Abstract
Common bacterial blight (CBB) is a severe disease of common bean and the use of resistant cultivars is the most effective control. The objectives were to determine (i) the most appropriate leaf type for CBB evaluation, (ii) the aggressiveness of two bacterial strains, (iii) the presence or absence of SAP6, BC420, and SU91 resistance QTL linked markers, and (iv) the most resistant genotypes. The CBB response in the primary and trifoliolate leaves of 21 genotypes of diverse origins and two checks was evaluated in two greenhouses. Mean trifoliolate leaf score (4.8) was higher than the primary leaf (2.5). The strain Xcp25 (3.2 primary, 5.4 trifoliolate) was more aggressive than ARX8AC (1.7 primary, 4.2 trifoliolate). Andean ‘Montcalm’ with SAP6 QTL was intermediate (6.0) to ARX8AC and susceptible (8.3) to Xcp25 in the trifoliolate leaf. New Andean RCS52-2, RCS53-3, and RCS63-5B with BC420 and SU91, and 08SH840 with SAP6 and SU91 QTL were intermediate (3.5–6.2) to both strains. But, Middle American VAX 3, VAX 4, and VAX 6 with SAP6 and SU91 QTL were resistant (2.3–2.5) to ARX8AC and intermediate to Xcp25 (3.4–6.5) in the trifoliolate leaf. Further efforts are required to pyramid higher levels of resistance from across Phaseolus species and introgressed in Andean common bean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Common bacterial blight (CBB), caused by Xanthomonas campestris pv. phaseoli Smith (Dye) [Xcp, synonym: X. axonopodis pv. phaseoli (Smith) Vauterin et al.] and X. fuscans sbsp. fuscans sp. nov. (Xff) is an important disease of common bean (Phaseolus vulgaris L.) in tropical and subtropical production regions worldwide (Gilbertson and Maxwell 1992; Singh and Schwartz 2010). The Gram-negative, aerobic, and motile bacterium infects leaves, pods, and seeds. Disease symptoms on leaves include water soaked spots that enlarge and coalesce causing necrotic lesions surrounded by a distinct yellow margin (Saettler 1989). In susceptible cultivars, the pathogen invades the entire leaf surface showing a necrotic burned appearance of the foliage. Early pod infection causes small, shriveled, and discolored seed adversely affecting their commercial quality and value, emergence, and seedling vigor (Ishimaru et al. 2005; Saettler 1989; Schuster and Coyne 1981). The disease is favored by temperatures between 28 and 30 °C with high humidity and frequent winds, and yield losses up to 50 % have been reported in favorable environments (Singh and Muñoz 1999; Singh and Schwartz 2010; Wallen and Jackson 1975; Yoshii et al. 1976).
The virulence can vary among bacterial species and strains, but the Xcp strains are more variable compared with Xff (Duncan et al. 2011; Mahuku et al. 2006; Mutlu et al. 2008). Duncan et al. (2011), for example, reported differences in aggressiveness among eight bacterial strains from the Americas and East Africa. They concluded that some strains of Xcp (e.g., Xcp25) were more aggressive than Xff strains, and the small-seeded (<25 g 100 seed) Middle American common bean genotypes with tepary bean (P. acutifolius A. Gray) derived and pyramided or combined resistance such as VAX 3 to VAX 6 and XAN 309 were most resistant to both bacterial species.
Bacteria are seed-transmitted and their epiphytic populations may survive on non-host species and weeds (Gent et al. 2005; Gilbertson et al. 1990; Jacques et al. 2005). An integrated CBB control involves the use of resistant cultivars, pathogen-free seed, bactericides, deep plowing of crop residues, and crop rotation with non-host species (Gent et al. 2005; Gilbertson et al. 1990; Saettler 1989). Of these, the use of resistant cultivars has been the most effective, economical, and environment friendly (Singh and Muñoz 1999).
