Abstract
The present investigation aims to isolate nitrogen fixing endophytic bacteria from cereals crops and their potential role in plant growth promotion of wheat (Triticum aestivum L.) for sustainable growth. In the present investigation, endophytic bacteria were isolated from different cereal crops growing in the Divine Valley of Baru Sahib, Himachal Pradesh, India and isolates were screened for nitrogen fixation. The nitrogenase activity exhibiting bacterial isolates were further screened for other plant growth promoting traits including solubilization of phosphorus, potassium, and zinc; production of indole-3-acetic acid, siderophores, ammonia, hydrogen cyanide and extracellular enzyme. The potential nitrogen fixing strains were molecularly identified and evaluated for the growth promotion of wheat. A total of 304 putative endophytic bacterial isolates were isolated from wheat, oats, barley, and maize using selective and complex growth media. Among 304 putative endophytic bacteria, 8 isolates exhibits nitrogenase activity. On the basis of nitrogenase activity and other plant promoting traits, two efficient strains i.e. EU-E1ST3.1 and EU-A2RNfb were molecularly identified using 16S rRNA gene sequencing and found that these strains belongs to genera Rahnella. The wheat inoculated with two selected nitrogen-fixing endophytic bacterial strains showed considerable enhancement in total chlorophyll, nitrogen, Fe and Zn content over the un-inoculated control. In comparison of two selected nitrogen-fixing endophytic bacterial strains, Rahnella aquatilis EU-E1ST3.1 was found to enhance better growth and physiological parameters and it might be developed as biofertilizers to establish a sustainable agriculture system. In the present investigation, the isolated potential nitrogen fixing endophytic bacteria could be used as biofertilizer or bioinoculant for growth of diverse cereal crops growing in hilly region for agricultural sustainability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cereals crops are one of the important food and it play an important role to fulfill the food requirement of growing population mainly in the developing nations. Among all the cereal crops, wheat is the most important staple food and major cereal crop worldwide. The wheat is a source of 45% of digestible energy and shares the 30% of total protein content in the diet of human. The U.S. Department of Agriculture in New Delhi estimated India with production forecast of wheat is 105 million metric tons (MMT) during 2020. The enhancing productivity and yield of wheat in modern agriculture relies on the utilization of chemical fertilizer (Patra et al. 2016). The usage of chemical fertilizers results in the gathering of harmful chemical residues that may cause salt effect (higher concentration of salt in the fertilizers resulting in the death or injury of the seedling), fertilizer burn (blackened seed and roots), toxicity effect, and soil acidification. The usage of chemical fertilizers disturb the ecological balance of environment as they alter the soil structure, contamination of groundwater, and eutrophication (enhanced growth of algal bloom and production of toxins) (Lin et al. 2019). Therefore, an alternative approach which is eco-friendly is necessary for enhancing the productivity of wheat.
Sustainable agriculture maintains the productivity of soil by utilization of natural resources without degrading the environment. Recently, the more focus is laid on the formulation of biofertilizer by scientists for maintaining sustainable agriculture. Plant growth-promoting endophytic microbes colonize inner tissues of different plants. The endophytic microbes support the growth and protect the plants against biotic and abiotic stresses (White et al. 2019). In terms of benefiting the host through fixation of atmospheric nitrogen, endophytic microbes are better than the rhizospheric microbes. Endophytic microbiomes are less vulnerable to competition in comparison to microbial communities of rhizospheric region. Apart from fixation of atmospheric nitrogen endophytic microbes also participates in the solubilization of mineral phosphorus (inorganic and/or organic phosphates), potassium, and zinc. Endophytic microbes produce phytohormones and siderophores (chelate Fe) and exhibit biocontrol activity through synthesis of antifungal and antibacterial compounds (Gupta et al. 2012).
The nitrogen fixation is particularly important worldwide for cereal crops including wheat, maize and rice. Endophytic microbes fix the nitrogen into the available forms of ammonia and nitrate. The nitrogen fixing microbes potentially enhances the production of non-leguminous crops while reducing the requirement of chemical fertilizers (Bhattacharjee et al. 2008). The cereal crops require nitrogen for the synthesis of protein chlorophyll pigment and other compounds. In India, since 1980s the use of biofertilizers has started and has been considered as an alternative to the chemical fertilizer. The association of endophytic microbes are generally emphasized because of their beneficial effects for plant such as they stimulate the plant growth, provide protection against various environmental stresses and their potential utilization in environmental restoration (Babu et al. 2013). In the last few decade, a number of endophytic bacteria belonging to different genera have been reported from wheat including Azorhizobium caulinodans, Klebsiella pneumonia, Achromobacter xylosoxidans, Acinetobacter lwoffi, Bacillus amyloliquefaciens, B. subtilis, Paenibacillus hispanicus, Pantoea alhagi, and Pedobacter chitinilyticus (Brady et al. 2009; Toubal et al. 2018).
