Abstract
Sugarcane (Saccharum spp. hybrid) cultivar Yuetang 03-373 is a hybrid with superior agronomic characteristics that was obtained from the cross between Yuetang 92-1287 (♀) and Yuetang 93–159 (♂). In this study, transversely cut discs of immature leaves were used to induce adventitious shoots for the first time. Embryonic callus was induced from leaf explants on Murashige and Skoog (MS) medium with 0.5 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.1 mg/l α-naphthaleneacetic acid (NAA). On MS medium containing 0.75 mg/l 6-benzyladenine and 0.1 mg/l NAA, 73.1% of explants from which callus was induced differentiated into adventitious shoot within 30 d and the mean number of adventitious shoot buds was 128.3 per explant. This is many times than regular axillary shoot propagation (mean 5.6 shoots) on the MS medium supplemented with 1.25 mg/l Kinetin (KIN) + 0.1 mg/l NAA. On rooting MS medium containing 0.4 mg/l indole-3-butyric acid (IBA), 0.2 mg L–1 NAA and 50 g/l sucrose, all shoots rooted. Well-rooted plantlets showed 100% survival in five tested substrates after 60 d. Using this efficient in vitro regeneration system, scaled-up production was achieved to mass produce sugarcane cultivar Yuetang 03-373 for national markets. The results of our genetic diversity study using DNA-based ISSR markers showed that there was no heritable variation in the induction of shoot organogenesis through immature leaves, which provided a shortcut for future vegetative propagation of sugarcane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum officinarum L.; Poaceae) is a perennial herb that grows in tropical and subtropical regions around the world. Globally, sugarcane is the source of approximately 80% of sugar (sucrose) and 35% of ethanol (OECD/FAO 2018), making it an important cash crop and a major sugar crop worldwide. Modern sugarcane cultivars result from interspecific crosses (Huang et al. 2022). The yield and quality of sugarcane can be improved through breeding and the promotion of new varieties with high yield and strong stress resistance (Abreu et al. 2020). Sugarcane can be efficiently converted into sugar and other renewable energy sources using solar energy, and used to make ethanol (Sanchez et al. 2020), acetate (Tondro et al. 2020), and industrial enzymes (Mason et al. 2020). The leaves can be used as animal feed and are a good raw material for sugar-related industries (de Oliveira et al. 2005; Shang et al. 2022). Yuetang 03-373 is a superior sugarcane hybrid cultivar that was obtained via a cross between Yuetang 92-1287 (♀) and Yuetang 93–159 (♂). This cultivar has several superior agronomic characteristics: early-mid maturity, high sugar content (14–16%), high and stable yield, strong perennial roots, strong stress resistance (wind, drought, disease and pest), wide adaptability, and excellent agronomic traits (Wu et al. 2011). After field trials were successfully conducted, Yuetang 03-373 is now planted in sugarcane-growing areas of China such as Guangdong, Guangxi, Guizhou and Yunnan. However, because there is no robust technology to proliferate plants, it is difficult to expand this variety to other suitable cultivation areas (Liu et al. 2020). One way to achieve plants at an industrial scale is through the use of in vitro culture, i.e., tissue culture. The tissue culture of Yuetang 03-373 was previously achieved using axillary buds as explants, but the shoot proliferation coefficient (SPC) was not so high (5.2 shoots per explant) (Chen et al. 2017). Consequently, further optimization and improvement of tissue culture of this variety is needed.
Somatic embryogenesis and shoot organogenesis as plant tissue culture techniques, can be used to solve several challenges and limitations of traditional breeding and proliferation. This technology can produce many virus-free sugarcane plantlets on a large scale (Lakshmanan et al. 2005; Kaur and Sandhu 2015; Kaur and Kapoor 2016). In sugarcane, somatic embryos (SEs) have been induced from inflorescences (Desai et al. 2004b), young leaves (Khan and Khatri 2006; Maulidiya et al. 2020; Passamani et al. 2020; Reis et al. 2021), midrib segments (Franklin et al. 2006). Callus induction, shoot organogenesis in vitro are viable pathways for clonal propagation and the preservation of germplasm, while virus-free sugarcane plantlets can be obtained via the latter two pathways (Roumagnac 2021). These techniques also allow for the large-scale proliferation of plants (Suprasanna et al. 2011) and the possibility of improving germplasm (Dorairaj et al. 2018).