Sources of CBB resistance have been identified in the common bean primary, secondary, and tertiary gene pools (Mohan 1982; Schuster et al. 1983; Singh and Muñoz 1999; Urrea et al. 1999; Welsh and Grafton 2001; Zapata et al. 1985). Low levels of resistance occur in some small-seeded genotypes such as Colima 9, PI 207262 (synonymous with G1320), and Tamaulipas 9B (Duncan et al. 2011; Singh and Muñoz 1999) and medium-seeded genotypes (25–40 g 100 seed) such as great northern Montana No. 5 (Miklas et al. 2003; Singh and Muñoz 1999) which are common bean landraces of Middle American origin. Intermediate levels of resistance occur in scarlet runner bean (P. coccineus L.) (Freytag et al. 1982; Miklas et al. 1994; Mohan 1982; Park and Dhanvantari 1987; Singh and Muñoz 1999). But, the highest levels of resistance have been found in the tepary bean such as G 40001 and G 40020 (Michaels 1992; Schuster et al. 1983; Singh and Muñoz 1999; Urrea et al. 1999; Zapata et al. 1985).
The CBB resistance is inherited quantitatively and controlled by >20 quantitative trait loci (QTL) (Bai et al. 1997; Geunhwa et al. 1997; Miklas et al. 1996, 2000a; Tar’an et al. 2001). Readers interested in more information on the major and minor effects CBB resistance QTL should also refer to reviews by Kelly et al. (2003), Miklas and Singh (2007), and Miklas et al. (2006a). The three major effects sequence characterized amplified region (SCAR) markers, namely SAP6 on Pv10 (Miklas et al. 2000a), SU91 on Pv08 (Pedraza et al. 1997), and BC420 on Pv06 (Tar’an et al. 2001; Yu et al. 2000) linkage groups are associated with resistance QTL. In addition, more recently Viteri et al. (2014) identified a new CBB resistance Xa11.4 QTL on Pv11.4 linkage group in VAX 1 interspecific breeding line derived from tepary bean G 40001 (Singh and Muñoz 1999; Singh et al. 2001). The SAP6 is linked with the QTL from great northern Montana No. 5 and present in great northern Nebraska No. 1 Sel. 27 (Miklas et al. 2003) and Colima 9 and Tamaulipas 9B (Duncan et al. 2011). SU91 (Pedraza et al. 1997) and BC420 (Tar’an et al. 2001; Yu et al. 2000) are associated with resistance QTL derived from tepary bean PI 319443 (synonymous to G 40020). The BC420, SAP6, and/or SU91 QTL have been used for marker-assisted selection (Miklas et al. 2006b, c; O’Boyle et al. 2007; Yu et al. 2000) and genetics studies (Durham et al. 2013; Vandemark et al. 2008, 2009; Viteri et al. 2014; Zapata et al. 2011).
Although efforts for improving CBB resistance in large-seeded (>40 g 100 seed) Andean beans have been carried out since the 1960s, in general, these possess lower levels of resistance compared with some small-seeded Middle American counterparts (Duncan et al. 2011; Lema et al. 2007; Singh and Muñoz 1999). Thus, the development of large-seeded breeding lines and cultivars with higher levels of CBB resistance is extremely important for production areas with severe CBB problems such as the Midwestern United States, southern Canada, central and southern Brazil, Argentina, Spain, and Africa. Selection for higher CBB resistance may depend on aggressiveness of Xcp and Xff isolates, bacterial density used, plant parts inoculated, post-inoculation time for evaluation, and the environment (Duncan et al. 2011; Lema et al. 2007; Viteri et al. 2014). Bacterial densities ranging from 105 to 108 CFU mL−1 have been used for successful infection and disease development in genetic diversity (Duncan et al. 2011; López et al. 2006; Mkandawire et al. 2004), genetics (Arnaud-Santana et al. 1994; Coyne and Schuster 1974; Urrea et al. 1999; Vandemark et al. 2008, 2009; Viteri et al. 2014; Zapata et al. 2011), breeding (Asensio-S-Manzanera et al. 2006; Miklas et al. 1994, 2006b, c; O’Boyle et al. 2007; Yu et al. 2000), and pathology (Duncan et al. 2011; López et al. 2006; Mkandawire et al. 2004; Mutlu et al. 2008) studies in common bean and other Phaseolus species. Differences in CBB response in leaves, pods, and seeds controlled by different genes/QTL with varying heritability were reported in the common and tepary beans (Arnaud-Santana et al. 1994; Coyne and Schuster 1974). Furthermore, CBB resistance QTL explained different percentages of the phenotypic variance for resistance depending upon the genotype, bacterial strain, plant part inoculated, number of days post inoculation for evaluation, and environment (Geunhwa et al. 1997; Miklas et al. 1996; Viteri et al. 2014). However, often the breeding and pathology studies are based on the disease response in the trifoliolate leaves, evaluated from seven to 21 days post inoculation (Duncan et al. 2011; Vandemark et al. 2008, 2009; Zapata et al. 1985). Researchers seldom used the disease response in the primary leaves (Lema et al. 2007). The objectives of this research were to determine (i) the most appropriate leaf type for CBB evaluation, (ii) the aggressiveness of two bacterial strains, (iii) the presence or absence of SAP6, BC420, and SU91 resistance QTL linked markers, and (iv) the most resistant genotypes.
