR) before and following ischemic stroke. Sixty SHRs were housed for 18 months with cognitive assessments every 6 months and post-surgery. MRI scans were performed at baseline and throughout the study. On day 3 post-stroke, rats were randomized to receive either angiotensin II type 2 receptor (AT2R) agonist Compound 21 (C21) or plain water for 8 weeks. SHRs demonstrated a progressive cognitive decline and significant MRI abnormalities before stroke. Perioperative mortality within 72 h of stroke was low. Stroke resulted in significant acute brain swelling, chronic brain atrophy, and sustained sensorimotor and behavioral deficits. There was no evidence of anhedonia at week 8. C21 enhanced sensorimotor recovery and ischemic lesion resolution at week 8. SHRs represent a clinically relevant animal model to study aging and stroke-associated VCID. This study underscores the importance of translational disease modeling and provides evidence that modulation of the AT2R signaling via C21 may be a useful therapeutic option to improve sensorimotor and cognitive outcomes even in aged animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension and aging are major contributing factors to stroke and most forms of cognitive impairment and dementia [1,2,3,4,5,6,7,8,9]. VCID encompasses cognitive impairment due to any vascular event including ischemic stroke. Recent improvements in acute stroke care have led to a decrease in stroke mortality and unfortunately, an increase in stroke-induced long-term disability including sensorimotor and cognitive deficits [10, 11]. Poststroke cognitive impairment (PSCI), a leading VCID, develops progressively after stroke and is proportionally associated with age [1, 3, 12, 13]. PSCI causes extensive reductions in quality of life and increases the burden of care, with many patients progressing to dementia [14, 15]. Preclinical models demonstrated that chronic hypertension and advanced age separately accelerated the development of cognitive decline and hippocampal neurodegeneration after stroke [16,17,18,19,20,21], but the interaction of age and hypertension on the progression of PSCI remained to be determined.
The brain renin-angiotensin system (RAS) is involved in the pathogenesis of stroke [22,23,24,25,26,27,28,29,30,31,32,33,34]. We and others have demonstrated that C21, the first selective non-peptide AT2R agonist, provides a neurovascular protective effect and enhances sustained functional improvement after stroke [23, 31, 33,34,35,36,37,38,39,40,41,42,43,44]. Direct stimulation of AT2R via C21 has been shown to improve functional recovery after stroke without affecting blood pressure in hypertensive animals, even at higher doses [38, 45]. Our recent studies provided evidence RAS modulation was an effective strategy to preserve cognitive function in comorbid disease models, even when initiated 3 days after stroke [23, 35,36,37]. This contrasts with most post-stroke clinical interventions where treatment is initiated acutely after stroke and discontinued thereafter, missing an opportunity for improved long-term outcomes. The impact of RAS modulation by AT2R agonism in aged hypertensive rats, especially after stroke, was unclear.
Most preclinical stroke research failed to include aging and hypertension, limiting the translation of preclinical studies [46]. Middle-aged SHRs have been studied as a model of cerebral small vessel disease, showing similar characteristics to human cerebral small vessel disease. SHRs (30–35 weeks old) exhibited cognitive deficits, and brain vascular and structural changes [47]. However, longitudinal studies looking at the additive effect of stroke are lacking. In the current study, we aimed to investigate the temporal development of cognitive impairment VCID in 18-month-old SHRs before and following a stroke induced by a short ischemic insult. We hypothesized that the development of VCID in hypertensive rats will progressively evolve, and stroke will accelerate the progression of PSCI. This study is also designed to explore the significance of delayed chronic stimulation of the AT2 receptor in preventing the development of long-term PSCI in an aged hypertensive population.
Materials and Methods
Experimental Design
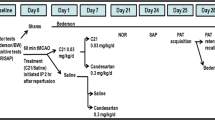
A total of 60 young male hypertensive rats (7-week-old SHRs) were obtained from Charles River laboratories. Rats were double housed in a pathogen-free, temperature-controlled environment with free access to food and water, and on a 12:12-h dark:light cycle. The experimental design and flowchart are shown in Fig. 1 and Supplementary Fig. 1. Rats were maintained up until the age of 18 months. During the aging process, body weight and cognitive function were monitored. Eighteen-month-old rats were subjected to either 30-min temporary middle cerebral artery occlusion (tMCAO) or sham surgery. On post-surgery day 3, rats were randomized to blindly receive either an oral dose of C21 (0.12 mg/kg/day; incorporated in drinking water) or plain drinking water. Treatment was initiated on day 3 and continued for a total of 8 weeks. A series of sensorimotor and cognitive assessments and MRI scans were performed at baseline and throughout the study (Fig. 1; Supplementary Fig. 1). A separate cohort of young SHRs (n = 5) was used as sham vehicle control for cognitive assessment by novel object recognition test-NOR as described below. Treatments were administered in a blinded manner and randomized by cage (housed in pairs). Treatment bottles were prepared by an individual not involved in the surgical procedure or behavioral assessments. Rats were numbered upon arrival and maintained the same number throughout the study.
Stroke Model and Treatment Strategy
Eighteen-month-old SHRs, averaging around 382 g, were subjected to a 30-min tMCAO surgery using silicon-coated nylon suture (Doccol 404356) as previously described [27, 36]. In our previous studies [36, 48], we successfully established a 60-min tMCAO model with high survivability and sufficient neurological deficits in young SHRs. However, we thought opting for a shorter occlusion time would translate into better survivability in these vulnerable animals. It has been reported that occlusion times longer than 22 min consistently resulted in adequate lesion size in young SHRs [49]. A separate cohort of five rats was used to validate 30-min occlusion time, suture size, and surgical condition in 18-month-old hypertensive rats.
Visible ischemic lesion at day 3 magnetic resonance imaging (MRI) scans was used as a pre-set inclusion criterion to ensure only animals with a degree of ischemic injury were included in the analysis. The dose for C21 was adopted from our previous studies [23, 35,36,37,38]. Oral treatment with C21 was initiated on day 3 after stroke to avoid the window of acute neuroprotection.
Power Analysis
Power analysis was based on the 8-week recognition index in which aged SHRs were subjected to chronic hypoperfusion and treated with vehicle or C21 [23]. It was predicted that 10/group would provide at least 95% power to detect a difference between groups (saline vs. C21) for aged SHRs (0.30 ± 0.15 vs. 0.60 ± 0.15, respectively).
