Abstract
MicroRNAs (miRNAs) are short sequenced non-coding RNAs that posttranscriptionally regulate gene expression. We investigated circulating miRNA expression levels in acute stroke patients and its relationship with future vascular event. We included acute ischemic stroke patients who admitted to a university hospital between May 1, 2011, and July 31, 2012, and the patients with vascular risk factors but not incident stroke as controls. We collected 5 ml of venous blood, and circulating miRNA levels were evaluated by quantitative real-time polymerase chain reaction. Five miRNAs (miR-17, miR-21, miR-106a, miR-126, and miR-200b), which had been reported to be related to atherosclerosis, were measured. The levels of miRNAs were compared with the presence of acute stroke, vascular risk factors, stroke subtypes, and stroke recurrence after index stroke. A total of 120 patients were included in the study, with 83 acute stroke patients. The levels of miR-17 were significantly increased in acute stroke patients, and the levels of miR-126 had positive correlation with cerebral atherosclerosis (r = 0.254, p = 0.021). Among the 83 stroke patients, eight experienced stroke recurrence during follow-up and higher level of miR-17 was associated with shorter event-free survival (p = 0.047). This study shows that the miR-17 level was elevated in acute ischemic stroke and associated with future stroke recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of stroke is increasing rapidly in concert with societal aging and is related to high mortality and disability. Although diagnostic accuracy has been aided by the advents of new brain imaging modalities, little progress has been achieved with respect to the development of blood biomarkers that predict stroke outcome or the burden of underlying atherosclerosis [1]. The introduction of various blood biomarkers of ischemic heart disease has enormously improved treatment strategy and clinical study performance on myocardial infarction, and thus, efficient blood biomarkers of ischemic stroke and cerebral artery atherosclerosis based on disease pathophysiology are urgently required [2]. MicroRNAs (miRNAs) are small non-coding RNAs with 20–25 nucleotides that posttranscriptionally regulate gene expression. Recently, circulating miRNAs have been studied in several disease backgrounds, and their expression patterns have been found to reflect disease subtype and prognosis. These findings extend the scope of miRNA from being representative of tissue-specific local machinery to being systemic representative of disease burden [3].
Several miRNAs have been studied in stroke using experimental models and small groups of patients [4–6]. Studies on animal models showed dynamic changes in the miRNA expression, and in particular, miR-200b and miR-298 levels were increased both in ischemic and hemorrhagic stroke models [4]. Another study on young stroke patients reported that miRNA expression patterns vary according to stroke mechanism [5]. In a recent study, symptomatic and asymptomatic carotid plaques harvested after carotid endarterectomy were found to exhibit different miRNA expression profiles [7]. However, little available data supports a relation between circulating miRNA levels in acute stroke and its prognosis.
We hypothesized that miRNAs related to atherosclerosis or hypoxia would be altered in the blood of acute ischemic stroke patients and their initial expression levels will be able to reflect atherosclerosis activity and to predict future vascular event. In this regard, we selected five miRNAs related with atherosclerosis by literature review and evaluated their expression patterns in acute stroke patients and control subjects with vascular risk factors.
Methods
Patient Inclusion and Clinical Variables
Between May 1, 2011, and July 31, 2012, acute ischemic stroke patients who admitted to Seoul National University Hospital were enrolled, and those patients who were admitted to control vascular risk factors or other neurological problems but without incident stroke during the same time period served as a control group. We obtained basic demographic data, clinical history including vascular risk factors, stroke subtypes, laboratory data and brain MR imaging (MRI)/MR angiography (MRA). Vascular risk factors included hypertension (systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg or on antihypertensive medication), diabetes mellitus (fasting blood sugar >7.0 mmol/L or hemoglobin A1c >6.5 % or on glucose-lowering medication), heart disease with potential embolic source, and smoking status. Stroke subtype was determined by Trial of Org 10172 in Acute Stroke Treatment classification as (1) large artery atherosclerosis (LAA) with significant stenosis in a relevant artery, (2) cardioembolic infarction (CE) with a documented potential cardioembolic source without relevant artery stenosis, and (3) small vessel occlusion (SVO), when an infarction had a typical location and size of lacunar stroke without large artery stenosis or a potential embolic source [8]. Patients with other stroke etiology, such as a genetic cause, a hypercoagulable state, combined malignancy, vasculitis, or an infection-related condition were excluded. Written informed consent was obtained from each patient before inclusion, and this study was approved by the institutional review board of Seoul National University Hospital (0906-028-282) and performed conform the declaration of Helsinki.