Simple Sequence Repeats (SSR) labeling is a molecular labeling technology based on specific primer PCR developed in recent years, also known as microsatellite DNA (Microsatellite DNA), which is a kind of tandem repeat sequence of dozens of nucleotides composed of several nucleotides (generally 1–6) as repeat units (Tikendra et al. 2021). Since the sequences on either side of each SSR are generally relatively conserved, single-copy sequences. It has been found that there is a high degree of variation in the number of repeating units in microsatellites, which is manifested as euploidy variation in the number of microsatellites or the possibility that the sequences in the repeating unit sequence are not identical, resulting in polymorphisms at multiple loci (Saker et al. 2000). SSR marker technology has been widely used in the construction of genetic maps, the indexing of target genes, the drawing of fingerprints, breed identification, pedigree analysis, genetic distance analysis between populations, evolution and genetic diversity (Bansal et al. 2024). In the current study, the objective of this study was to induce shoots from immature leaf discs of sugarcane variety Yuetang 03-373, and evaluate of genetic integrity using ISSR markers revealed complete endomorphism and indicating their variations among the regenerants.
Materials and methods
Surface disinfection of immature leaf discs
Six month old Yuetang 03-373 plants were grown outside in the field at the breeding base of Zhanjiang Research Center at the Southern Breeding Industry Research Institute of Guangdong Academy of Sciences, in China. The plants were identified by the institutional and/or licensing committee that approved these experiments, and all experiments were performed in accordance with relevant guidelines and regulations. Shoot tips were cut, outer leaf sheaths were peeled off, and the surfaces of remaining leaf tissues were sprayed with 75% ethanol. Leaf sheath segments were disinfected in 75% ethanol for 30 s, washed three times with sterilized distilled water (SDW), soaked in 0.1% HgCl2 solution for 5 min, washed 3–4 times with SDW, then dabbed dry on sterilized Kraft paper (Ertai, Dongguan, Guangdong, China). The outer leaf layer was peeled off, leaving a leaf explant with only immature leaves (circles) that were cut into slices (explants) about 1 mm thick (Fig. 1a). Leaf circles were cut into two equally sized halves that were used for subsequent experiments.
Induction of callus and adventitious shoots from immature leaf discs of sugarcane cultivar Yuetang 03-373. a, Freshly cut immature leaf disc; b, from half-discs, yellow callus was induced after incubation in the dark for 25 days on ideal callus induction medium (CIM; MS with 0.5 mg/l 2,4-D and 0.1 mg/l NAA); c, after culture in the dark on CIM for 30 days, some callus became purple; d, after culture in the dark on CIM for 30 days, then transfer to light for one week, shoot buds formed on the surface of callus; e-f, after culture in the dark on CIM for 30 days, then transfer to MS medium with 0.75 mg/l BA and 0.1 mg/l NAA in light within 30 days, some adventitious shoots and SEs developed multiple shoot clump. Bars: a-c, 1.0 mm; d-e, 0.5 mm; f, 0.5 cm
Callus induction
Explants were plated on Murashige and Skoog (MS) medium (Chembase, Beijing, China) (Murashige and Skoog 1962) supplemented with different concentrations and combinations of plant growth regulators (PGRs): 6-benzyladenine (BA), α-naphthaleneacetic acid (NAA), 2,4-dichlorophenoxyacetic acid (2,4-D), kinetin (KIN), and thidiazuron (TDZ) (Sigma-Aldrich ®, Saint Louis, MO, USA), and cultured in the dark. Six explants were plated in each culture jar (Honghua, Xuzhou, Jiangsu, China). Each treatment had 30 culture jars. Callus induction was observed after 30 days (Table 1; Fig. 1). All media (pH 5.8) were supplemented with 30 g/l sucrose (Sinopharm, Beijing, China) and 7.5 g/l carrageenan (Aladdin Co., Shanghai, China). The temperature of the growth culture room was 28 ± 2 °C. After culture in the dark for 30 days, culture jars were transferred to light (80 µmol m-2s-1; 12-h photoperiod), and callus induction was investigated (Table 1). Somatic embryogenesis and shoot organogenesis were directly observed under a stereomicroscope (Nikon SMZ745T, Tokyo, Japan) at different culture times and photographs were taken.
Differentiation of adventitious shoots
Callus (about 0.5 g) that was derived from MS medium supplemented with 0.5 mg/l 2,4-D and 0.1 mg L–1 NAA, i.e., callus induction medium (CIM), in the dark for 30 d was inoculated onto MS medium with different combinations and concentrations of PGRs: 0.1, 0.5, and 1.0 mg/l BA (or KIN); 0.75 mg/l BA (or KIN) and 0.1 mg/l NAA (Table 2). Six callus clumps were cultured for each PGR combination, and each treatment included 10 culture jars. After culture in light for 30 days, organ differentiation from callus was investigated under a Nikon SMZ745T stereomicroscope and photographs were taken.
Shoot proliferation
Callus clumps that were induced on CIM after 30 days were transferred to ORM to differentiate SEs and adventitious shoots. Both SEs and adventitious shoots generally developed shoot clusters, and multiple shoots were divided into smaller explants, each with 2–3 shoots, that were inoculated onto MS medium with different concentrations and combinations of PGRs (Table 3). Each treatment included 60 shoot clumps. SPC (i.e., the number of new shoots that formed from each original shoot) was calculated after 30 days.