Assessment of Functional Outcome
Weight monitoring is an exceedingly helpful tool to assess animals’ health and welfare. Body weight was recorded monthly throughout the aging process, daily after surgery, and weekly afterward. All rats were provided with a supplementary Nutra-Gel Complete Nutrition (Bio-Serv®) to support their recovery, and Ringer’s lactate solution if necessary. In a separate cohort of two aged SHRs, arterial blood pressure was recorded throughout 14 days by telemetry. Arterial pressure was monitored in 10-min intervals. Seven-day-average arterial pressure was used to assess their mean arterial pressure (MAP), and circadian rhythm.
Behavioral Assessment
A diverse set of neurobehavioral tests were performed, recorded, and analyzed in a blinded fashion (Supplementary Fig. 1). Neurobehavior analyses were designed to evaluate sensorimotor function (modified neuro-severity score (mNSS), beam walk), non-spatial working memory (NOR), spatial memory (Y-maze spontaneous alteration test-SAT), associative learning and reference memory (passive avoidance test-PAT), locomotor activity and anxiety-like behavior (Open field-OF), and depression-related behavior (sucrose preference test-SPT). Cognitive assessments were conducted on separate days to avoid interference and fatigue. All behavioral analyses were performed/analyzed by a blinded investigator.
Assessment of Sensorimotor Function
We have previously reported that aged SHRs (14-m old) subjected to chronic hypoperfusion and young SHRs subjected to stroke and treated with C21 fully recover from motor deficits [23, 45]. To determine whether this is the case in aged animals (18-m old) subjected to stroke, the sensorimotor function was evaluated via mNSS, adopted from Yu et al. [50], and beam walk tests on days 1, 7, and 14, and weeks 5 and 8. mNSS is a 14-point neurological test that is designed to test sensory and motor function, abnormal movement patterns, absence of reflexes, and beam balance. Each domain is assigned a certain score that reflects its neurological status with lower scores indicating better performance. A maximum of 14 points indicates a severe neurological deficit [50]. Beam walk test was performed to assess body balance and motor coordination after surgery. Briefly, rats were placed on an elevated beam and allowed to move across the beam toward the narrow end for 60 s. Latency to cross the beam was used as an endpoint to evaluate motor function. A maximum score of 60 s was assigned in case of inability to cross the beam.
Assessment of Cognitive Function
NOR test was performed, as we previously reported [23, 35, 36] to evaluate non-spatial working memory related to both cortical and hippocampal damage [51,52,53]. The time spent exploring each object was noted and used to calculate the discrimination index (DI), which was defined as time spent exploring the novel object minus the time spent exploring the familiar object divided by the total exploration time.
PAT was conducted as we previously reported and involves two trials: an acquisition trial, when animals are exposed to an aversive stimulus, and a test trial, to check their latency to enter the arm where an aversive stimulus was previously introduced [23, 35, 36]. A shorter latency to enter the shock arm indicates a long-term memory deficit, while a longer latency suggests better memory. Those who did not cross over to the dark compartment were assigned a latency of 300 s.
SAT relies on the natural tendency of rats to explore a novel environment. Rats were introduced to the center of a Y-shaped maze and allowed to explore all three arms freely for a total of 7 min. Cognitively intact rats tend to explore the least recently visited arm and alternate their entries between all three arms. Alterations are described as successful entries into all three arms in consecutive triplet sets (i.e., ABC, ACB, BAC, BCA). An entry occurs when all four limbs are within the arm. ANY‐maze software was used to video record and automatically detect the total number of arm entries, and the number of triads. The percentage of spontaneous alternation was defined as the ratio of actual alternation to possible alternation.
OF was performed to evaluate anxiety and exploratory behavior. Rodents tend to have an aversion toward open areas. Typically, animals seek shelter closer to the walls, known as thigmotaxis. While an anxious animal would spend more time in the periphery, a low degree of thigmotaxis can be interpreted as a higher degree of risk-taking behavior [54, 55]. Rats were individually placed into the open field activity monitors (43.2 × 43.2 cm, Med Associates St. Albans, VT), and all behavioral events were controlled using Med-Associates software. Horizontal distance traveled (cm) was recorded during a 30-min test session. Distance traveled across the peripheral zone and central zone was analyzed as an indicator of locomotor activity and anxiety-like behavior.
In our prior work with young hypertensive animals, we revealed significant anhedonia development in our singly housed animals, leading us to use double housing for the duration of this study. To test whether this tactic was effective, SPT was performed at week 8 to assess anhedonia and depression-related behavior. Briefly, animals were separated from their partners and provided with a 1.5% sucrose solution for habituation. Twenty-four hours later, rats were presented with two bottles, normal drinking water and 1.5% sucrose solution, for 24 h. The positions of these two bottles were switched halfway to eliminate side bias. Water and sucrose intake were measured and used to calculate sucrose preference over water. Rodents show a preference toward sweetened solutions or food, while those with anhedonia, a key sign of depression, lose that interest.
Integrated Memory Score
Z-scoring, a dimensionless mathematical tool, was used to combine the outcomes of NOR, PAT, and SAT to obtain an integrated memory score [56, 57]. For each behavioral measure, the Z-score was calculated using the Z = X-mean of control/standard deviation (SD) of control. X is the individual data point of each parameter (RI for NOR, latency sec for PAT and % spontaneous alternation for SAT) for each animal. The vehicle treated sham group was used as the control to calculate the mean and SD. After a Z-score was calculated for each test, an integrated memory score was determined for each animal.
Magnetic Resonance Imaging (MRI) Acquisition and Processing
MRI scans were performed to assess the pathological structural changes in the brain. All rats underwent two types of MRI scans, T2-weighted (T2WI) and fluid-attenuated inversion recovery (FLAIR) scans. A detailed description of imaging is given in Supplementary Material. The Core Imaging Facility for Small Animals (CIFSA) performed all MRI scans at the Augusta University Cancer Center. Total brain and hemispheric ventricular and ischemic lesion volumes were evaluated using the Mango multi-image analysis software. For each brain scan, 35 slices were obtained with a thickness of 0.8 mm for each slice.
Assessment of Plasma Biomarkers for Neurodegeneration, Vascular Injury, and Demyelination
Plasma biomarkers were selected based on the recommendations of MarkVCID and Discovery (Determinants of Incident Stroke Cognitive Outcomes and Vascular Effects on RecoverY) Consortiums [58, 59]. Neuroaxonal injury (neurofilament light chain-NfL), neurodegeneration (total tau), and inflammation (glial fibrillary acidic protein-GFAP) were measured using the highly sensitive Meso Scale Discovery (MSD) S-Plex Neurology Panel (catalog number K15639S-1). Vascular injury (vascular endothelial growth factor VEGF-A/C, and basic fibroblast growth factor — bFGF) was assessed using the MSD V-Plex Angiogenesis Panel 1 (catalog number K15190D-1). Aβ42/40 levels were measured as a marker of amyloid burden with the V-Plex Ab peptide panel (catalog number K15199E-1) [60]. Samples were processed blindly by the Emory University Multiplexed Immunoassay Core.