In the present investigation, endophytic bacteria were isolated using the culture-dependent technique from different cereal crops growing in Baru Sahib, Himachal Pradesh, India. The main objective of the present investigation was to isolate the nitrogen-fixing endophytic bacteria with other multiple plant growth-promoting attributes from cereal crops and the selected isolates of nitrogen-fixing endophytic bacteria were evaluated under in vitro and in vivo conditions for the growth of wheat..
Materials and methods
Isolation and enumeration of endophytic bacteria
A total 15 samples of plants (wheat, oat, barley, and maize) root and stem were collected at maturity stage from the research field of Dr. Khem Singh Gill Akal College of Agriculture, Eternal University, growing at Baru Sahib (30.7537° N, 77.2965° E) located in Sirmour, Himachal Pradesh, India. The healthy plant samples were collected in sterilized polythene bags, labelled, and transported immediately to the laboratory from the field. The plant samples were washed under the running tap water to remove the surface bacteria on roots. The root and stem samples were cut into small pieces of about 1 cm in size. The endophytic bacteria were isolated using surface sterilization method as described by Conn and Franco (2004). The macerate thus prepared was serially diluted and plated on different growth media including nutrient agar, trypticase soy agar, Lurie Bertani agar, King’s B agar, Jensen’s agar and Azotobacter medium. The plates were incubated at 30–37 °C for 3–5 days and observed periodically for bacterial growth. The morphologically, different bacteria from different media were selected, sub-cultured and purified for further studies. The purified microbial cultures were stored in slants at 4 °C and glycerol stock (25%) at -80 °C.
Screening bacterial endophytes for plant growth promoting attributes
The bacterial endophytes were screened firstly for fixation of nitrogen using the acetylene reduction assay. Further nitrogen fixing bacterial isolates were screened for different plant growth promoting attributes including solubilization of phosphorus, potassium, zinc, production of indole acetic acid (IAA) siderophores, ammonia, hydrogen cyanide (HCN) and extracellular enzyme production. The screening of bacteria for plant growth promoting attributes has been done with three replicates using standard protocol as described earlier (Rana et al. 2020).
Acetylene reduction assay
The nitrogen-fixing attribute of endophytic bacterial isolates was checked using the acetylene reduction assay technique (Han and New 1998). The selected endophytic bacterial cultures were inoculated in nitrogen free bromothymol blue medium at 30 °C for 5–7 days. The cotton plugs of each test tube were replaced with Suba seal and gas phase were replaced with 10% of gas mixture consisting of nitrogen, air, and acetylene in the ratio of 90:10:10, v/v. The test tube containing endophytic bacterial cultures and 10% of gas mixture were again incubated for 24 h at 30 °C and the ethylene produced was measured by a Perkin Elmer F-11 gas chromatograph.
Phosphorus solubilization
The qualitative screening of P-solubilization of nitrogen fixing endophytic bacterial isolates were carried out on to Pikovskaya agar medium supplemented with insoluble source of phosphorus i.e. tricalcium phosphate, rock phosphate and apatite (Pikovskaya 1948). The quantitative estimation of selected endophytic bacterial isolates for phosphorus solubilization was performed according to the method illustrated previously by Murphy and Riley (1962). One mL bacterial suspension was inoculated in the 25 mL of National Botanical Research Institute phosphate medium broth (NBRIP) for 7–14 days at 30 °C. After the desired incubation, the endophytic bacterial suspension was centrifuged at 12,000 rpm for 15 min. and the content of supernatant was estimated for phosphorus. At 600 nm the optical density (OD) was measured and P concentration was calculated and expressed in mg L− 1.
Zinc solubilization
The Zn-solubilization qualitative screenings of endophytic bacteria were carried out on nutrient agar supplemented with zinc compounds (0.1%). The quantitative estimation of zinc solubilization of endophytic bacterial isolates was performed according to the method described by Saravanan et al. (2007). The endophytic bacterial isolates were grown overnight in the nutrient broth suspension. The 100 µl of culture grown overnight was transferred into the nutrient broth (50 mL) amended with 0.1% of insoluble zinc phosphate and incubated at 30 °C for 7–14 days. After the 7 days, the samples were centrifuged and using the membrane filter (0.45 μm) the supernatant was filtered. The estimation of soluble zinc content was performed using atomic absorption spectrophotometry (AAS).