Atherosclerotic Burden and Clinical Outcome Assessment
Brain MRI was performed using a 3.0-T unit (Signa, GE medical systems, Milwaukee, WI, USA) and an eight-channel head coil including T1- and T2-weighted, diffusion weighted, fluid-attenuated inversion recovery, gradient echo images, and time-of-flight MRA. The degree of cerebral atherosclerosis was determined using brain MRA findings with previously reported scoring system [9]. In brief, stenosis was graded into three levels: 0 indicating <50 % stenosis, 1 indicating 50 to 99 % stenosis, and 2 indicating occlusion, which was assessed separately from anterior cerebral, middle cerebral, posterior cerebral, internal carotid, vertebral, and basilar arteries [9]. The atherosclerosis score was defined as the sum of the scores of these arteries on three-dimensional MRA [9]. High-resolution vessel wall MRI was additionally performed among the 12 stroke patients with intracranial atherosclerosis by previously described protocol, and enhanced atheroma from T1 enhancement protocol was considered as vulnerable plaque [10]. The target atherosclerotic vessel for high-resolution MRI evaluation was determined from initial MRA image review. The black blood technique with fat pre-regional saturation pulses of 80-mm thickness to saturate incoming arterial flow was used, and high-resolution MR sequences using a 3.0-T unit (Verio, Siemens Medical Solution, Erlangen, Germany) included T1-weighted images acquired with repetition time (TR)/echo time (TE) = 600/12 ms, T2-weighted images acquired with TR/TE = 2910/70 ms, proton density (PD) images acquired with TR/TE = 2500/30 ms, and T1-weighted images with gadolinium enhancement. All images were taken with a field of view = 120 × 120 mm, slice thickness 2 mm, matrix size = 384 × 269, and number of average = 4. Patient functional status was measured by National Institutes of Health Stroke Scale on admission and by modified Rankin’s score at discharge, and further dichotomized as good outcome when discharge modified Rankin’s score was 0 or 1. All stroke patients were monitored regularly after discharge every 2 or 3 months to detect recurrence of cerebral infarction.
MicroRNA Sample and Expression Levels
A peripheral blood sample of 5 ml was harvested in an EDTA bottle from each patient. For acute stroke patients, blood sample was harvested within 7 days after symptom onset. Plasma samples were immediately extracted after centrifugation at 1500g for 15 min at 4 °C and stored at −70 °C until required for analysis. miRNAs were extracted from 200 μl of plasma aliquot using a miRNeasy kit (Qiagen, GmbH, Germany). About 150 ng of RNA mixture was collected from each plasma sample, and miRNA quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the SYBR GreenER™ miRNA qRT-PCR kits (Invitrogen, Carlsbad, CA) and the respective primers (Applied Biosystems, Foster City, CA). We determined to evaluate five different miRNAs: miR-17 [11, 12], miR-21 [12, 13], miR-106a [14], miR-126 [15, 16], and miR-200b [6, 17], which had been associated with vascular pathologies and atherosclerosis as illustrated in Supplementary Table 1. We used miR-16 as a standard because several studies have shown its stable expression in human blood [3, 5, 18]. Each sample was tested three times, and relative miRNA expression levels were determined using the formula 2−ΔCT (ΔCT = mean Ct [miRNA] − mean Ct [miRNA-16]).