Statistical Analysis
Comparisons between surgery groups, or treatment within stroke animals were performed using a two-sample t-test or Mann–Whitney U tests if needed. Differences in mortality for surgery (sham vs. stroke) and treatment (vehicle vs. C21) were assessed using a Cochran-Mantel–Haenszel test of general association. Differences for stroke and C21 treatment were assessed using a two surgery (sham vs. stroke) × 2 treatment (vehicle vs. C21) ANOVA where the interaction determined the differential effect of C21 on the presence of stroke. Dunn’s multiple comparison test was used to interpret significant interactions. Repeated measures of three-way ANOVA (RMANOVA) was used to assess differences between surgery and treatment across time and all interactions were explored. Within surgery, two-way RMANOVA was used to assess the relationship of C21 treatment over time for sham and stroke animals separately in order to aid the interpretation of significant interactions. Plasma biomarkers were analyzed by two-way ANOVA. ANOVA tables are shown on the graphs, and post hoc analyses with Tukey’s multiple comparison were marked on the graphs if significance was observed. For small sample sizes, the normality tests have little power to reject the null hypothesis that “sample distribution is a normal distribution,” and therefore, data with small sample sizes most often pass normality tests. Thus, data was not tested for normality. However, the data was plotted and assessed for outlying or influential observations and there were none. SAS© 9.4 (SAS Institute, Inc, Cary, NC) was used for all analyses. Significance was determined at predetermined alpha of 5%. Graphs were prepared using the GraphPad software.
Results
Aged Hypertensive Rats Demonstrate Preserved Overall Health Despite Brain Pathology and Progressive Cognitive Decline Consistent with VCID
Aged hypertensive rats illustrated a high level of survivability throughout the aging process, with a survival rate of 86.7% (52 out of 60 rats) (Fig. 1). Aged SHRs maintained good health and showed a continuous weight gain over the course of 18 months (Fig. 2A). Cognitive status was evaluated at 6 and 12 months via NOR. At 12 months, there was a significant decline in total exploration time suggesting changes in cognitive behavior.
Aged hypertensive rats exhibited a progressive cognitive decline and brain abnormalities that are consistent with vascular cognitive impairment. A Average body weight (g) over an 18-month follow-up. B Total exploration time in two-object novel object recognition test at 6 months (n = 60) versus 12 months (n = 56). Paired t-test; p-value < 0.0001. C Qualitative analysis of brain pathological changes at 18 months of age, assessed via brain MRI scans (n = 55). D Representative of two light/dark cycles for 7-day average mean arterial pressure readings. Readings were monitored via a telemetry transmission device (n = 2)
Imaging results showed that 18-month-old hypertensive rats displayed a high incidence of brain pathology and evidence of significant abnormalities consistent with vascular cognitive impairment. The most prevalent abnormality was enlarged ventricles (72%) followed by hematoma, old ischemic lesion, white matter hyperintensities, and hydrocephalus (Fig. 2C).
Aged Hypertensive Rats Demonstrate a Normal Circadian Rhythm Despite Severely Elevated Mean Arterial Pressure
Aged SHRs displayed an elevated MAP that ranged between 177 and 183 mm Hg and retained a normal circadian rhythm during the dark/light cycle (Fig. 2D).
Aged Hypertensive Rats Are Able to Survive Stroke Induced by a Short Ischemic Insult
There was no intraoperative mortality, but one rat died under anesthesia before undergoing either surgery. Mortality within 72 h was 14% in the stroke group (four rats out of 27) and 0% in the sham group. We anticipated a high mortality rate after surgery; however, overall survivability was better than expected. In the stroke group, the survival rate to week 8 was 100% and 88.9% in C21- and vehicle-treated groups, respectively (Fig. 3A). In the sham-operated group, the probability of survival was 75% in the C21 group and 88.9% in the control group (Fig. 3A).
Aged hypertensive rats demonstrated high survivability, and a prolonged sensorimotor deficit after stroke, which was ameliorated with C21 treatment. A The survival rate of aged SHRs after a 30-min tMCAO over an 8-week follow-up; Cochran-Mantel–Haenszel test of general association among surgery, treatment, and mortality; p-value = 0.93. The acute sensorimotor deficits were assessed 24 h after stroke via B mNSS and D beam walk test (n = 16 in sham group; n = 21 in tMCAO group, Mann Whitney test; p-value < 0.0001 for B and unpaired t-test; p-value = 0.0035 for D). C Sensorimotor deficits persisted over time. Three-way repeated measures of ANOVA (surgery X time X treatment) resulted in a significant interaction (p = 0.025) indicating that the relationship between surgery and treatment differs over time. Two-way repeated measures of ANOVA within the stroke group indicated that there is a significant interaction between treatment and time such that the C21 treated group improves over time but not the vehicle group. In the sham group, this effect was not observed. E Three-way repeated measures of ANOVA of beam walk data indicated a surgery and time effect (p-value = 0.0030 for surgery and < 0.0001 for time). Two-way ANOVA analysis within sham or stroke groups suggested a time effect in the stroke group (p = 0.0004) and a trend in the sham group (p = 0.053) (c) but no treatment effect with C21
Stroke Causes an Acute and Sustained Sensorimotor Deficit, Which Was Alleviated with C21 Treatment
Animals subjected to a brief tMCAO showed a significant sensorimotor deficit 24 h after stroke (Fig. 3B and D). Rats in the stroke vehicle group had a prolonged sensorimotor deficit throughout the study (Fig. 3C and E). Analysis of mNSS for the impact of surgery and treatment over time (three-way RANOVA) indicated a significant interaction suggesting that the relationship between surgery and treatment differs over time (Fig. 3C). When the impact of time and treatment was analyzed within sham or stroke groups individually by two-way RANOVA, a significant interaction between treatment and time was noted in stroked animals such that the C-21 treated group improved over time but not the vehicle group. In the sham group, this effect was not observed. Analysis of beam walk results for the impact of surgery and treatment over time (three-way RANOVA) indicated a surgery and time effect (Fig. 3E). two-way ANOVA analysis within sham or stroke groups suggested a time effect in the stroke group (p = 0.0004) and a trend in the sham group (p = 0.053) but no treatment effect with C21.