Indole-3-acetic acid production
The quantitative estimation production of IAA was performed by inoculation of bacterial culture in Luria Bertani (LB) broth in two sets according to the method of Gordon and Weber (1951). Set I consisted of LB broth (50 mL) containing 100 µg mL− 1 tryptophan and Set II consisted of LB broth (50 mL) without tryptophan. Both sets were inoculated with 1 mL bacterial culture and incubated at 30 °C for 7–14 days on incubator shaker. After the desired incubation, Salkowski reagent was added in both the sets and observed for IAA production (Patten and Glick 2002; Salkowski 1885). The supernatant of microbial culture was collected by centrifugation at 3000 rpm for 20 min and was used for detection of indole acetic acid.
Extracellular enzymes production
The bacterial endophytes were also screened for different extracellular enzyme activity. The amylase and protease activity of bacterial endophytes were screened using the nutrient agar medium supplemented with 0.025% starch and skim milk agar. The bacterial endophytes were grown on Luria Bertani agar supplemented with 0.2% pectin and carboxy methyl cellulose containing media for the screening of pectinase and cellulose. The xylanase and phytase activity of the bacterial endophytes were determined by using xylan (1.0%) agar medium and phytase screening medium amended with sodium phytate, respectively. The standard protocol was used for screening of bacteria for extracellular enzymes production as described earlier by Yadav et al.(2016).
Molecular characterization of endophytic nitrogen fixing bacteria
The selected bacterial strains were molecularly identified by genomic DNA (gDNA) isolation and 16S rRNA gene amplification. The gDNA of the selected potent bacterial strains were isolated according to the method described by Yadav et al. (2015). The quality of extracted genomic DNA was assessed by the electrophoresis on agarose gel (0.8%) and the quantity of genomic DNA was confirmed by spectrophotometer and gel images were digitalized. Afterwards, isolated gDNA of bacterial strains were subjected to 16S rRNA gene amplification using the universal primers 27 F (5’-ACGGCTACCTTGTTACGACTT-3’) and 1492R (5’-AAGGAGGTGATCCAGCCGCA-3’) to obtain fragment of nearly 1500-bp. The PCR amplification was carried out in 100 µL volume with the thermal profile 94 ºC, 2 min; 35 cycles 94 ºC, 1 min; 55 ºC, 1 min; 72 ºC, 2 min; 1 cycle of 72 ºC, 10 min. Following the amplification, amplified PCR products were purified using QIA quick purification kit (Qiagen) and the purified PCR products were sent to Xcelris lab Ltd., Ahmedabad for sequencing. The partial sequences of 16S rRNA gene results obtained after sequencing were subjected to BLASTn program to obtain the bacterial strains identities. The 16 S rRNA gene partial sequences were deposited to NCBI GenBank and accession numbers were assigned.
Evaluation of plant growth promoting ability of endophytic bacteria
On the basis of nitrogenase activity and other plant growth promoting traits two nitrogen fixing endophytic bacteria were selected for the evaluation on wheat (cultivar PBW 343 + Lr24 + GPC) in a pot and field experiment. Under both the conditions, the experiment was carried out in triplicates with total four treatments viz. T1 (EU-E1ST3.1); T2 (EU-A2RNfb); T3 (nitrogen fixing biofertilizer containing Azotobacter chroococcum) and T4 (control) and randomized block design was followed. The pot experiment was carried out in plastic pots containing 4 kg non-sterile soil and the pots were placed 50 cm apart from each other, for the reduction of cross-contamination. In each pot, bacterized wheat seeds were sown and total three plants in each pot were maintained till the harvesting. The field trail was conducted at the research field of Eternal University, Baru Sahib, Sirmour district. The experimental site situated between 30.7537° N latitude, 77.2965° E longitudes in Himachal Pradesh, India. The experiment was laid out as a complete randomized design with three blocks as replicates in a plot size of 16.5 m2 (11 × 1.5 m2) in which each bed was 0.5 m apart. Before the sowing, the wheat seeds were sugar coated (1:1 ratio) and bacterized in the endophytic bacterial suspensions which were grown overnight in the nutrient broth medium. After 90 days of sowing, wheat plants were harvested for the analysis of growth and physiological parameters.
Plant growth parameters and physiological analysis
The length of root and shoot, fresh weight of root and shoot of uprooted plants, and weight of seeds were recorded. The dry weight of plant was recorded by drying in hot air oven at a temperature of 65 °C. After harvesting the crop the grain yield was recorded and expressed in q ha− 1. Using the standard protocol by Moran and Porath (1980) the total chlorophyll content in the leaves of wheat plants were estimated. The Fe and Zn content in the different treated wheat grains were estimated using the standard protocol by Katyal and Sharma (1980). The nitrogen content in grains of wheat was estimated as described by Kjeldahl (1883). All the plant growth and physiological parameters were done using three replicates.