Materials and methods
Common bean genotypes
Twenty-one common bean genotypes of different origins with varying levels of CBB response and Andean ‘Montcalm’ and Middle American ‘Othello’ as checks were evaluated. Montcalm has large dark red kidney seed and determinate growth habit Type I (Singh 1982). Othello (Burke et al. 1995) has medium size pinto seed and growth habit Type III (Viteri et al. 2014). Of 16 Andean beans evaluated, the interspecific breeding line XAN 159 derived from tepary bean PI 319443 has gray speckled seed (24 g 100 seed) and growth habit Type I. Other 15 Andean breeding lines with tepary bean derived and pyramided resistance included 08SH840, CXR 1, GNX 6, RCS52-1, RCS52-2, RCS53-2, RCS53-3, RCS53-5, RCS63-3, RCS63-4, RCS63-5A, RCS63-5B, USDK-CBB-15, USWK-CBB-17, and Wilkinson 2. Of small-seeded Middle American genotypes, the interspecific breeding line VAX 1 with cream striped seed and growth habit Type III was derived from tepary bean accession G 40001; and VAX 3, VAX 4, VAX 5, and VAX 6 have tepary G 40001 derived (via VAX 1, which is synonymous with PVPA9576-1) and pyramided (via XAN 263 or XAN 309 that possess tepary PI 319443 and common bean great northern Montana No. 5) resistance (Singh and Muñoz 1999; Singh et al. 2001). Breeding lines 08SH840 and CXR 1 derive their CBB resistance from VAX 6 (Dr. Tim Porch, personal communication 2013). The GNX 6 and all the RCS series breeding lines have VAX 3 and Wilkinson 2 in their pedigree (Asensio-S-Manzanera et al. 2006). USDK-CBB-15 and USWK-CBB-17 have XAN 159 (McElroy 1985) and great northern Montana No. 5 (Miklas et al. 2003) resistance (Miklas et al. 2006b, c).
Greenhouse evaluation
A randomized complete bock design with three replications was used in a factorial set up. Two different greenhouses were used, each with two consecutive plantings. Bacterial strains ARX8AC (recovered from an Andean bean in Embarcación, Salta, Argentina in 2008) and Xcp25 (recovered from an Andean bean in Wisconsin, USA before 2003), at densities of 1.7 and 3.2 × 108 CFU mL−1, and 23 common beans were randomized in each planting. Three seeds were sown in a 16.5 × 20.3 cm plastic pot for each genotype, bacterial strain, and bacterial density.
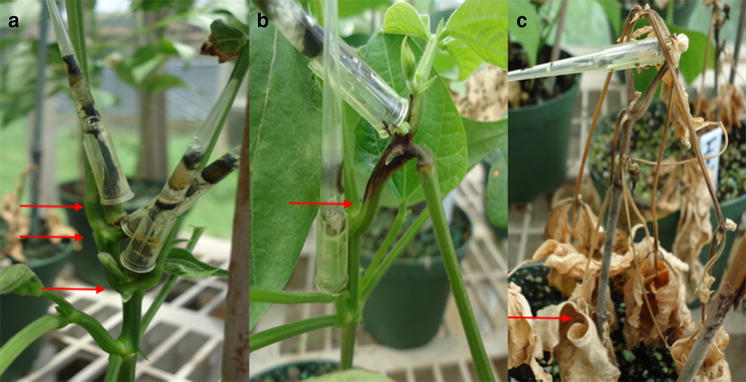
Bacterial cultures were multiplied as needed, and adjusted to densities of 1.7 and 3.2 × 108 CFU mL−1 for inoculation according to Viteri et al. (2014). The primary leaf was inoculated 10 days after sowing and the first trifoliolate leaf on the same plant at 23 days, using a sterilized florist frog (i.e., multiple needles) following the method described by Lema et al. (2007) and Viteri et al. (2014). Inoculated plants were kept in 70 % humidity in the first and 85 % in the second greenhouse. Humidifiers situated under the greenhouse benches and wetting of greenhouse floors after each inoculation were used to achieve high humidity. Plants were grown at a mean day temperature of 24 °C and mean night temperature of 18 °C in the first greenhouse. In the second greenhouse, mean day temperature was 26 °C and mean night temperature was 20 °C with 12 h of light in both greenhouses. Disease severity for each plant individually in the primary leaf was evaluated 14 days post inoculation, whereas the trifoliolate leaf was evaluated at 21 days on a 1–9 scale according to Lema et al. (2007). Genotypes with CBB scores of 1–3 were considered resistant, 4–6 intermediate and 7–9 susceptible.