Stroke Induces Brain Edema at Day 3 and Chronic Brain Atrophy in the Ipsilateral Hemisphere at Week 8
MRI analyses revealed a significant reduction in the brain ventricular volume after stroke in both hemispheres on day 3 (Fig. 4A–C, G). This was prior to treatment being initiated and indicated acute cerebral edema in response to ischemic injury. There was a significant increase in the ipsilateral but not contralateral ventricular volume at week 8, evidence of chronic brain atrophy (Fig. 4D–G).
Stroke resulted in acute cerebral edema on day 3, and chronic brain atrophy in the ipsilateral hemisphere at week 8. Brain MRI scans were performed on day 3 (A–C) and week 8 (D–F) to assess changes in brain ventricular volume. On day 3, minor stroke reduced A total ventricular volume in both hemispheres (unpaired t-test p-value = 0.0022, B contralesional ventricular volume (unpaired t-test p-value = 0.0055, and C ipsilateral hemispheres (unpaired t-test p-value = 0.0027. (n = 8 for Sham, and n = 14 for tMCAO). At post-stroke week 8, there were no significant differences in the D total ventricular volume (unpaired t-test p-value = 0.1685) and E contralesional ventricular volume (unpaired t-test p-value = 0.4200). F A significant increase in the ipsilateral ventricular volume was observed at week 8 (unpaired t-test p-value = 0.0191). n = 14 for Sham, and n = 17 for tMCAO. G Representative images of brain MRI scans of sham- and tMCAO-operated rats at baseline, day 3, and week 8. Since there was no significant effect in the treatment groups (Supplementary Fig. 3), data were pooled based on the surgery group and regardless of treatment
C21 Improves Ischemic Resolution at the Chronic Phase After Stroke
Animals were randomly assigned to treatment on day 3. Lesion volume (day 3 — prior to treatment) in animals that received C21 was lower than in the vehicle group (Fig. 5A–B). When the change in lesion volume reduction was assessed as a percent reduction relative to lesion volume at post-stroke day 3, C21 reduced lesion volume by more than 70% at post-stroke week 8, as compared to around 44% in the control group (Fig. 5C).
C21 was associated with a better lesion resolution. Lesion volume was assessed at post-stroke day 3 and week 8. A Representative images of brain MRI scans at day 3 and week 8 after stroke. B Absolute lesion volume at day 3 and week 8 in both C21- and vehicle-treated animals; two-way-ANOVA p-value = 0.0050 for treatment, and p-value = 0.0004 for time. C C21 was associated with a significant > 70% reduction in lesion size (as compared to 44% in the control group). Percent reduction was calculated as the change in lesion size between week 8 vs day 3. Unpaired t-test, p-value = 0.0034, n = 5 per group
C21 Treatment Improves Plasma Biomarkers of VCID
At 8 weeks, there was a trend for increased plasma NfL in the stroke vehicle group indicative of demyelination [61]. C21 reduced NfL levels in both sham and stroke groups (Fig. 6A). GFAP, a marker of inflammation, was significantly higher in the vehicle stroke group as compared to the sham vehicle and there was a trend (p = 0.06) for a decrease with C21 treatment (Fig. 6B) [62]. Post hoc analysis indicated a significant difference between sham and stroke vehicle groups. The stroke vehicle group exhibited higher total tau levels suggesting worsened neurodegeneration. C21 prevented this increase (Fig. 6C). Post hoc analysis showed that the stroke vehicle group was different as compared to the sham vehicle or stroke C21 groups. Human studies suggest that a lower Aβ42/40 ratio is associated with greater amyloid burden in the brain [60]. In this study, plasma Aβ42/40 ratio indicated an interaction in which C21 treatment improved the ratio in the stroke group while lowering it in the sham group (Fig. 6D) [60]. There was no notable difference in angiogenic markers VEGF A, VEGF C, or FGF (not shown).
Changes in plasma biomarkers indicate neuroaxonal pathologies after stroke. A Neuroaxonal injury (neurofilament light chain-NfL), B inflammation (glial fibrillary acidic protein-GFAP), and C neurodegeneration (total tau) were measured using the highly sensitive Meso Scale Discovery (MSD) S-Plex Neurology Panel. D Aβ42/40 levels were measured with the MSD V-Plex Ab peptide panel. Stroke caused an increase in NfL, GFAP and total tau and C21 treatment prevented these increases. While there was no change in Aβ42/40 ratio by stroke, C21 improved this ratio. Two-way ANOVA analysis tables are given on each figure and where applicable, significant post hoc results are marked on the graphs. n = 5–6/group
Stroke Exacerbates the Progression of Cognitive Decline in Aged Hypertensive Rats
PAT, performed at week 5, demonstrated a significant reduction in the latency to cross the shock arm in the stroke vehicle group, indicating that stroke exacerbated aversive learning. There was a significant surgery and treatment interaction such that treatment improved latency in the stroke but not sham groups. (Fig. 7A). Multiple comparisons by post hoc analysis indicated that only the stroke vehicle group was different from the others.
Stroke impaired associative learning and exploratory behavior in aged hypertensive rats. Post-stroke cognitive decline was assessed at weeks 4 (Y-maze), 5 (NOR, and PAT), and 8 (NOR). A There was a significant surgery and treatment interaction such that treatment improved latency in the stroke but not sham groups. Multiple comparisons by Dunn’s multiple comparisons test indicated that only vehicle stroke group is different than other groups. n = 7 for vehicle-treated sham, n = 6 for C21-treated sham, n = 8 for vehicle-treated stroke, and n = 9 for C21-treated stroke. Y-maze data was inconclusive due to the high variability. Most rats had an alternation of < 50%, even before the surgery (B) and in the sham group at week 4 (C) suggesting a severe cognitive decline as a result of advanced age and/or hypertension; n = 8 for vehicle-treated sham, n = 6 for C21-treated sham, n = 8 for vehicle-treated stroke, and n = 9 for C21-treated stroke. D There was a progressive reduction in total exploration time over rats’ lifespans. One-way repeated measures ANOVA (p-value < 0.0001 for 6 months vs 12; p-value = 0.015 for 12 vs 18 months; p-value = 0.94 for 18 vs 19 months; p-value = 0.33 for 19 vs 20 months). Total exploration time (measured at 18 m of age before stroke and 8 weeks after stroke) was not significantly changed by time or treatment in the sham groups (E). Total exploration time was decreased in the stroke groups (F)
The Y-maze alternation test revealed a wide range of variability with more rats being within or less than 50% alternation at baseline and post-stroke week 4 (Fig. 7 B and C). In NOR test, there was a significant deterioration of total exploration time before and after stroke surgery (Fig. 7D). Analysis of total exploration time as an index of NOR showed that the exploratory behavior significantly decreased in the stroke but not sham groups (Fig. 7 E and F). DI was similar across groups at baseline before surgery and at week 5 (Fig. 8 A and B). % change in DI at 8 weeks after surgery showed high variability, especially in the sham C21 group and a significant decline in the stroke vehicle group (Fig. 8C). Integrated memory score which was calculated as a combined Z-score of DI (NOR), latency (PAT), and spontaneous alternation (Y-maze) showed surgery and treatment main effects (Fig. 8D). Post hoc analysis indicated a trend for lower memory scores in the stroke vehicle group as compared to the sham vehicle group (p = 0.07). C21 treatment prevented this decline in the stroke (p = 0.03) group.