Statistical analysis
The obtained data was subjected to one-way ANOVA statistical analysis. Mean comparisons were conducted using the critical difference (5%) and least significant difference (LSD) test (P = 0.05). LSD and standard error results were calculated.
Results
Isolation and enumeration of endophytic bacteria
A total of 304 endophytic bacteria were isolated from the root and stem of cereal crops of wheat, oat, barley, and maize. The abundance of endophytic bacterial population in the root and stem of different cereal crops ranged from 0.01×107 to 4.40×107 CFU g− 1 sample. The lowest CFU was observed in root samples of oats as 0.02×107 CFU g− 1 root and highest observed was 4.40×107 CFU g− 1 in root samples of wheat (Table 1). Similarly, in case of stem samples the lowest CFU was observed in oats (0.01×107 CFU g− 1) and highest was observed in stem samples of wheat (4.40×107 CFU g− 1). The Jensen agar media supported maximum growth of endophytic bacterial population followed by nutrient agar whereas; Azotobacter media supported minimum growth of endophytic bacterial population.
Screening bacterial endophytes for plant growth promoting attributes
Among all 304 endophytic bacterial isolates screened for nitrogen fixation PGP attributes, 8 exhibited nitrogenase activity. Strain EU-E1ST3.1 showed the maximum nitrogenase activity (272.00±0.13 nmoles C2H4 mg− 1 protein hr− 1) followed by EU-A2RNfb (22.18±0.10 nmoles C2H4 mg− 1 protein hr− 1). Among eight different nitrogen fixing strains 5, 6, and 4 isolates demonstrated solubilization of phosphorus, potassium, and zinc, respectively. Seven six and seven isolates produced siderophores hydrogen cyanide, and ammonia, respectively. IAA production activity was showed by five nitrogen fixing bacterial isolates. The bacterial endophytes isolated from different cereal crops exhibited concurrent production of extracellular enzymes. Among 8 nitrogen fixing bacterial isolates 3 and 5 strains showed amylase and pectinase activity, respectively. Total 5 and 7 isolates demonstrated with protease and xylanase activity, respectively. Cellulase and phytase activity was revealed by 4 strains of bacterial endophytes (Table 2).
Molecular characterization of endophytic nitrogen fixing bacteria
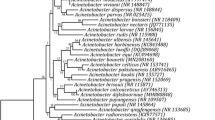
Based upon the 16S rRNA gene sequencing the phylogeny of endophytic bacterial isolates was characterized. PCR amplification of the 16S rRNA gene was performed for examining the differentiation among species. Using the BLASTn algorithm of NCBI database sequences similar to the query sequence within the same organism or in different organisms was studied. The 16S rRNA gene sequence of two selected endophytic bacterial isolates showed > 99% similarity with isolates within of GenBank database. The BLASTn analysis of EU-E1ST3.1, and EU-A2RNfb 16S rRNA sequences revealed 99% similarity with those of the genus Rahnella aquatilis CIP 78.65T. The 16S rRNA gene sequencing demonstrated that strain EU-E1ST3.1 and EU-A2RNfb belonged to the phyla Proteobacteria and class γ—proteobacteria. The 16 S rRNA sequences of strains EU-E1ST3.1, and EU-A2RNfb were deposited in the NCBI GenBank database and accession number were assigned as MN294544 and MN294538.
Plant growth parameters and physiological analysis
Pot experiment
The analysis of variance revealed the highly significant (p < 0.01) differences among all growth and physiological parameters except chlorophyll content in a pot experiment (Table 3). The different isolates of nitrogen-fixing endophytic bacteria had variable effects on the elongation of root and shoot of wheat. The inoculation with nitrogen-fixing endophytic bacterial isolate Rahnella aquatilis EU-E1ST3.1 resulted in the maximum enhancement in shoot length and root length of wheat after 90 days about 1.20 and 1.11 folds compared with uninoculated control. The endophytic bacterial inoculation and NF biofertilizer demonstrated with the positive impact on the Fe and Zn concentration of wheat seeds. The treatment of NF biofertilizer proved better for enhanced Fe (3.66 folds) and Zn (2.07 folds) content in the seeds of wheat significantly better than the uninoculated control. Maximum Fe content (91.9 mg kg− 1) was observed in wheat seeds inoculated with Rahnella aquatilis EU-E1ST3.1 over uninoculated control (33.2 mg kg− 1). The treatment of Rahnella aquatilis EU-E1ST3.1 also resulted in maximum Zn content (55.4 mg kg− 1) over uninoculated control (Fig. 1).