Rooting
Multiple shoots were cut into clusters with 2–3 shoots and transferred to MS medium with different concentrations of IBA and/or NAA, as well as sucrose (Table 4). Each treatment included 60 shoot clusters. After 21 days, rooting percentage, root number, and root length were investigated. Rooting percentage was calculated as: number of rooted plantlets / number of inoculated shoots (or somatic embryos) × 100%.
Acclimatization and transplanting
Culture jars with well-rooted plantlets were placed in a greenhouse (temperature range: 10–35 °C; light intensity ≤ 3000 µmol m-2 s-1; 45–95% relative humility) for 10 days and then lids were opened for 15 d to expose plants to ambient environmental conditions. Roots were carefully washed with tap water, and plantlets were soaked in 0.1% rooting solution that contained NAA and IBA (Peste Sciences Co., Zhengzhou, China) for 1–2 min. After drying roots slightly in air, plantlets were potted in seedling trays (Hongxiang Co., Dezhou, Shandong, China), at 2–3 plantlets per cell, in the following 10 substrates (ratios = v/v): peat: sand (1:1); vermiculite: sand (1:1); perlite: sand (1:1); peat: vermiculite (1:1); perlite: vermiculite (1:1); peat: perlite (1:1); yellow mud: sand (1:1); perlite: vermiculite: sand (1:1:1); perlite: peat: sand (1:1:1); peat: vermiculite: sand (1:1:1). Each treatment included 60 cells. After 60 days, survival percentage of transplanted plants was calculated as [number of surviving plants / total number of transplanted plants] ×100%.
Assessment of genetic integrity
To assess the genetic integrity of in vitro raised plantlets, inter simple sequence repeat (ISSR) markers were employed. Genomic DNA was isolated from 10 plantlets, including three randomly selected mother plants (8-week-old plantlets used as explant source) and three plantlets each obtained from shoots multiplied on media containing 0.4 mg/L IBA and 0.2 mg/L NAA, using CTAB method (Doyle and Doyle 1987). The quality and quantity of extracted DNA were estimated using NanoDrop 1000 (Thermo Fischer Scientific, USA). PCR conditions were optimized for each primer, and PCR reactions (10 µl reaction volume) containing 1X reaction mixture (One PCRTM, GeneDireX Inc. USA), 10 µM primer, and 40 ng template DNA were set up. The PCR was carried out in a thermal cycler (Gene Pro, Hangzhou Bioer Technology Co., China) with specific conditions, including initial denaturation at 94 °C for 5 min, followed by 37 cycles of denaturation at 94 °C (30 s), primer annealing at 37–56.7 °C (45 s), and primer extension at 72 °C (60 s), with a final extension at 72 °C for 5 min. PCR amplicons were resolved on a 2.5% agarose gel in 1X TAE buffer. The size of the amplified product was determined by co-electrophoresing a 100 bp DNA ladder. Amplification profiles were visualized using a Gel Documentation System (GenoSens 2100, Clinx Science Instruments Co., China), and the data were noted.
Large-scale proliferation and cultivation
Based on in vitro results, a large-scale proliferation and regeneration system was established by replacing glass culture jars with domestically manufactured PVP plastic bags (11 cm × 13.5 cm) (Jinhongda, Shenzhen, China), which can withstand high temperatures (> 130 °C), thus allowing them to be autoclaved and used in plant tissue culture, broadly following a previously described procedure (Zheng et al. 2022). 100 leaf sheath segments were used as explants, after they were disinfected in 75% ethanol for 30 s and soaked in 0.1% HgCl2 solution for 5 min, washed 3–4 times with SDW, the immature leaves (circles) that were cut into slices (explants) about 1 mm thick, they were inoculated on the MS medium supplemented with 0.5 mg/l 2,4-D and 0.1 mg/l NAA, in the dark on CIM for 30 days. Callus clumps were transferred to optimal regeneration medium (ORM), which was MS medium with 0.75 mg/l BA and 0.1 mg/l NAA, and cultured in light to induce SEs and/or adventitious shoots i.e., callus induction medium (CIM) Plantlets were produced in vitro in three steps: first on CIM in the dark for 30 days, then on ORM in the light for 30 days, followed by eight 30-d subcultures of shoots/SEs in light. Shoots were transferred to PVP plastic bags (10 shoots/bag) containing rooting medium (MS with 0.4 mg/l IBA and 0.2 mg/l NAA) and placed in light conditions for 14 days in the culture room, followed by 14 days of natural conditions in a greenhouse. After rooted plantlets elongated by 20 cm, they were transplanted to a field with an inter-plant space of 15 cm and an inter-row space of 80 cm. Plants were irrigated daily with tap water. After six months, plant growth and development were investigated.
Data analysis
Data are reported as the mean ± standard deviation (SD). Statistical analyses followed the procedure described by Xiong et al. (2019), using Duncan’s multiple range test (P ≤ 0.05) with SPSS v. 19.0 (IBM, New York, NY, USA).