Stroke exacerbated cognitive decline in aged hypertensive rats. The discrimination index (DI) measure of NOR was similar in all groups at baseline before stroke surgery (A) and at week 5 (B). C At week 8, change from baseline was evident in the stroke groups. D Integrated memory score calculated as the mean of z-scores for RI (NOR), latency (PAT), and % spontaneous alternation (Y-maze) of each animal allowed the combination of the results of three different cognitive tests. Stroke caused a decrease in the integrated memory score and C21 treatment also showed a main effect as determined by two-way ANOVA. Post hoc analysis showed a trend between stroke vehicle versus sham vehicle (p = 0.07) and a significant difference between vehicle and C21 in the stroked animals (p = 0.03). n = 6–9
Aged Hypertensive Rats Exhibit Altered Exploratory Behavior and Significant Hyperactivity After Stroke with No Indication of Anxiety-Like Behavior
Gross locomotor activity and anxiety-like behavior were evaluated via an open field test at week 5. Animals in the stroke group covered significantly more distance in the open field compared to the control group. Neither the treatment group nor the interaction between surgery and treatment showed any difference (two-way ANOVA p-value = 0.01 for surgery) (Fig. 9A). Further analysis demonstrated that all rats traveled comparable distances in the inner and outer zones, indicating no difference in locomotion (Fig. 8 B and C). Aged rats in all groups covered more distance in the center zone which is unusual for rodents that typically spend more time in the periphery [54, 55]. This altered exploratory behavior suggests more risk-taking behavior in the aged animals.
Aged SHRs showed signs of risk-taking behavior and stroke-mediated hyperactivity with no signs of anxiety- and depression-like behavior. The open field test was performed at week 5 to assess locomotor activity and anxiety-like behavior. A Aged hypertensive rats demonstrated stroke-induced hyperactivity as was assessed via the total distance traveled. B and C SHRs traveled more distance in the central zone which is not a typical rodent behavior and indicates risk-taking behavior. Stroke did not affect the exploratory behavior. D Sucrose preference test was performed at week 8 to assess anhedonia which was evaluated via the percentage of preferring sucrose over plain drinking water. All rats regardless of their group assignment displayed an interest in consuming more sucrose than water. The variability was greater in the stroke vehicle group and C21 improved sucrose preference only in the stroke group resulting in an interaction
Aged Hypertensive Rats Do Not Display Depression-Related Behavior at the Chronic Phase After Stroke
A sucrose preference test was performed at week 8 to assess anhedonia and depression-related behavior. While it was not significant, the variability was greater in the stroke vehicle group. C21 treatment prevented this effect in the stroke group resulting in a surgery and treatment interaction (Fig. 8D).
Discussion
Animal models of stroke typically failed to include major comorbidities such as advanced age and hypertension, which likely contributed to a consistent lack of translation of experimental treatments into clinical care. It has been reported that only 11.4% of preclinical studies of stroke included advanced age and/or other comorbidities [46]. These comorbidities have been overlooked due to uncertain mortality and increased cost [63]. Recently completed SPAN (Stroke Preclinical Assessment Network) preclinical trial highlighted the importance of incorporating age and disease models in the search for novel treatments for acute ischemic stroke [64, 65]. A similar approach is yet to be applied to VCID research which includes cognitive impairment due to vascular disease and PSCI [12, 13]. In the current study, to the best of our knowledge, we are the first to report the development of VCID in 18-month-old SHRs prior to and following a stroke.
Aged hypertensive rats displayed a progressive cognitive decline, brain atrophy, and abnormalities that are consistent with VCID. As early as 12 weeks, exploratory behavior of animals changed as indicated by lower exploration times in NOR. Brain pathological changes were present in most animals before stroke. Enlarged ventricular volume was the most prevalent abnormality. Hypertension and/or advanced age are well-known to be contributors to brain atrophy in patients [66]. Enlarged ventricular volume and brain atrophy were observed in older individuals, and in those with elevated systolic blood pressure [67]. This agrees with our findings where most aged hypertensive animals exhibited brain atrophy and early cognitive decline as early as 12 months old validating the clinical relevance of our model to study VCID and the development of PSCI. Moreover, in this study, we had a relatively low mortality rate after surgery. We previously reported a higher mortality rate after stroke in diabetic animals as well as young hypertensive animals [36]. High mortality rate in aged animals following stroke was observed in several other studies as well [68,69,70,71]. In the current study, there was no intraoperative mortality and overall mortality in the 8-week follow-up was low. This is likely due to the reduction in occlusion time from 60 to 30 min. A 30-min tMCAO effectively produced a visible ischemic lesion in day 3 MRI, as well as significant sensorimotor deficit 24 h after stroke. High survivability in aged hypertensive rats after stroke encourages preclinical studies in a more clinically relevant model of stroke.
Stroke resulted in acute and prolonged sensorimotor deficits throughout the study. This uniquely differs from what has been previously reported in young hypertensive and aged normotensive animals, where animals recovered to their baseline in the weeks after stroke [23, 35]. This is consistent with the manifestation of stroke in patients. The delayed-chronic stimulation of AT2R profoundly blunted the long-term sensorimotor deficit. MRI analysis revealed an acute ischemic lesion formation, cerebral edema, and long-term brain atrophy in the ipsilateral hemisphere after stroke. Delayed C21 treatment was associated with a 70% reduction in lesion volume at the chronic phase after stroke but had no effect on brain atrophy.
Aged hypertensive rats showed early signs of cognitive decline at 12 months. We anticipated that this cognitive decline would progress over time and worsen after stroke. Long-term cognitive assessments were performed at post-stroke week 4 and beyond. PAT revealed an impaired long-term reference memory at week 5 which was prevented by C21 treatment. This impairment was not observed in Y-maze in part due to the high variability. NOR also indicated a decline in the discrimination index in the stroked animals, but C21 treatment did not prevent this decline, again in part due to high variability in treatment groups. To address this problem and evaluate the cognitive status of animals more comprehensively, we applied integrated behavioral Z-scoring which has been reported to increase the sensitivity and reliability of behavioral phenotyping in rodents [56, 57, 72]. This approach has been mostly used in anxiety research. Using the combination of Z-scores for each test, we were able to assess the overall cognitive/memory function which showed a progressive decline after stroke and treatment with C21 was effective in the stroke but not the sham group.