Field experiment
In a field experiment the analysis of variance also revealed the highly significance (p < 0.01) differences among all growth and physiological parameters except chlorophyll content (Table 4). The data of field experiment indicated that the inoculation with selected endophytic bacterial isolates had significant but variable impact on the growth and yield of wheat. The isolate Rahnella aquatilis EU-E1ST3.1 and R. aquatilis EU-A2RNfb produced significantly higher shoot length with 1.09 folds and root length 1.43 folds greater than the uninoculated control. Further, treatments of Rahnella aquatilis EU-E1ST3.1 and R. aquatilis EU-A2RNfb resulted in higher fresh and dry weight of plant than the uninoculated control. The increase in seed weight was also observed with wheat plant inoculated with Rahnella aquatilis EU-E1ST3.1.
The nitrogen uptake by wheat plant, seeds was the highest in treatment Rahnella aquatilis EU-E1ST3.1 with 1.25 folds. The treatment of endophytic bacterial isolates and NF biofertilizer independently resulted in significantly more N accumulation in the seeds than the uninoculated control. The enhanced nitrogen content in wheat seeds inoculated with Rahnella aquatilis EU-E1ST3.1 also significantly resulted with the increased protein content (Fig. 2). Further, the grain yield of wheat was observed to be maximum in treatment of Rahnella aquatilis EU-E1ST3.1 compared with the uninoculated control. The treatment of Rahnella aquatilis EU-E1ST3.1 resulted with 1.22 folds higher grain yield of wheat as compared with the uninoculated control. The treatment of NF biofertilizer also significantly resulted with higher nitrogen, protein content and grain yield of wheat over the uninoculated control but lesser than Rahnella aquatilis EU-E1ST3.1. The endophytic bacterial isolate Rahnella aquatilis EU-E1ST3.1 potentially enhanced the grain yield greater than uninoculated control (Fig. 3).
Effect of nitrogen fixing endophytic bacteria on the growth and physiological parameters (a) shoot length; (b) root length; (c) fresh weight; (d) dry weight; (e) weight of seeds per plant; (f) chlorophyll content; (g) iron content; (h) zinc content and (i) nitrogen content of wheat under in vivo conditions. NF: biofertilizer: Nitrogen fixing biofertilizer containing Azotobacter chroococcum
Discussion
The present study deals with the assessment of the nitrogen-fixing endophytic bacteria isolated from cereal crops grown in the research field of Baru Sahib, district Sirmour, Himachal Pradesh, India. A total of 304 bacterial endophytes were isolated from cereal crops including wheat, oat, barley, and maize by using different media and standard method of isolation. According to the different plants species the endophytic bacterial diversity varies and total endophytic bacterial communities have been studied well in rice and wheat (Brady et al. 2009; Toubal et al. 2018). The present study revealed the endophytic bacterial CFU count was found to be maximum from the root region. Similar findings of abundance of bacterial endophytes have been reported root region of Triticum aestivum cv. by Hereward (Robinson et al. 2016). The synthesis of hydrolytic enzymes such as pectinases, xylanases, and cellulases at the favorable site of root apical zone helps the endophytic bacteria to penetrate into the intercellular tissues of plants (Naveed et al. 2014). In present study, the endophytic bacteria were observed to secrete pectinases, cellulases, amylases, xylanases, proteases, and phytases. The present findings are in comparison to those Elbeltagy et al. (2000). The synthesis of pectinases enzyme may confer an advantage for spreading of bacterial endophytes into the intercellular tissues of host plant whereas, the plant cell wall also consist of major portion of cellulose and middle lamella mostly contains the pectin (Hung and Annapurna 2004). The higher colonization of endophytic microbes within the root segment was analyzed through in-situ laser ablation electrospray ionization mass spectrometry (Agtuca et al. 2020). Though, nitrogen-fixing endophytic bacteria have been explored very less in cereal crops. The current study is a systematic study of nitrogen-fixing endophytic bacteria residing within root and stems parts of cereal crops and was also aimed at characterization of nitrogen-fixing endophytic bacteria.