Molecular marker assays
Five disks punched out from the primary leaf from each plant (approximately 30 mg) were collected before inoculation with bacterial strains. The DNA extraction was carried out using the Dellaporta protocol (Dellaporta et al. 1983), and assays for the SAP6 (Miklas et al. 2000a) was carried out according to Viteri et al. (2014). For BC420 (Yu et al. 2000) and SU91 markers (Miklas et al. 2000b; Pedraza et al. 1997), a multiplex PCR was carried out according to Duncan et al. (2011). All PCR reactions were performed in a PTC-100 thermocycler (MJ Research Inc., Walthman, MA, USA) and PCR products were run in 1.4 % agarose gel stained with 2 % of ethidium bromide. Due to the dominant characteristics of the markers, the presence or absence of different-sized fragments was recorded visually.
Statistical analysis
Analysis of variance, mean disease score, and Fisher’s least significant difference at P ≤ 0.05 were calculated for each data set. Data was analyzed using the SAS 9.3 PROC GLM procedures (SAS 2008). Bartlett’s test of the data from the two greenhouses displayed homogeneity of variances; therefore, a combined analysis of both greenhouses also was carried out (Steel and Torrie 1980). A mix model was used (McIntosh 1983) where replicates were random and greenhouses, genotypes, bacterial strains and densities, and consecutive plantings were fixed effects. The main effects and their interactions were tested using their own pooled error to increase the resolving power to detect smaller, but significant (P ≤ 0.05) differences for CBB response among common bean genotypes.
Results
Mean squares due to greenhouse, leaf type, bacterial strain, bacterial density, genotype, and their interactions were significant (P ≤ 0.05) (Table 1). Trifoliolate leaf had significantly higher CBB scores (3.1–6.4) compared to primary leaf (1.3–5.3). Similarly, bacterial strain Xcp25 (scores 2.1–5.3 in primary and 4.6–6.4 in trifoliolate) was significantly more aggressive than ARX8AC (scores 1.3–2.0 in primary and 3.1–5.3 in trifoliolate) in both leaves (Table 2). But, there were no significant differences (P ≥ 0.05) in CBB scores between bacterial densities in primary and trifoliolate leaves in the first greenhouse. The CBB scores, in general, were higher in the second greenhouse, and significant difference (P ≤ 0.05) in bacterial density were noted only in the primary leaf for Xcp25 (Table 2). Because for germplasm and cultivar improvements researchers are usually interested in determining the highest or best response of genotypes to pathogens, therefore, only data from the second greenhouse were used for reporting the response of 23 common beans to both Xcp strains in this manuscript.
Othello was susceptible in primary and trifoliolate leaves to both Xcp strains (Table 3). Montcalm had intermediate or resistant score in response to ARX8AC, but was susceptible to Xcp25 in both leaves regardless of the bacterial densities. All genotypes, with the exception of Othello and Montcalm, had resistant CBB scores in the primary leaf in response to ARX8AC at both bacterial densities. In contrast, at the high density of Xcp25, Wilkinson 2, 08SH840, RCS52-1, RCS52-2, RCS53-2, RCS53-3, RCS63-5B, VAX 3, and VAX 5 had intermediate and all other genotypes had susceptible scores in trifoliolate leaf (Table 3). Among the interspecific breeding lines derived from tepary bean, VAX 1 had significantly lower scores (2.2–6.5) compared to XAN 159 (3.0–7.2) in both leaves to both strains. Among the Andean breeding lines with pyramided resistance, USWK-CBB-17 and GNX 6 were intermediate to ARX8AC and susceptible to Xcp25 at both bacterial densities in the trifoliolate leaf. Thus, of 17 Andean common beans tested 08SH840, RCS53-3, and RCS63-5B followed by RCS52-2 with either a resistant or intermediate CBB score were most resistant to both bacterial strains at both densities. But, on the average, Middle American VAX 3 to VAX 6 had even higher levels of resistance than these recently developed Andean breeding lines (Table 3).