Statistical Analysis
Continuous values are expressed as means ± standard deviations and categorical values as numbers of the patients. Continuous values were tested for normality using the Shapiro-Wilk test and converted to natural logs when appropriate. The Student’s t test or Mann-Whitney U test was used to analyze continuous variables and chi-squared test to analyze categorical values. Clinical and laboratory variables including miRNA levels were compared between acute stroke patients and control group. Multivariate logistic regression analysis was performed to evaluate independent miRNA change after acute infarction and included age, gender, and those variables with p value <0.05 from bivariate analysis. Among the stroke patients, miRNA levels were compared in terms of stroke subtype, neurological outcome, and the presence of atherosclerosis. Receiver operating characteristic curves (ROC) were generated to determine sensitivity and specificity values of acute ischemic stroke diagnosis by miRNA level. Pearson’s correlation analysis was used to examine relationship between clinical variables including atherosclerosis score and miRNA levels. The levels of miRNA were compared between the patients with plaque enhancement and those with non-enhanced plaque from high-resolution vessel wall MRI. Finally, clinical and laboratory variables were compared between the patients who experienced stroke recurrence and those without recurrence. Kaplan-Meier survival curves with log-rank test were applied to compare stroke recurrence-free survival between the patients with higher miR-17 level and those with lower levels by GraphPad Prism ver. 5 (GraphPad, La Jolla, CA, USA). All the statistical analyses were performed using SPSS ver. 19 (SPSS, Chicago, IL) unless otherwise indicated, and statistical significance was accepted for p value <0.05.
Results
Plasma Levels of miR-17 were Significantly Higher in Acute Cerebral Infarction Patients
A total of 120 patients were enrolled in this study, including 83 acute ischemic stroke patients and 37 controls. Stroke subtypes included 35 LAA, 17 SVO, and 31 CE. The levels of miR-17 (p = 0.013) and miR-106a (p = 0.022) were elevated in acute ischemic stroke patient (Table 1), and the increase in miR-17 remained significant after multivariate logistic regression analysis adjusted for age, gender, diabetes mellitus, white blood cell count, and systolic blood pressure (Table 2). The area under the ROC of miRNA-17 was 0.642 (37.4 % sensitivity and 89.2 % specificity at a cutoff of 0.1265, p = 0.013, Supplementary Fig. 1). miRNA expression levels were not significantly different regardless of gender, vascular risk factors, or neurological outcome (Supplementary Table 2), but miR-126 level was higher among the patients with atherosclerosis than those without atherosclerotic stenosis (p = 0.044, Supplementary Table 2).
miRNA Expressions Reflected Stroke Subtypes and Atherosclerosis Burden
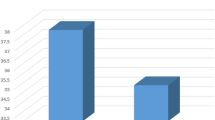
When stroke subtypes were dichotomized into CE and non-CE stroke groups including LAA and SVO, miR-126 (p = 0.018) and miR-200b (p = 0.024) levels were found to be higher in non-CE patients (Fig. 1, Supplementary Table 3). Atherosclerosis scores from MRA positively correlated with miR-21 (r = 0.223, p = 0.043) and with miR-126 levels (r = 0.254, p = 0.021, Supplementary Table 4), whereas stroke onset, infarction volume, and stroke severity were not. The levels of miR-126 also positively correlated with white blood cell count, but negatively correlated with age (Supplementary Table 4). Plaque enhancement from vessel wall MRI was associated with elevated levels of miR-17 (p = 0.048), miR-126 (p = 0.030), and miR-200b (p = 0.030, Fig. 2, Supplementary Table 5).
The microRNA levels by stroke subtype. miR-126 levels were elevated in non-cardioembolic stroke patients (including large artery atherosclerosis patients and patients with small vessel occlusion) than in cardioembolic stroke patients (n = 83, Mann-Whitney U test, p = 0.018). Bars represent median values (*P < 0.05). CE cardioembolism, SVO small vessel occlusion, LAA large artery atherosclerosis
The microRNA levels according to plaque enhancement from high resolution vessel wall MR imaging. Representative high-resolution vessel wall MR imaging from two different pontine infarction patients due to basilar artery atherosclerosis show non-enhancing plaque (a, arrow) and enhancing plaque (b, arrow) in basilar artery. The levels of miR-17 (p = 0.048), miR-126 (p = 0.030), and miR-200b (p = 0.030) were significantly elevated in stroke patients with enhanced plaque in patients with non-enhanced plaque (n = 12, Mann-Whitney U test). *P < 0.05
The Higher miR-17 Level was Associated with Future Stroke Recurrence
Among 83 stroke patients, eight patients experienced recurrent cerebral infarction during median follow-up period of 24 months. Stroke patients who experienced recurrent stroke during follow-up period exhibited higher levels of miR-17 (p = 0.013) and miR-106a (p = 0.015, Table 3). Other clinical variables and laboratory data were not different between the two groups. When stroke patients were dichotomized by the median value of miR-17 level (cutoff value = 0.1031), higher level of miR-17 was associated with shorter event-free survival (p = 0.047, Fig. 3).