Results
Shoot induction from immature leaf discs
When immature leaf discs (explants) (Fig. 1a) were cut in half and cultured on MS medium with 0.1–3.5 mg/l 2,4-D for 30 d in the dark, callus formed on cut surfaces of select treatments (Fig. 1b). Although callus was induced on 100% of explants in some treatments, such as 0.1–1.5 mg/l 2,4-D, they displayed several developmental deficiencies, including explant browning, explant necrosis, the formation of hard callus, and purple pigment formation (Fig. 1c). The ideal callus induction medium, based on induction percentage and the quality of callus formed, was MS with 0.5 mg/l 2,4-D and 0.1 mg/l NAA (Table 1), i.e., CIM. When callus was transferred to several media, shoots or SEs formed, and in several cases, they were visually indistinguishable, so the shoot and SE data was combined (Table 2). In terms of receptivity (% of explants inducing shoots or SEs), and productivity (number of shoots or SEs per explant), ORM was determined to be MS with 0.75 mg/l BA and 0.1 mg/l NAA. When callus was transferred to CIM, shoot buds formed on the surface of callus (Fig. 1d), and when these were transferred to ORM, shoots developed into shoot clusters (Fig. 1e and f).
Shoot proliferation, rooting and transplantation
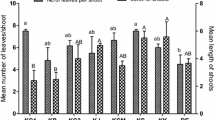
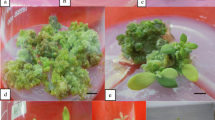
Most multiple shoots (i.e., highest SPC) (Fig. 2a) were induced in MS with 1.25 mg/l KIN and 0.1 mg/l NAA. Several other treatments produced hyperhydric shoots (Table 3). The optimal rooting medium, as assessed by the number of shoots that formed roots, number of roots per shoot, and root length, was MS with two auxins (0.4 mg/l IBA and 0.2 mg/l NAA) and 50 g/l sucrose (Table 4). Resulting plantlets had well-developed leaves and root system (Fig. 2b and c). When plantlets were divided into clusters of three plantlets per tray of seedling trays with different substrates (Fig. 2d and e), the optimal substrate was, as assessed by survival, plant height, stem diameter and leaf number, perlite: peat (1:1) or perlite: sand (1:1) (Table 5).
Shoot proliferation, rooting and transplanting of sugarcane cultivar Yuetang 03-373 a, Multiple shoots were proliferated on MS medium with 1.25 mg/l KIN and 0.1 mg/l NAA for 30 days; b, shoots rooted on MS medium with 0.4 mg/L IBA, 0.2 mg/l NAA, and 50 g/L sucrose; c, rinsed plantlets from in vitro culture; d-e, plantlets with well-developed roots were transferred to cells (8 × 5) in seedling trays (30 cm × 50 cm) containing different substrates (ratios = v/v): A, peat: sand (1:1); B, vermiculite: sand (1:1); C, perlite: sand (1:1); D, peat: vermiculite (1:1); E, perlite: vermiculite (1:1); F, peat: perlite (1:1); G, yellow mud: sand (1:1); H, perlite: vermiculite: sand (1:1:1); I, perlite: peat: sand (1:1:1); J, peat: vermiculite: sand (1:1:1). Bars: a-b, 1.0 cm; C, 0.5 cm. d-e, 3 cm
Large-scale production, and growth characteristics and yield of plants
Using PVP plastic culture bags, the optimized in vitro culture protocols that were developed and described above were adopted (Fig. 3a-c). Culture bags were transferred to a greenhouse for 15 days (Fig. 3d), then to seedling trays (Fig. 3e), and finally to a field (Fig. 3f, g). It was possible to mass produce approximately 5 million plants per year (from callus induction until transplantable age) of sugarcane var. Yuetang 03-373. Production was approximately 40-fold higher than a previous protocol that induced shoots from shoot tips or axillary buds (Chen et al. 2017). Field-grown plants did not display any visible morphological deficiencies. Throuth successfully three test, the mean yield was 92,045 plants/ha, yield of sucrose stems was 92.88 tons/ha, cane stem yield was 100,290 kg/ha, and sugar content was 15,420 kg/ha.