We also noted that rats exhibited a lack of interest in exploring either object suggestive of apathy which is a common finding in individuals with cognitive demise. The lack of interest in exploring objects could be explained, perhaps, by either depression or the lack of ambulation. However, these were ruled out since animals did not exhibit anhedonia in the sucrose preference test, and the open field test demonstrated stroke-induced hyperactivity. We were able to rule out that the decrease in exploration time upon repeated testing over eight weeks was an inherent limitation in the NOR itself. When we assessed the exploration time changes in a group of young sham SHRs in a separate study, using the same NOR assessment methods, we found no significant change in exploration time over eight weeks (Supplemental Fig. 4). Furthermore, we did not see a decrease in exploration time with repeated testing in our previous studies in young diabetic and hypertensive animals [45, 73]. Thus, we propose that decreased exploration time indicative of apathy can supplement traditional indices measured in NOR.
One of the most noteworthy findings in this study is the ability of C21 to mediate beneficial effects even when treatment was delayed. Chronic treatment with RAS modulators prevented demyelination, enhanced cognitive function and prevented the development of PSCI in hypertensive animals [23]. These effects were persistent even with delayed treatment, 7 days after stroke, and believed to be independent of blood pressure [23]. A similar pattern was observed in diabetic animals when treatment with C21 was initiated 72 h after stroke and continued for 4 and 8 weeks [36, 37]. This study further corroborates these findings and points toward the importance of long-term treatment after stroke. Moreover, to improve the translational potential of our findings, this study followed the recommendations of the MarkVCID and Discovery Consortiums [58, 59] that focus on the identification of fluid and imaging biomarkers of VCID and PSCI for diagnosis and monitoring of the disease progression. We have found that the Aβ42/40 ratio was decreased and biomarkers of neuroaxonal injury (NfL GFAP and total tau) were increased in the plasma after stroke which were prevented by C21 treatment. The evidence supporting the role of RAS modulation is mixed at this point, and the scientific community has virtually abandoned this pathway as a viable stroke treatment. However, we firmly believe that more investigation is warranted. First, the focus should shift toward the development of post-stroke cognitive impairment as a target for later intervention after stroke. Second, chronic treatment after stroke, beyond the usual 5–7-day treatments studied in clinical trials, should be pursued to improve long-term outcomes. Focusing beyond the acute intervention after stroke could potentially be an alternative strategy to enhance long-term functional recovery after stroke. A well-designed clinical trial addressing these important aspects of RAS modulation should target the later development of long-term PSCI.
We recognize several shortcomings of our study. First, animals were randomized to treatment on day 3 after the presence of ischemic injury was confirmed. It turned out that day 3 lesion volume was smaller in the rats assigned to C21. Second, lesion resolution at week 8 was better in the C21-treated animals. The smaller infarct size and/or enhanced resolution may have contributed to the better outcomes in this group. It is important to note that C21 did not influence brain atrophy measured by MRI. Third, we do not have control nonhypertensive rats in this study. However, we previously showed that C21 was effective in 15-m-old Wistar rats [35]. Finally, we lack histological data that would have provided mechanistic information on lesion progression, C21 effects, and recovery. Brain specimens, especially brains from stroked animals, were too fragile for tissue processing which resulted in loss of tissue. This precluded proper comparisons of groups. Alternatively, we evaluated plasma biomarkers of neurodegeneration, demyelination, and amyloid burden that are currently being evaluated in MarkVCID and DiscoverY consortiums [58,59,60,61,62]. Collectively, we provide evidence that aged hypertensive rats demonstrate high survivability pre- and post-stroke. A short ischemic insult resulted in cerebral edema and brain atrophy as well as acute and prolonged sensorimotor deficits. Based on decreased associative learning and altered exploratory activity indicated by greater risk-taking behavior at week 5 and decreased exploration time and declines in discrimination index and composite memory score at week 8, we conclude that age-related cognitive decline progressively worsened after stroke in SHRs. There was no evidence of post-stroke depression, likely due to double housing and maintaining the same partner throughout the study. Use of a Z-scoring-based memory score improved classical cognitive assessment tools and allowed for the combination of test scores that evaluate different domains of memory and cognition. Delayed stimulation of AT2R resulted in a sustained improvement in sensorimotor function and a significant reduction in lesion volume at the chronic phase after stroke. Moreover, C21 treatment improved cognitive behavior. PSCI is a leading cause of VCID. Collectively, our novel results suggest that SHRs serve as a good comorbid disease model to integrate into VCID research and AT2R stimulation is a viable therapeutic target.
Data Availability
No datasets were generated or analysed during the current study.
Change history
05 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12975-024-01263-8
References
Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995–2010. Stroke. 2013;44(1):138–45.
Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006–18.
Levine DA, et al. Risk factors for poststroke cognitive decline: the REGARDS study (Reasons for Geographic and Racial Differences in Stroke). Stroke. 2018;49(4):987–94.
Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. Int J Stroke. 2012;7(1):61–73.
Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension. Circ Res. 2019;124(7):1025–44.
Gorelick PB, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–713.
Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke. 2012;43(11):3137–46.
Foulquier S, et al. Hypertension-induced cognitive impairment: insights from prolonged angiotensin II infusion in mice. Hypertens Res. 2018;41(10):817–27.
Reitz C, et al. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64(12):1734–40.
Yang Q, et al. Vital signs: recent trends in stroke death rates — United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;66(35):933–9.
Lackland DT, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke. 2014;45(1):315–53.
Zlokovic BV, et al. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 2020;16(12):1714–33.
Corriveau RA, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36(2):281–8.
Mijajlovic MD, et al. Post-stroke dementia — a comprehensive review. BMC Med. 2017;15(1):11.
Leys D, et al. Poststroke dementia. Lancet Neurol. 2005;4(11):752–9.
Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69(3):143–79.
Hullinger R, Puglielli L. Molecular and cellular aspects of age-related cognitive decline and Alzheimer’s disease. Behav Brain Res. 2017;322(Pt B):191–205.
Caprioli A, et al. Spatial learning and memory in the radial maze: a longitudinal study in rats from 4 to 25 months of age. Neurobiol Aging. 1991;12(5):605–7.