Survival curve analysis predicting event free survival between the groups with higher and lower miR-17 level. When stroke patients were dichotomized by the median value of miR-17 levels (cutoff value = 0.1031), the patients group with higher miR-17 showed shorter stroke recurrence-free survival than those patients with lower miR-17 levels (p = 0.047, log-rank test)
Discussion
This study shows acute ischemic stroke was associated with elevated plasma levels of miR-17, and its higher level was related to future stroke recurrence. The miRNA expression patterns of stroke subtypes differed; miR-126 levels correlated with the degrees of cerebral atherosclerosis from brain MRA and were significantly lower in CE stroke patients than in atherosclerotic stroke patients, emphasizing its role in vascular dysfunction. This is the first study suggesting prognostic value of miRNA in cerebral infarction.
Two hypotheses could be proposed to explain the origins of circulating microRNAs in stroke patients. First, apoptotic microparticles from brain endothelial cell, vulnerable atheromatous plaque, or injured nervous tissue might be a potential source, as they have stable microenvironment protected by hydrophobic lipid membrane [19]. Second, miRNAs might be produced after acute ischemia because they have tissue protective function [20, 21]. This study shows that miR-17 was elevated among acute cerebral infarction patients: around 25 % of risk factor combined controls. Angiogenesis with increased vascular endothelial growth factor have been observed after acute cerebral ischemia, and the miRNA 17-92 cluster is known to augment angiogenesis by suppression of antiangiogenic thrombospondin-1 and connective tissue growth factor [11, 20]. Reactive angiogenesis after acute ischemic stroke may underlie miR-17 elevation after acute cerebral infarction [22]. It remains to be determined whether circulating microRNAs are actively secreted protective material or by-products from vulnerable atheroma.
This study suggested prognostic implication of miR-17 in stroke patients, although the exact pathomechanism is yet illusive. The level of circulating miR-17 may reflect systemic atherosclerosis activity in stroke patients, which is related to future vascular event. Many studies have shown that miR-17 harbors oncogenic potential and elevated miR-17 level is associated with poor prognosis in cancer patients by increased angiogenesis [23, 24]. The progression of atherosclerosis is a complex pathological process that involves inflammatory cell infiltration, smooth muscle cell hyperplasia, and reactive angiogenesis [25]. Vulnerable plaque from high-resolution vessel wall MRI was associated with elevated miR-17, miR-126, and miR-200b levels compared to non-enhanced plaque, suggesting their role in atheroma neoangiogenesis and inflammation.
The miR-126 levels were increased in patients with atherosclerosis and significantly higher in non-CE stroke group. Endothelial cells are known to express abundant miR-126 to protect vascular integrity, which decreases inflammatory cell adhesion by inhibiting the expression of vascular cell adhesion molecule-1.16 Therefore, the elevation of miR-126 levels in patients with advanced atherosclerosis could be counter-reaction against atheroma progression. However, increased baseline miR-126 levels were also reported to be associated with incident myocardial infarction from a cohort study, suggesting bidirectional role of miR-126 in atherosclerosis pathophysiology [26].
This study has several limitations. First, all the patients were Korean and the number of included patients was small because we did not include every stroke patient within the study period. We excluded stroke etiologies other than LAA, CE, or SVO, and patients with an undetermined stroke subtype, which might result in selection bias. Second, the miRNAs studied were selected from previous studies performed on various vascular diseases, which might not be an ideal way of deriving sensitive and specific biomarkers assessing brain neuronal damage. However, selected miRNAs reflected atherosclerosis burden and activity among stroke patients, which will help to predict future vascular event. Third, although atherosclerosis is a systemic disease, we only evaluated cerebral vessels to determine atherosclerosis score, which could have underestimated overall atherosclerosis burdens.