Large-scale production of sugarcane cultivar Yuetang 03-373 and field cultivation a, Shoot proliferation and rooting were achieved in the tissue culture room in PVC plastic bags for 30 days; b, multiple shoots were proliferated in MS medium with 1.25 mg/l KIN and 0.1 mg/l NAA for 30 days; c, shoots were rooted in MS medium with 0.4 mg/l IBA, 0.2 mg/l NAA and 50 g/l sucrose for 30 days; d, rooted plantlets in plastic bags were acclimatized in a greenhouse for 15 days; e, acclimatized plantlets were transferred to seeding trays (30 cm × 50 cm) with 40 cells (8 × 5) in a peat: sand (1:1, v/v) substrate for further plant development and hardening; f, two-month old plants were transferred directly to the field with an inter-plant spacing of 15 cm and an inter-row spacing of 80 cm. Irrigation tubing was placed alongside all rows of field-grown plants; g, six month-old mature plants. Bars: b-c, 1 cm; d, 5 cm;
ISSR primer polymorphisms
Three pairs of primers detected a total of 50 alleles in 10 individual samples. Among them, the minimum number of alleles was 1 (mSSCIR68, mSSCIR74) (Fig. 4a and b), the maximum number of alleles was 3 (mSSCIR43), and the average number of alleles per point was 1.6667 (Fig. 4c).
Population genetic structure analysis
The total number of effective alleles (Ne, the more evenly distributed the alleles in the population, the closer Ne is to the number of alleles actually detected) was 5.0000, the numerical variation range was 1.0000, and the average number of effective alleles per locus was 1.0000. The Shannon index (I) has a range of 0.0000 (Fig. 5a). The optimal delta K value calculated in STRUCTURE HARVESTER according to the method of Evanno et al. (2005) is 2, indicating the presence of 2 gene pools out of 10 samples (Table 6). As can be seen from the graph, when K = 2, the gene composition of the 10 samples is derived from gene pools 1 and 2 (Fig. 5b).
Discussion
The success of the plant regeneration pathway depends on a variety of factors such as the sugarcane variety used (Gandonou 2005; Kaur and Kapoor 2016), the media sterilization method (Maribel et al. 2020), the type of explant (Lakshmanan et al. 2006), PGRs (Reis et al. 2021), light (Neto et al. 2020), pH (Ali et al. 2008), sucrose content (Lu et al. 2020), activated charcoal (Mittal et al. 2016), gelling agents (Manchanda and Gosal 2012), type of basal medium (Kaur and Gosal 2009), and several other factors. The literature on sugarcane tissue culture reveals wide differences in callus induction, somatic embryogenesis, and plant regeneration capacity of different genotypes or cultivars. Callus induction and/or somatic embryogenesis have been achieved from immature inflorescences (Desai et al. 2004a), young leaves (Franklin et al. 2006; Ali et al. 2007; Joshi et al. 2013; Dorairaj et al. 2018), stem tips (Ali et al. 2008), and stem bases (Kaur and Kapoor 2016). These studies revealed that callus could be induced from several explants, although there were vast differences in callus induction, somatic embryogenesis and plant regeneration capacity. Immature leaves are a popular and commonly used explant for the induction of callus and SEs (Desai et al. 2004b; Franklin et al. 2006; Ali et al. 2007; Joshi et al. 2013; Dorairaj et al. 2018). Even though 2,4-D has been widely used to induce callus, it can inhibit the development of SE into normal plants (Reis et al. 2021). In this study on Yuetang 03-373, high concentrations of 2,4-D generally induced embryonic callus while low concentrations of 2,4-D (0.1–1.5 mg L–1) directly induced SEs as well as 100% callus from leaf explants. Our histological analysis revealed that callus, SEs, and adventitious shoot buds originated exclusively from epidermal cells (Fig. 3).
Carrageenan, a natural seaweed polysaccharide, has been used in plant tissue culture as a solidifying agent for in vitro media (Hurtado and Biter 2007; Prajapati et al. 2014). Since it is very cheap and easy to obtain, compared to agar, replacing agar with carrageenan in tissue culture can reduce the costs of plant tissue culture. As before, for the industrial scale-up of Dracaena cambodiana (Zheng et al. 2022), we replaced glass jars with PVP plastic bags, which greatly improved work efficiency and reduced the cost of large-scale production of sugarcane plantlets.
From among 10 tested substrates for the acclimatization of in vitro-derived Yuetang 03-373 plants, five allowed for 100% transplant survival. This indicated that the cultivar was easy transplanted. In the last large-scale proliferation and transplantation, we just used peat soil: sand (1:1, v/v) substrate for further plant development and hardening. In the previous report, they only use pure river sand as the substrate for plantlet transplantation and transplantation survival percentage was 98% within one month (Chen et al. 2017).
Regeneration from callus may cause a certain amount of somaclonal variation (Ghosh et al. 2021). Even though we did not observe any noticeable phenotypic variation, somaclonal variation might impact plant proliferation or yield, so detailed trials that assess such variation (Rajeswari et al. 2009; Martínez-Estrada et al. 2017) would need to be conducted in the future. Here we first induce calli from the leaves, and then shoot organogenesis, so that we can reproduce a large number of small shoots in larger adventitious shoots, and their shoot induced coefficient can be as large as 128 within 30 d, through which we can produce out large-scale shoots via shoot proliferation (the reproductive coefficient is only 5.6). Through our ISSR experiment, it was found that it did not undergo hereditary mutation, so that we obtained a large number of shoots through the initial shoot organogenesis, and then carried out shoot propagation, which is much faster than traditional shoot propagation, and can reduce genetic variation, which is conducive to large-scale reproduction, We have mapped a fast and simple propagation model that can be used to propagate sugarcane seedlings on a large scale (Fig. 6).