As could be expected, the susceptible check pinto Othello lacked the three CBB resistance linked QTL, while Montcalm and VAX 1 only possessed the SAP6 QTL (Table 3). Four each of Andean (USDK-CBB-15, USWK-CBB-17, 08SH840, CXR 1) and Middle American (VAX 3, VAX 4, VAX 5, VAX 6) genotypes had SAP6 and SU91 QTL. In contrast, all 12 genotypes with BC420 and SU91 were Andean common bean (Table 3).
Discussion
Differences observed in CBB severity between the two greenhouses could be due to variation in temperature and moisture conditions. Although the conditions for plant growth and disease development in both greenhouses were adequate, higher temperatures in the second greenhouse accompanied with higher moisture conditions (85 %) due to humid dirt floor could have increased disease severity in the second greenhouse. Duncan et al. (2011) also observed differences in CBB severity between greenhouses. CBB was more severe in warmer temperatures (28–30 °C), extended period of moisture, and prevalence of winds under field conditions (Gilbertson and Maxwell 1992; Saettler 1989).
In common bean, most CBB evaluations are carried out in trifoliolate leaves in the greenhouse and/or field (Duncan et al. 2011; Singh and Muñoz 1999). In this study, the trifoliolate leaf had significantly higher mean CBB scores, which facilitated separation among resistant, intermediate, and susceptible genotypes. Lema et al. (2007) also reported higher CBB scores in the trifoliolate leaf compared to the primary leaf. Trifoliolate leaves were more susceptible than primary leaves probably due to increasing plant age (Coyne and Schuster 1974). However, inoculations in the primary leaf in common bean also are used for other bacteria (e.g., Pseudomonas syringae pv. phaseolicola Van Hall), fungi [e.g., Colletotrichum lindemuthianum (Sacc. & Magnus) Lams.-Scrib., Uromyces appendiculatus (Pers.: Pers.) Unger], and viruses (e.g., Bean common mosaic virus, an aphid-vectored potyvirus) that readily reproduce in younger tissues resulting in a successful infection and disease development (Mills and Silbernagel 1992; Strausbaugh et al. 2003; Terán et al. 2013). The importance of evaluating CBB response in the primary leaf lies in the identification of susceptible genotypes such as Othello that could be eliminated in the earlier stages. Thus, only resistant genotypes would then be inoculated and evaluated in the trifoliolate leaf with the same and/or different pathogen isolates, reducing expense and work load especially when dealing with high plant populations. Similarly, genotypes such as Montcalm could be eliminated due to CBB response in the primary leaf to aggressive strains such as Xcp25. Thus, genotypes exhibiting lower CBB scores in both leaves to bacterial strains of different aggressiveness would be selected. They may have better and/or more genes/QTL conferring resistance in different plant parts for a prolonged growth period.
Bacterial strain Xcp25 was significantly more aggressive than ARX8AC in both leaves. Duncan et al. (2011), Lema et al. (2007), and Viteri et al. (2014) also found Xcp25 to be most aggressive. Bacterial density was significant only for Xcp25 in the primary leaf in the second greenhouse where higher disease severity was noted. However, some genotypes (e.g., CXR 1, VAX 1, and VAX 3) were resistant at low density and intermediate at high density of ARX8AC in the trifoliolate leaf. Thus, highly disease-conducive environments may be required for determining appropriate bacterial density for germplasm screening, breeding, and genetics studies. But, Lema et al. (2007) reported that the use of higher densities of aggressive bacterial strains such as Xcp25 could eliminate valuable genotypes (e.g., GNX 6) with resistance to less aggressive strains such as ARX8AC. Thus, for separation of resistant, intermediate, and susceptible responses, appropriate bacterial density should be determined for the plant parts to be inoculated.