Burger C, et al. Changes in transcription within the CA1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiol Learn Mem. 2007;87(1):21–41.
Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2(1):51–61.
Kruyer A, et al. Chronic hypertension leads to neurodegeneration in the TgSwDI mouse model of Alzheimer’s disease. Hypertension. 2015;66(1):175–82.
Jackson L, et al. Within the brain: the renin angiotensin system. Int J Mol Sci. 2018;19(3):876. https://doi.org/10.3390/ijms19030876.
Ahmed HA, et al. Role of angiotensin system modulation on progression of cognitive impairment and brain MRI changes in aged hypertensive animals - a randomized double- blind pre-clinical study. Behav Brain Res. 2018;346:29–40.
Fagan SC, et al. Hypertension after experimental cerebral ischemia: candesartan provides neurovascular protection. J Hypertens. 2006;24(3):535–9.
Kozak A, et al. Candesartan augments ischemia-induced proangiogenic state and results in sustained improvement after stroke. Stroke. 2009;40(5):1870–6.
Guan W, et al. Acute treatment with candesartan reduces early injury after permanent middle cerebral artery occlusion. Transl Stroke Res. 2011;2(2):179–85.
Guan W, Kozak A, Fagan SC. Drug repurposing for vascular protection after acute ischemic stroke. Acta Neurochir Suppl. 2011;111:295–8.
Guan W, et al. Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS ONE. 2011;6(9):e24551.
Alhusban A, et al. AT1 receptor antagonism is proangiogenic in the brain: BDNF a novel mediator. J Pharmacol Exp Ther. 2013;344(2):348–59.
Ishrat T, et al. Candesartan reduces the hemorrhage associated with delayed tissue plasminogen activator treatment in rat embolic stroke. Neurochem Res. 2013;38(12):2668–77.
Alhusban A, et al. Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. J Hypertens. 2015;33(1):170–80.
Ishrat T, et al. Role of matrix metalloproteinase activity in the neurovascular protective effects of Angiotensin antagonism. Stroke Res Treat. 2014;2014:560491.
Fouda AY, et al. Brain-derived neurotrophic factor knockdown blocks the angiogenic and protective effects of angiotensin modulation after experimental stroke. Mol Neurobiol. 2017;54(1):661–70.
Fouda AY, et al. Contralesional angiotensin type 2 receptor activation contributes to recovery in experimental stroke. Neurochem Int. 2022;158:105375.
Ahmed HA, et al. Angiotensin receptor (AT2R) agonist C21 prevents cognitive decline after permanent stroke in aged animals—a randomized double- blind pre-clinical study. Behav Brain Res. 2019;359:560–9.
Jackson L, et al. Delayed administration of angiotensin II type 2 receptor (AT2R) agonist compound 21 prevents the development of post-stroke cognitive impairment in diabetes through the modulation of microglia polarization. Transl Stroke Res. 2020;11(4):762–75.
Jackson-Cowan L, et al. Delayed administration of angiotensin receptor (AT2R) agonist C21 improves survival and preserves sensorimotor outcomes in female diabetic rats post-stroke through modulation of microglial activation. Int J Mol Sci. 2021;22(3):1356. https://doi.org/10.3390/ijms22031356.
Eldahshan W, et al. Stimulation of angiotensin II receptor 2 preserves cognitive function and is associated with an enhanced cerebral vascular density after stroke. Vascul Pharmacol. 2021;141:106904.
McCarthy CA, et al. Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PLoS ONE. 2014;9(4):e95762.
Ishrat T, et al. Low-dose candesartan enhances molecular mediators of neuroplasticity and subsequent functional recovery after ischemic stroke in rats. Mol Neurobiol. 2015;51(3):1542–53.
Jing F, et al. Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J Cereb Blood Flow Metab. 2012;32(2):248–55.
Fuchtemeier M, et al. Vascular change and opposing effects of the angiotensin type 2 receptor in a mouse model of vascular cognitive impairment. J Cereb Blood Flow Metab. 2015;35(3):476–84.
Gelosa P, et al. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens. 2009;27(12):2444–51.
Bennion DM, et al. Post-stroke angiotensin II type 2 receptor activation provides long-term neuroprotection in aged rats. PLoS ONE. 2017;12(7): e0180738.
Ahmed HA, et al. RAS modulation prevents progressive cognitive impairment after experimental stroke: a randomized, blinded preclinical trial. J Neuroinflammation. 2018;15(1):229.
McCann SK, Lawrence CB. Comorbidity and age in the modelling of stroke: are we still failing to consider the characteristics of stroke patients? BMJ Open Science. 2020;4(1):e100013.
Kaiser D, et al. Spontaneous white matter damage, cognitive decline and neuroinflammation in middle-aged hypertensive rats: an animal model of early-stage cerebral small vessel disease. Acta Neuropathol Commun. 2014;2:169.
Eldahshan W, et al. Angiotensin II type 2 receptor stimulation with compound 21 improves neurological function after stroke in female rats: a pilot study. Am J Physiol Heart Circ Physiol. 2019;316(5):H1192–201.
Ejaz S, et al. What is the optimal duration of middle-cerebral artery occlusion consistently resulting in isolated cortical selective neuronal loss in the spontaneously hypertensive rat? Front Neurol. 2015;6:64.
Yu J, et al. Reliability of behavioral tests in the middle cerebral artery occlusion model of rat. Lab Anim. 2019;53(5):478–90.
Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20(23):8853–60.
Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13(2):93–110.
Buckmaster CA, et al. Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. J Neurosci. 2004;24(44):9811–25.
Tucker LB, McCabe JT. Measuring anxiety-like behaviors in rodent models of traumatic brain injury. Front Behav Neurosci. 2021;15: 682935.
Valvassori SS, Varela RB, Quevedo J. Animal models of mood disorders: focus on bipolar disorders and depression. In Animal models for the study of human disease. Elsevier Inc; 2020. pp. 991–1001. https://doi.org/10.1016/B978-0-12-809468-6.00038-3.
Guilloux JP, et al. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods. 2011;197(1):21–31.
Kraeuter AK. The use of integrated behavioural z-scoring in behavioural neuroscience - a perspective article. J Neurosci Methods. 2023;384:109751.
Rost NS, et al. Cognitive impairment and dementia after stroke: design and rationale for the DISCOVERY study. Stroke. 2021;52(8):e499–516.
Wilcock D, et al. MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols. Alzheimers Dement. 2021;17(4):704–15.
Perez-Grijalba V, et al. Plasma Abeta42/40 ratio detects early stages of Alzheimer’s disease and correlates with CSF and neuroimaging biomarkers in the AB255 study. J Prev Alzheimers Dis. 2019;6(1):34–41.