Conclusion and limitations
Transversely cut discs of immature leaves were used as explants of sugarcane var. Yuetang 03-373 to induce callus, shoots for the first time. On MS medium with 0.5 mg/l 2,4-D and 0.1 mg/l NAA, 100% of leaf explants induced embryogenic callus. On MS medium with 0.75 mg/l BA and 0.1 mg/l NAA, 62% of induced callus differentiated into adventitious shoots within 30 d (coefficient highest 128). All the adventitious shoots developed multiple shoots on the shoot proliferation medium MS medium supplemented with 1.25 mg/l KIN and 0.1 mg/l NAA and SPC reached 5.6 with each 30-day subculture. Histological observations indicated that both SEs and adventitious shoot buds were derived from parenchymatous meristems in the outer or inner epidermal layers of the cut edges of leaf discs. On optimal rooting medium (MS with 0.4 mg/l IBA, 0.2 mg/l NAA and 50 g/l sucrose), 100% shoots rooted. Well-rooted plantlets showed 100% survival in five out of 10 tested substrates after 60 days. An efficient in vitro regeneration system was thus established. This study also scaled up production using large-scale technology to mass produce sugarcane var. Yuetang 03-373 for the national markets. A summary of the process for efficient plant proliferation and regeneration of sugarcane var. Yuetang 03-373 is described in Fig. 6. It was possible to mass produce approximately 5 million plants per year. Production was approximately 40-fold higher than a previous protocol, which induced shoots from shoot tips or axillary buds (Chen et al. 2017).
Data availability
Not applicable.
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid;
- BA:
-
6-benzyladenine;
- CIM:
-
Callus induction medium;
- IBA:
-
Indole-3-butyric acid
- KIN:
-
Kinetin
- MS medium:
-
Murashige and Skoog (1962) medium
- NAA:
-
α-naphthaleneacetic acid;
- ORM:
-
Optimal regeneration medium
- PGR:
-
Plant growth regulator
- SPE:
-
Shoot proliferation coefficient;
- TDZ:
-
Thidiazuron
References
Abreu L, Grassi M, Carvalho LMD, Silva JJBD, Pereira G (2020) Energy cane vs sugarcane: watching the race in plant development. Indust Crops Prod 156:112868
Ali A, Naz S, Iqbal J (2007) Effect of different explants and media composition for efficient somatic embryogenesis in sugarcane (Saccharum Oficinarum). Pak J Bot 39:1961–1977
Ali A, Shagufta N, Fayyaz AS, Javed I (2008) An efficient protocol for large scale production of sugarcane through micropropagation. Pak J Bot 40:139–149
Bansal S, Sharma MK, Joshi P, Malhotra EV, Latha M, Malik SK (2024) An efficient direct organogenesis protocol for in vitro clonal propagation of rubia cordifolia l. Industrial Crops Prod 208:117856
Chen YG, Tan JN, Guan JY, Luo JP, Huang HY, Luo QW, Yang JX, Li QW (2017) Tissue culture and rapid propagation of Yuetang 03-373, a new sugarcane variety. Guangdong Agricult Sci 44:13–18
De-Oliveira MED, Vaughan BE, Rykiel EJ (2005) Ethanol as fuel: energy, carbon dioxide balances, and ecological footprint. Biosci 55:593–602
Desai NS, Suprasanna P, Bapat VA (2004a) Partial desiccation of embryogenic calli improves plant regeneration in sugarcane (Saccharum spp). J Plant Biotech 6:229–233
Desai NS, Suprasanna P, Bapat VA (2004b) Simple and reproducible protocol for direct somatic embryogenesis from cultured immature inflorescence segments of sugarcane (Saccharum spp). Curr Sci 87:764–768
Dorairaj S, Venkatachalam V, Jeevaraj T, Dhandapani E, Chinnaswamy A, Ramamoorthy S, Markandan M (2018) Efficient direct plant regeneration from immature leaf roll explants of sugarcane (Saccharum officinarum L.) using polyamines and assessment of genetic fidelity by Scot markers. Vitro Cell Dev Biol - Plant 54:1–14
Doyle JJ, DJ L (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Evanno G, Regnaut S, Goudet J (2005) Detecting the number ofclusters of individuals using the software STRUCTURE: asimulation study. Mol Ecol 14:2611–2620
Franklin G, Arvinth S, Sheeba CJ, Kanchana M, Subramonian N (2006) Auxin pretreatment promotes regeneration of sugarcane (Saccharum spp. hybrids) midrib segment explants. Plant Grow Reg 50:111–119