The 16S rRNA gene sequencing defines the phylogenetic position of endophytic bacteria and demonstrated three strains of nitrogen-fixing endophytic bacteria belonged to the genera Rahnella and phylum Proteobacteria. In addition, Patel and Archana (2017) also reported Proteobacteria, Actinobacteria, and Firmicutes were the most dominant phylum of nitrogen-fixing bacterial endophytes on the basis of 16S rRNA gene sequence analysis from maize, wheat, pearl millet, sorghum, and rice. In the present investigation, Rahnella aquatilis EU-E1ST3.1, identified as potential nitrogen-fixing endophytic bacteria and isolated from oat exhibited multiple plant growth promoting attributes. Rahnella aquatilis ATCC 33,989 was isolated from wheat and maize rhizosphere and had the ability to reduce acetylene (Berge et al. 1991). The bacteria Pseudomonas and Rahnella were identified as seed associated endophytes with Picea abies plant growth promoting and antagonistic activity (Cankar et al. 2005). In another report, Rahnella sp. strain EU-A3SNfb was associated with wild wheat relatives (Negi et al. 2022). In a study, endophytic Rahnella sp. isolated from Ipomoea batatas reported to exhibit the nitrogen fixing ability and also synthesizes indole acetic acid (Khan and Doty 2009). In a study, Chen et al. (2007) reported Rahnella aquatilis act as biological control agent as significantly inhibit the development of crown galls in grapevine caused by Agrobacterium vitis. Our results showed, in cereal crops the nitrogen fixation is mostly dominated by bacterial endophytes belonging to phylum Proteobacteria. Many of the bacterial endophytes belonging to different genera as Pantoea, Bacillus, Brevundimonas, Methylobacterium, and Pseudomonas have been isolated from different plants as nitrogen fixers (Kour et al. 2020b; Rana et al. 2020).
Phosphorus and potassium are the other macronutrients after nitrogen which promotes development of plants. Phosphorus and potassium are abundant in the soil, though available in bound forms to other minerals as phosphorus mostly available is fixed as insoluble iron, aluminum phosphates, and calcium phosphates (Wang et al. 2020). Several studies reported the endophytic bacteria belonging to the genera Pseudomonas, Bacillus, Burkholderia have the potential to solubilize phosphorus and potassium (Kour et al. 2020a). Rahnella aquatilis isolated from soybean rhizosphere exhibited the stronger ability to solubilize the hydroxyapatite and di-calcium phosphate with a drop in the pH. The high pressure liquid chromatography technique identified gluconic acid as the major organic acid synthesized by Rahnella aquatilis (Kim et al. 1997). In the current study, endophytic bacteria Rahnella aquatilis EU-E1ST3.1 demonstrated with highest solubilization of phosphorus 346.8±0.01 mg L− 1 and also showed potassium solubilization. In a study, Rahnella aquatilis isolated from rice plant revealed with the capability to fix atmospheric nitrogen, solubilizes the higher concentration of phosphorus 71.68±6.4 mg L− 1 and also showed significantly higher IAA production in presence of tryptophan. Under the field conditions, Rahnella aquatilis enhances the growth of rice with the maximum reduction in the emission of methane (Rani et al. 2020).
The production of phytohormones plays an essential role in the growth enhancement of plants. There was a diverse level of production of IAA for bacterial endophytes belonging to different genus Acetobacter, Bacillus, Enterobacter, Klebsiella, Sphingomonas reported to produce IAA (Kour et al. 2020a). L-tryptophan is an amino acid with an indole group and it act as a precursor for the IAA production. The addition of L-tryptophan in the medium enhances production of IAA. The previous literature, demonstrated certain bacteria synthesizes the enzyme tryptophanase that catalyzes the breakdown of the indole group from tryptophan. Rahnella sp. has been reported by Vyas et al. (2010) from Hippophae rhamnoides showed with various plant growth promoting attributes solubilization of phosphorus, produces IAA, and siderophores. The previous literature had majorly focused on the symbiotic association between Rhizobium and leguminous plants (Zahran 1999). Presently, more attention is laid on the identification of nitrogen-fixing endophytic microbes in cereal crops (Verma et al. 2019). Besides focusing on the nitrogen fixation, solubilization of phosphorus and potassium, in the current investigation we also examine the IAA synthesizing potential of nitrogen-fixing endophytic bacteria. The results of present study revealed, most of the bacterial endophytes produce the rooting hormone IAA, but their production rates were quite different. Our finding suggests the maximum production of IAA is generated by Rahnella aquatilis EU-E1ST3.1 in the medium amended without L-tryptophan. Whereas the strain Rahnella sp. EU-A3SNfb showed production of IAA is stimulated by the presence of L-tryptophan in the medium.
In a study by Kumar et al. (2009) the data showed Rahnella aquatilis as cold tolerant bacteria stimulated the biomass of plant with higher chlorophyll and protein content, improved phytoextraction of metals, produced siderophores, indole acetic acid, and also solubilized phosphate. The result of present study are in agreement with earlier report describing Rahnella sp. JN27 enhances the growth of plant with the ability of producing IAA, siderophores, ACC deaminase and solubilizing phosphate. The siderophores production is related with the capability of chelating the unavailable form of Fe3+ (Yuan et al. 2014). The synthesis of siderophores and volatile based compound ammonia and HCN is an important attribute which also act as biocontrol agent by suppression of phytopathogens. The present study showed endophytic bacteria Rahnella aquatilis EU-E1ST3.1 promoted production of the siderophores, ammonia, and HCN. Similar result was obtained in a study by Bakhshandeh et al. (2015) Rahnella aquatilis under in vitro conditions solubilized phosphate and under the pot and field experiment Rahnella aquatilis promoted rice plants growth with an increment in content of leaf chlorophyll and development of root.