Summarizing, we studied five atherosclerosis-related miRNAs in acute cerebral infarction patients, and found that plasma miR-17 levels were increased in acute ischemic stroke and its higher level was associated with future vascular event after index stroke. The levels of miR-126 were increased in patients with atherosclerosis and correlated with atherosclerosis burden. Future studies are warranted to determine the pathomechanism responsible for these increases and to evaluate biomarker potentials.
References
Whiteley W, Wardlaw J, Dennis M, Lowe G, Rumley A, Sattar N, et al. The use of blood biomarkers to predict poor outcome after acute transient ischemic attack or ischemic stroke. Stroke. 2012;43(1):86–91.
Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–62.
Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–77.
Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30(1):92–101.
Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, et al. Expression profile of microRNAs in young stroke patients. PLoS ONE. 2009;4(11):e7689. doi:10.1371/journal.pone.0007689.
Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, et al. MicroRNAs induced during ischemic preconditioning. Stroke. 2010;41(8):1646–51.
Cipollone F, Felicioni L, Sarzani R, Ucchino S, Spigonardo F, Mandolini C, et al. A unique microRNA signature associated with plaque instability in humans. Stroke. 2011;42(9):2556–63.
Adams Jr HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41.
Lee EJ, Choi KH, Ryu JS, Jeon SB, Lee SW, Park SW, et al. Stroke risk after coronary artery bypass graft surgery and extent of cerebral artery atherosclerosis. J Am Coll Cardiol. 2011;57(18):1811–8.
Kim JM, Jung KH, Sohn CH, Moon J, Han MH, Roh JK. Middle cerebral artery plaque and prediction of the infarction pattern. Arch Neurol. 2012;69(11):1470–5.
Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, et al. Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet. 2006;38(9):1060–5.
Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, et al. Circulating microRNAs in patients with coronary artery disease. Circ Res. 2010;107(5):677–84.
Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-α in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011;108(25):10355–60.
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MircroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–33.
Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71.
Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105(5):1516–21.
Nagalla S, Shaw C, Kong X, Kondkar AA, Edelstein LC, Ma L, et al. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117(19):5189–97.
Tsujiura M, Ichikawa D, Komatsu S, Shiozaki A, Takeshita H, Kosuga T, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102(7):1174–9.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324(5935):1710–3.
Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14(3):249–56.
Prakash R, Li W, Qu Z, Johnson MA, Fagan SC, Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke. 2013;44(10):2875–82.
Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20(12):1603–14.
Yu G, Tang JQ, Tian ML, Li H, Wang X, Wu T, et al. Prognostic values of the miR-17-92 cluster and its paralogs in colon cancer. J Surg Oncol. 2012;106(3):232–7.
Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110(14):2032–8.
Zampetaki A, Willeit P, Tilling L, Drozdov I, Prokopi M, Renard JM, et al. Prospective study on circulating microRNAs and the risk of myocardial infarction. J Am Coll Cardiol. 2012;60(4):290–9.
Funding
This work was supported by a grant of the Korea Health technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A111393). The funding has no role in design, collection, analysis, or interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional: Seoul National University Hospital, 0906-028-282) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Conflict of Interest
Jeong-Min Kim, Keun-Hwa Jung, Kon Chu, Soon-Tae Lee, Jaejun Ban, Jangsup Moon, Manho Kim, Sang Kun Lee, and Jae-Kyu Roh, MD, PhD declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jeong-Min Kim and Keun-Hwa Jung contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 172 kb)
Rights and permissions
About this article
Cite this article
Kim, JM., Jung, KH., Chu, K. et al. Atherosclerosis-Related Circulating MicroRNAs as a Predictor of Stroke Recurrence. Transl. Stroke Res. 6, 191–197 (2015). https://doi.org/10.1007/s12975-015-0390-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-015-0390-1