The intermediate or resistant response of Montcalm and GNX 6 to ARX8AC in contrast to their susceptible response to Xcp25 in the trifoliolate leaf should be of interest to breeders. The interest lies in finding out if there was or was not any significant crossover interaction between the common bean genotypes and bacterial strains. In this case, there were no crossover interactions between the Xcp strains and the common bean genotypes. For example, all genotypes with a resistant or intermediate response to Xcp25 also had a similar or better response to ARX8AC. In general, only higher CBB scores were observed for Xcp25 compared to ARX8AC. Nonetheless, it is important to isolate and characterize bacterial strains from production regions of interest and use appropriate representative strains for germplasm screening and cultivar development (Duncan et al. 2011; López et al. 2006; Mkandawire et al. 2004). For example, use of less aggressive strains for regions with aggressive strains may lead to erroneous results. Therefore, use of at least one each of less aggressive and aggressive representative strain inoculated in different plant parts (e.g., primary and trifoliolate leaves, and pods) on the same plant should help select strain-specific as well as genotypes with broad-spectrum resistance.
Montcalm and VAX 1 only possessed the SAP6 QTL (Duncan et al. 2011; this study). But, VAX 1 had higher levels of resistance than Montcalm. Thus, indicating that VAX 1 had additional CBB resistance genes/QTL, which may be worth identifying and tagging. In fact, as noted earlier VAX 1 possesses a new CBB resistance Xa11.4 QTL located on Pv11.4 linkage group and identified by the closest marker SNP47467 (Viteri et al. 2014). Furthermore, not all genotypes with the same set of SCAR markers (e.g., USDK-CBB-15, USWK-CBB-17, 08SH840, CXR 1, and VAX 3 to VAX 6 with SAP6 and SU91) had similar CBB response, especially to Xcp25. Therefore, it would be prudent to combine marker-assisted selection (O’Boyle et al. 2007; Yu et al. 2000) with direct CBB screening (Asensio-S-Manzanera et al. 2006) to develop genotypes with the highest levels of resistance (Duncan et al. 2012; Miklas et al. 2006b, c).
In summary, the use of contrasting greenhouse conditions, bacterial strains of different aggressiveness, different bacterial densities, and inoculations in different leaves facilitated separation of genotypes with different levels of CBB responses. Thus, the newly developed Andean genotypes (e.g., 08SH840, RCS52-2, RCS53-3, and RCS63-5B) were not susceptible to any bacterial strain in the primary and trifoliolate leaves, and had significantly higher levels of CBB resistance than Montcalm, XAN 159, Wilkinson 2, USDK-CBB-15, and USWK-CBB-17 developed earlier (McElroy 1985; Miklas et al. 2006b, c; Singh and Muñoz 1999). However, none of these new Andean breeding lines had as high resistance as Middle American VAX 3 to VAX 6. Thus, further efforts are required to pyramid higher levels of resistance from across Phaseolus species and introgressed in Andean common bean.