Conclusion
In conclusion, the result of this study clearly indicates endophytic bacteria Rahnella aquatilis EU-E1ST3.1 enhanced highest nitrogen content in the wheat grains. The current study also validates, for the first time the Rahnella aquatilis EU-E1ST3.1 with highest nitrogenase activity and other multiple plant growth promoting attributes had stimulatory effect on growth of wheat. Mechanistically, the inoculation of Rahnella aquatilis EU-E1ST3.1 strain in wheat enhances the nutrient conditions thereby, increasing the Fe and Zn content. Rahnella aquatilis EU-E1ST3.1 reported in this study is revealed as a potential candidate for the formulations of bioinoculant as it not only enhanced accumulation of biomass but also yield of seed. The nitrogen-fixing endophytic bacteria could be used as biofertilizer or bioinoculant for growth of cereal crops growing in hilly region for agricultural sustainability.
Data availability
Not applicable.
References
Agtuca BJ, Stopka SA, Tuleski TR, do Amaral FP, Evans S, Liu Y et al (2020) In-situ metabolomic analysis of Setaria viridis roots colonized by beneficial endophytic bacteria. Mol Plant Microbe Interact 33:272–283
Babu AG, Kim J-D, Oh B-T (2013) Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. J Hazard Mater 250:477–483
Bakhshandeh E, Rahimian H, Pirdashti H, Nematzadeh G (2015) Evaluation of phosphate-solubilizing bacteria on the growth and grain yield of rice (Oryza sativa L.) cropped in northern Iran. J Appl Microbiol 119:1371–1382
Berge O, Heulin T, Achouak W, Richard C, Bally R, Balandreau J (1991) Rahnella aquatilis, a nitrogen-fixing enteric bacterium associated with the rhizosphere of wheat and maize. Can J Microbiol 37:195–203
Bhattacharjee RB, Singh A, Mukhopadhyay S (2008) Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: prospects and challenges. Appl Microbiol Biotechnol 80:199–209
Brady CL, Venter SN, Cleenwerck I, Engelbeen K, Vancanneyt M, Swings J et al (2009) Pantoea vagans sp. nov., Pantoea eucalypti sp. nov., Pantoea deleyi sp. nov. and Pantoea anthophila sp. nov. Int J Syst Evol Microbiol 59:2339–2345
Cankar K, Kraigher H, Ravnikar M, Rupnik M (2005) Bacterial endophytes from seeds of Norway spruce (Picea abies L. Karst). FEMS Microbiol Lett 244:341–345
Chen F, Guo Y, Wang J, Li J, Wang H (2007) Biological control of grape crown gall by Rahnella aquatilis HX2. Plant Dis 91:957–963
Conn VM, Franco CM (2004) Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Appl Environ Microbiol 70:6407–6413
Elbeltagy A, Nishioka K, Suzuki H, Sato T, Sato Y-I, Morisaki H et al (2000) Isolation and characterization of endophytic bacteria from wild and traditionally cultivated rice varieties. Soil Sci Plant Nutr 46:617–629
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26:192
Gupta G, Panwar J, Akhtar MS, Jha PN (2012) Endophytic nitrogen-fixing bacteria as biofertilizer. In: Lichtfouse E (ed) Sustainable agriculture reviews. Springer, Dordrecht, pp 183–221
Han SO, New P (1998) Variation in nitrogen fixing ability among natural isolates of Azospirillum. Microb Ecol 36:193–201
Hung PQ, Annapurna K (2004) Isolation and characterization of endophytic bacteria in soybean (Glycine sp). Omonrice 12:92–101
Katyal J, Sharma B (1980) A new technique of plant analysis to resolve iron chlorosis. Plant Soil 55:105–119
Khan Z, Doty SL (2009) Characterization of bacterial endophytes of sweet potato plants. Plant Soil 322:197–207
Kim KY, Jordan D, Krishnan HB (1997) Rahnella aquatilis, a bacterium isolated from soybean rhizosphere, can solubilize hydroxyapatite. FEMS Microbiol Lett 153:273–277
Kjeldahl C (1883) A new method for the determination of nitrogen in organic matter. Z Anal Chem 22:366
Kour D, Rana KL, Kaur T, Sheikh I, Yadav AN, Kumar V et al (2020a) Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum Bicolour L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocatal Agric Biotechnol 23:101501
Kour D, Rana KL, Sheikh I, Kumar V, Yadav AN, Dhaliwal HS et al (2020b) Alleviation of drought stress and plant growth promotion by Pseudomonas libanensis EU-LWNA-33, a drought-adaptive phosphorus-solubilizing bacterium. Proc Natl Acad Sci India Sect B Biol Sci 90:785–795