Gandonou C, Errabii T, Abrinii J, Idaomar M, Chibi F, Skali SNS (2005) Effect of genotype on callus induction and plant regeneration from leaf explants of sugarcane. Afr J Biotech 4:1250–1255
Ghosh A, Igamberdie VAU, Debnath SC (2021) Tissue culture-induced DNA methylation in crop plants: a review. Mol Biol Rep 48:823–841
Huang HR, Gao YJ, Malviya MK, Verma KK, Solanki MK, Huang YX, Li X, Deng Y, Yan J, Tang SY, Wang LW, Xu L (2022) Genetic diversity analysis of sugarcane (Saccharum spp. hybrids) among high-sucrose clones of GT series and commonly used parents by using microsatellite markers in Guangxi, China. Sugar Tech 24:397–407
Hurtado AQ, Biter AB (2007) Plantlet regeneration of Kappaphycus alvarezii var. Adik-Adik by tissue culture. J Appl Phycol 19:783–786
Joshi S, Jain M, Tillman B, Altpeter F, Gallo M (2013) Comparative analysis of direct plant regeneration from immature leaf whorl and floral explants for three elite US sugarcane (Saccharum spp. hybrids) genotypes. Vitro Cell Dev Biol - Plant 49:674–681
Kaur A, Gosal SS (2009) Desiccation of callus enhances somatic embryogenesis and subsequent shoot regeneration in sugarcane. Indian J Biotechnol 8:332–334
Kaur R, Kapoor M (2016) Plant regeneration through somatic embryogenesis in sugarcane. Sugar Tech 18:93–99
Kaur A, Sandhu JS (2015) High throughput in vitro micropropagation of sugarcane (Saccharum officinarum L.) from spindle leaf roll segments: cost analysis for agri-business industry. Plant Cell Tiss Org Cult 120:339–350
Khan IA, Khatri A (2006) Plant regeneration via organogenesis or somatic embryogenesis in sugarcane: histological studies. Pak J Bot 38:631–636
Lakshmanan P, Geijskes RJ, Aitken KS, Grof C, Smith B (2005) Sugarcane biotechnology: the challenges and opportunities. Vitro Cell Dev Biol - Plant 41:345–363
Lakshmanan P, Geijskes RJ, Wang L, Elliott A, Grof CPL, Berding N, Smith GR (2006) Developmental and hormonal regulation of direct shoot organogenesis and somatic embryogenesis in sugarcane (Saccharum spp. interspecific hybrids) leaf culture. Plant Cell Rep 25:1007–1015
Liu FY, Wu WL, Zhou RQ, Wen MF, Peng DY, Yang CQ, Pan FY (2020) Multiple regression analysis of influencing factors on yield of new sugarcane Yuetang 03-373 cane. Sugarcane Canesugar 1:69
Lu JJ, Ali A, He EQ, Yan GQ, Arak TU, Gao SJ (2020) Establishment of an open, sugar-free tissue culture system for sugarcane micropropagation. Sugar Tech 22:8–14
Manchanda P, Gosal SS (2012) Effect of activated charcoal, carbon sources and gelling agents on direct somatic embryogenesis and regeneration in sugarcane via leaf roll segments. Sugar Tech 14:168–173
Maribel RP, Rene CRE, Marcos ADG, Bello-Bello JJ (2020) Influence of vitrofural on sugarcane micropropagation using temporary immersion system. Plant Cell Tiss Org Cult 141:447–453
Martínez-Estrada E, Caamal-Velázquez JH, Salinas-Ruíz J, Bello-Bello JJ (2017) Assessment of somaclonal variation during sugarcane micropropagation in temporary immersion bioreactors by intersimple sequence repeat (ISSR) markers. Vitro Cell Dev Biol - Plant 53:553–560
Mason PJ, Furtado A, Marquardt A, Hodgson-Kratky K, Henry RJ (2020) Variation in sugarcane biomass composition and enzymatic saccharification of leaves, internodes and roots. Biotech Biofuels 13:201
Maulidiya A, Sugiharto B, Dewanti P, Handoyo T (2020) Expression of somatic embryogenesis-related genes in sugarcane (Saccharum officinarum L). J Crop Sci Biotech 23:207–214
Mittal P, Devi R, Gosal SS (2016) Effect of genotypes and activated charcoal on high frequency in vitro plant regeneration in sugarcane. Ind J Biotech 15:261–265
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant 15:473–497
Neto AR, Chagas EA, Costa B, Chagas PC, Vendrame WA (2020) Photomixotrophic growth response of sugarcane in vitro plantlets using different light intensities and culture vessel types. Vitro Cell Dev Biol - Plant 56:1–11
OECD/FAO (2018) OECD-FAO Agricultural Outlook 2018–2027. OECD Publishing, Paris/FAO. https://doi.org/10.1787/agr_outlook-2018-en