Kautz TF, Mathews JJ, Parent D, Sudduth TL, Liu Q, Wiedner C, Wang C-P, et al. Validation and utility of plasma neurofilament light as a biomarker for vascular cognitive impairment. Alzheimer's & Dementia. 2022;18(S6):e065811. https://doi.org/10.1002/alz.065811.
Gonzales MM, et al. A population-based meta-analysis of circulating GFAP for cognition and dementia risk. Ann Clin Transl Neurol. 2022;9(10):1574–85.
Candelario-Jalil E, Paul S. Impact of aging and comorbidities on ischemic stroke outcomes in preclinical animal models: a translational perspective. Exp Neurol. 2021;335:113494.
Lyden PD, et al. A multi-laboratory preclinical trial in rodents to assess treatment candidates for acute ischemic stroke. Sci Transl Med. 2023;15(714):eadg8656.
Morais A, et al. Embracing heterogeneity in the multicenter Stroke Preclinical Assessment Network (SPAN) trial. Stroke. 2023;54(2):620–31.
Kern KC, et al. Blood pressure control in aging predicts cerebral atrophy related to small-vessel white matter lesions. Front Aging Neurosci. 2017;9:132.
Goldstein IB, et al. Ambulatory blood pressure and brain atrophy in the healthy elderly. Neurology. 2002;59(5):713–9.
Shapira S, et al. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925(2):148–58.
Ritzel RM, et al. Aging alters the immunological response to ischemic stroke. Acta Neuropathol. 2018;136(1):89–110.
Liu F, et al. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29(4):792–802.
Liu F, McCullough LD. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotechnol. 2011;2011:464701.
Meng B, et al. The investigation of hippocampus-dependent cognitive decline induced by anesthesia/surgery in mice through integrated behavioral Z-scoring. Front Behav Neurosci. 2019;13:282.
Jackson L, et al. Microglia knockdown reduces inflammation and preserves cognition in diabetic animals after experimental stroke. J Neuroinflammation. 2020;17(1):137.
Acknowledgements
Authors thank Vicore Pharma (Göteborg, Sweden) for the supply of Compound 21. We would like to acknowledge the core imaging facility for small animals (CIFSA) of the Georgia Cancer Center for their assistance in MRI analysis, the small animal behavioral core for assistance with PAT and OF tests, the animal facility staff at Augusta University and Charlie Norwood Department of Veterans Affairs Medical Center, Augusta, GA for exemplary care of our aged animals, and Ms. Maribeth A. Johnson for statistical analyses.
Funding
This study was supported by the VA Merit Review (BX000347), VA Senior Research Career Scientist Award (IK6 BX004471), NIH RF1 NS083559 (formerly R01 NS083559), and R01 NS104573 (multi-PI, SCF as co-PI) to AE.
Author information
Authors and Affiliations
Contributions
SCF and AE conceived and designed research; BP, AA, PK, MBK, WL, DCH and TWJ reviewed experimental plan and/or performed experiments; AA, BP, TWJ, AB, SCF, and AE analyzed data; AA, PRS, SCF, and AE interpreted results of experiments and prepared figures; AA drafted manuscript; AA, PRS, SCF, and AE edited and revised manuscript; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experiments were performed following the National Institutes of Health Guide for the Care and Use of Laboratory Animals and ARRIVE guidelines. Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Charlie Norwood Veterans Affairs Health Care System, and Augusta University, Augusta, Georgia.
Consent for Publication
All authors give their consent and approval for publication of this manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary file1
Supplementary Fig. 1. Schematic depiction of experimental design. Adapted from “Mouse Experimental Timeline”, by BioRender.com (2023). Retrieved from https://app.biorender.com/biorender-templates (PNG 572 kb)

Supplementary file2
Supplementary Fig. 2. Stroke resulted in a significant weight loss at 24 hours, and C21 helped them maintain their weight. (A) On average, rats in the sham group lost 4.9 grams, while those in the tMCAO group lost 22.9 grams. Unpaired t-test; p-value <0.0001. (B) Post-stroke 5-day average body weight was comparable between groups. For the sham group it was 378g and 375g, in vehicle- and C21-treated rats, respectively. Vehicle-treated tMCAO group averaged around 354g, while those treated with C21 averaged around 357g; Upon treatment initiation, C21-treated group started to maintain/gain weight, and vehicle-treated animals continued to lose weight 3-way Repeated measure ANOVA (p-value=0.0395 for all interaction). Significant effect of treatment observed in both Sham and stroke groups (3-way Repeated measure ANOVA p-value=0.044 and 0.041, respectively). (C) Weight trend from baseline to post-stroke week 8; Baseline: 2-way ANOVA (p-value=0.18 for surgery, p-value=0.62 for treatment, and 0.14 for their interaction). A significant surgery-time interaction observed from day 1 through 5; 3-way repeated measure ANOVA (p-value=0.0039). There was a significant effect of time from week 2 throughout the end of the study; 3-way repeated measure ANOVA (p-value=0.022). (PNG 190 kb)

Supplementary file3
Supplementary Fig. 3. C21 had no effect in stroke-induced acute cerebral edema, and chronic brain atrophy. Brain MRI scans were performed on day 3 (A-B) and week 8 (C-D) to assess changes in brain ventricular volume. Stroke significantly lowered the ventricular volume in both hemispheres at day 3 MRI scans; 2-way ANOVA (p-value=0.0026 for surgery, and 0.1195 for treatment in the contralesional hemisphere) (A) and (p-value=0.0037 for surgery, and 0.613 for treatment in the ipsilateral hemisphere) (B). Stroke resulted in a significant increase in the ipsilateral ventricular volume at week 8, with no difference between the treatment groups; 2-way ANOVA (p-value=0.3436 for surgery, and 0.9128 for treatment in the contralesional hemisphere) (C) and (p-value=0.012 for surgery, and 0.1076 for treatment in the ipsilateral hemisphere) (D). (PNG 234 kb)

Supplementary file4
Supplementary Fig. 4. Decreased exploration time is observed in the aged but not young SHRs. Total exploration time in vehicle-treated young sham SHRs (A) and vehicle-treated aged sham SHRs. (PNG 93 kb)
Rights and permissions
About this article
Cite this article
Alshammari, A., Pillai, B., Kamat, P. et al. Angiotensin II Type 2 Receptor Agonism Alleviates Progressive Post-stroke Cognitive Impairment in Aged Spontaneously Hypertensive Rats. Transl. Stroke Res. (2024). https://doi.org/10.1007/s12975-024-01232-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12975-024-01232-1