Kumar KV, Srivastava S, Singh N, Behl H (2009) Role of metal resistant plant growth promoting bacteria in ameliorating fly ash to the growth of Brassica juncea. J Hazard Mater 170:51–57
Lin W, Lin M, Zhou H, Wu H, Li Z, Lin W (2019) The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 14:e0217018
Moran R, Porath D (1980) Chlorophyll determination in intact tissues using N, N-dimethylformamide. Plant Physiol 65:478–479
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Naveed M, Mitter B, Reichenauer TG, Wieczorek K, Sessitsch A (2014) Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ Exp Bot 97:30–39
Negi R, Kaur T, Devi R, Kour D, Sheikh I, Tyagi V et al (2022) First report on Rahnella sp. strain EU-A3SNfb, a plant growth promoting endophytic bacterium from wild wheat relative Aegilops kotschyi. Natl Acad Sci Lett 45:393–396
Patel JK, Archana G (2017) Diverse culturable diazotrophic endophytic bacteria from Poaceae plants show cross-colonization and plant growth promotion in wheat. Plant Soil 417:99–116
Patra S, Mishra P, Mahapatra S, Mithun S (2016) Modelling impacts of chemical fertilizer on agricultural production: a case study on Hooghly district, West Bengal, India. Model Earth Syst Environ 2:1–11
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Pikovskaya R (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Rana KL, Kour D, Kaur T, Sheikh I, Yadav AN, Kumar V et al (2020) Endophytic microbes from diverse wheat genotypes and their potential biotechnological applications in plant growth promotion and nutrient uptake. Proc Natl Acad Sci India Sect B Biol Sci 90:969–979
Rani V, Bhatia A, Nain L, Kaushik R (2020) Flooded paddy ecosystem harbors methanol oxidizing-plant growth promoting bacteria belonging to order Enterobacterales. Int J Curr Microbiol Appl Sci 9:685–696
Robinson RJ, Fraaije BA, Clark IM, Jackson RW, Hirsch PR, Mauchline TH (2016) Endophytic bacterial community composition in wheat (Triticum aestivum) is determined by plant tissue type, developmental stage and soil nutrient availability. Plant Soil 405:381–396
Salkowski E (1885) Ueber das Verhalten Der Skatolcarbonsäure Im Organismus. Z Physiol Chem 9(1):23–33
Saravanan V, Madhaiyan M, Thangaraju M (2007) Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 66:1794–1798
Toubal S, Bouchenak O, Elhaddad D, Yahiaoui K, Boumaza S, Arab K (2018) MALDI-TOF MS detection of endophytic bacteria associated with great nettle (Urtica dioica L.), grown in Algeria. Pol J Microbiol 67:67–72
Verma P, Yadav AN, Khannam KS, Mishra S, Kumar S, Saxena AK et al (2019) Appraisal of diversity and functional attributes of thermotolerant wheat associated bacteria from the peninsular zone of India. Saudi J Biol Sci 26:1882–1895
Vyas P, Joshi R, Sharma K, Rahi P, Gulati A, Gulati A (2010) Cold-adapted and rhizosphere-competent strain of Rahnella sp. with broad-spectrum plant growth-promotion potential. J Microbiol Biotechnol 20:1724–1734
Wang J, Li R, Zhang H, Wei G, Li Z (2020) Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol 20:1–12
White JF, Kingsley KL, Zhang Q, Verma R, Obi N, Dvinskikh S et al (2019) Endophytic microbes and their potential applications in crop management. Pest Manag Sci 75:2558–2565
Yadav AN, Sachan SG, Verma P, Tyagi SP, Kaushik R, Saxena AK (2015) Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India). World J Microbiol Biotechnol 31:95–108
Yadav AN, Sachan SG, Verma P, Kaushik R, Saxena AK (2016) Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three sub-glacial lakes of NW Indian Himalayas. J Basic Microbiol 56:294–307
Yuan M, He H, Xiao L, Zhong T, Liu H, Li S et al (2014) Enhancement of cd phytoextraction by two Amaranthus species with endophytic Rahnella sp. JN27. Chemosphere 103:99–104
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63:968–989
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict ofinterest
There are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rana, K.L., Negi, R., Sharma, B. et al. Potential effect of novel endophytic nitrogen fixing diverse species of Rahnella on growth promotion of wheat (Triticum aestivum L.). J. Crop Sci. Biotechnol. (2024). https://doi.org/10.1007/s12892-024-00254-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s12892-024-00254-3