Passamani LZ, Reis RS, Vale EM, Sousa KR, Aragão VPMV, Santa-Catarina C (2020) Long-term culture with 2,4-dichlorophenoxyacetic acid affects embryogenic competence in sugarcane callus via changes in starch, polyamine and protein profiles. Plant Cell Tiss Org Cult 140:415–429
Prajapati VD, Maheriya PM, Jani GK, Solanki HK (2014) Carrageenan: a natural seaweed polysaccharide and its applications. Carbohyd Poly 105:97–112
Rajeswari S, Thirugnanakumar S, Anandan A, Krishnamurthi M (2009) Somaclonal variation in sugarcane through tissue culture and evaluation for quantitative and quality traits. Euphytica 168:71–80
Reis RS, Vale EM, Sousa KR, Santa-Catarina C, Silveira V (2021) Pretreatment free of 2,4-dichlorophenoxyacetic acid improves the differentiation of sugarcane somatic embryos by affecting the hormonal balance and the accumulation of reserves. Plant Cell Tiss Org Cult 145:101–115
Roumagnac P (2021) Comparison of the virome of quarantined sugarcane varieties and the virome of grasses growing near the quarantine station. Viruses 13:922
Saker M, Bekheet S, Taha H, Fahmy A, Moursy H (2000) Detection of somaclonal variations in tissue culture derived date palm plants using isoenzyme analysis and RAPD fingerprints. Biol Plant 43:347–351
Sanchez N, Ruiz R, Plazas A, Vasquez J, Cobo M (2020) Effect of pretreatment on the ethanol and fusel alcohol production during fermentation of sugarcane press-mud. Biochem Eng J 161:107668
Shang Y, Xie C, Meng L, Cui F, Lu HQ, Li W, Li K (2022) Polyphenol profiles and antioxidant activities of non-centrifugal sugars derived from different varieties of membrane-clarified sugarcane juice. Sugar Tech 24:614–625
Suprasanna P, Patade VY, Desai NS, Devarumath RM, Kawar PG, Pagariya MC (2011) Biotechnological developments in sugarcane improvement: an overview. Sugar Tech 13:322
Tikendra L, Potshangbam AM, Dey A, Devi TR, Sahoo MR, Nongdam P (2021) RAPD, ISSR, and SCoT markers based genetic stability assessment of micropropagated Dendrobium Fimbriatum Lindl. Var. Oculatum hk. f.- an important endangered orchid. Physiol Mol Biol Plants 27:341–357
Tondro H, Musivan DS, Zilouei H, Bazarganipour M, Zargoosh K (2020) Biological production of hydrogen and acetone- butanol-ethanol from sugarcane bagasse and rice straw using co-culture of Enterobacter aerogenes and clostridium acetobutylicum. Biomass Bioenerg 142:105818
Wu WL, Liu FY, Pan FY, Yang JX, Deng HH, Wu JH, Chen YS, Liang QR (2011) Breeding of Yuetang 03-373, an early-mid new sugarcane variety. Guangdong Agricult Sci 38:41–43
Xiong YP, Liang HZ, Yan HF, Guo BY, Niu MY, Chen SY, Jian SG, Ren H, Zhang XH, Li Y, Zeng SJ, Wu KL, Zheng F, Teixeira da Silva, Jaime A, Ma GH (2019) NaCl-induced stress: physiological responses of six halophyte species in in vitro and in vivo culture. Plant Cell Tiss Org Cult 139:531–546
Zheng F, Xiong YP, Wu KL, Teixeira da Silva Jaime A, Zeng SJ, Lin XM, Ma GH (2022) Industrial scale-up of tissue-cultured Dracaena Cambodiana Pierre Ex Gagnep. Trees 36:1161–1167
Funding
This work was financially supported by the GDAS’ Project of Science and Technology Development (2018GDASCX-0926; 2022GDASZH-202203060), Science and Technology Planning Project of Zhanjiang (2021B01118), National Key R & D Program of China (2020YFD1000600; 2021YFC3100400), Zhanjiang Plan for Navigation (2020LHJH006).
Author information
Authors and Affiliations
Contributions
SYC, JNT, JYG, JPL, YCX, MYC, RZ, YL and QWL, YFZ and GHM designed the experiment and provided guidance for the study. SYC, and JNT prepared samples for all analyses. JPL and YCX conducted statistical analyses. SYC, GHM and JATdS co-wrote the manuscript. GHM and JATdS interpreted the data. GHM assessed the experimental results. All authors read and approved the manuscript for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Lu, Y., Luo, Q. et al. Adventitious shoot organogenesis from immature leaves of sugarcane (Saccharum spp. hybrid) cultivar Yuetang 03-373 improving shoot proliferation and industrial scale-up of plants. J. Crop Sci. Biotechnol. (2024). https://doi.org/10.1007/s12892-024-00255-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12892-024-00255-2