Abbreviations
- CBB:
-
Common bacterial blight
- QTL:
-
Quantitative trait locus or loci
- SCAR:
-
Sequence characterized amplified region
- Xcp :
-
Xanthomonas campestris pv. phaseoli
- Xff :
-
Xanthomonas fuscans sbsp. fuscans sp. nov
References
Arnaud-Santana E, Coyne DP, Eskridge KM, Vidaver AK (1994) Inheritance, low correlations of leaf, pod, and seed reactions to common bacterial disease in common beans, and implications for selection. J Am Soc Hortic Sci 119:116–121
Asensio-S-Manzanera MC, Asensio C, Singh SP (2006) Gamete selection for resistance to common and halo bacterial blights in dry bean intergene pool populations. Crop Sci 46:131–135
Bai Y, Michaels TE, Pauls KP (1997) Identification of RAPD markers linked to common bacterial blight resistance genes in Phaseolus vulgaris L. Genome 40:544–551
Burke DW, Silbernagel MJ, Kraft JM, Koehler H (1995) Registration of Othello pinto bean. Crop Sci 35:943
Coyne DP, Schuster MM (1974) Inheritance and linkage relations of reaction to Xanthomonas phaseoli (E.F. Smith) Dowson (common blight), stage of plant development and plant habit in Phaseolus vulgaris L. Euphytica 23:195–204
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: Version II. Plant Mol Biol Rep 1:19–21
Duncan RW, Singh SP, Gilbertson RL (2011) Interaction of common bacterial blight bacteria with disease resistance quantitative trait loci in common bean. Phytopathology 101:425–435
Duncan RW, Gilbertson RL, Singh SP (2012) Direct and marker-assisted selection for resistance to common bacterial blight in common bean. Crop Sci 52:1511–1521
Durham KM, Xie W, Yu K, Pauls KP, Lee E, Navabi A (2013) Interaction of common bacterial blight quantitative trait loci in a resistant inter-cross population of common bean. Plant Breed 132:658–666
Freytag GF, Bassett MJ, Zapata M (1982) Registration of XR-235-1-1 bean germplasm. Crop Sci 22:1268–1269
Gent DH, Lang JM, Schwartz HF (2005) Epiphytic survival of Xanthomonas axonopodis pv. allii and Xanthomonas axonopodis pv. phaseoli on leguminous hosts and onion. Plant Dis 89:558–559
Geunhwa J, Skroch PW, Coyne DP, Nienhuis J, Arnaud-Santana E, Ariyarathne HM, Kaeppler SM, Bassett MJ (1997) Molecular-marker-based genetic analysis of tepary bean-derived common bacterial blight resistance in different developmental stages of common bean. J Am Soc Hortic Sci 122:329–337
Gilbertson RL, Maxwell DP (1992) Common blight of bean. In: Chaube HS, Singh US, Mukhopadhay AN (eds) Diseases of international importance. Prentice Hall, Inglewood Cliffs, pp 18–39
Gilbertson RL, Rand RE, Hagedorn DJ (1990) Survival of Xanthomonas campestris pv. phaseoli and pectolytic strains of X. campestris in bean debris. Plant Dis 74:322–327
Institute SAS (2008) SAS/STAT user’s guide. SAS Institute Inc, Cary
Ishimaru C, Mohan SK, Franc GD (2005) Common bacterial blight. In: Schwartz HF, Steadman JR, Hall R, Forster RL (eds) Compendium of bean diseases. Am Phytopath Soc, St. Paul, pp 47–49
Jacques MA, Josi K, Darrasse A, Samson R (2005) Xanthomonas axonopodis pv. phaseoli var. fuscans is aggregated in stable biofilm population sizes in the phyllosphere of field-grown beans. Appl Environ Microbiol 71:2008–2015
Kelly JD, Gepts P, Miklas PN, Coyne DP (2003) Tagging and mapping of genes and QTL and molecular marker assisted selection for traits of economic importance in bean and cowpea. Field Crops Res 82:135–154
Lema M, Terán H, Singh SP (2007) Selecting common bean with genes of different evolutionary origins for resistance to Xanthomonas campestris pv. phaseoli. Crop Sci 47:1367–1374
López R, Asensio C, Gilbertson RL (2006) Phenotypic and genetic diversity in strains of common blight bacteria (Xanthomonas campestris pv. phaseoli and X. campestris pv. phaseoli var. fuscans) in a secondary center of diversity of the common bean host suggest multiple introduction events. Phytopathology 96:1204–1213
Mahuku GS, Jara C, Henríquez MA, Castellanos G, Cuasquer J (2006) Genotypic characterization of the common bean bacterial blight pathogens, Xanthomonas axonopodis pv. phaseoli and Xanthomonas axonopodis pv. phaseoli var. fuscans by rep-PCR and PCR-RFLP of the ribosomal genes. J Phytopathol 154:35–44
McElroy JB (1985) Breeding for dry beans, P. vulgaris L., for common bacterial blight resistance derived from Phaseolus acutifolius A. Gray. Ph. D. Dissertation. Cornell University, Ithaca, U.S.A
McIntosh MS (1983) Analysis of combined experiments. Agron J 75:153–155
Michaels TE (1992) Genetic control of common blight resistance in lines derived from P. vulgaris/P. acutifolius crosses. Annu Rep Bean Improv Coop 35:40–41
Miklas PN, Singh SP (2007) Common bean. In: Kole C (ed) Genome mapping and molecular breeding in plants: pulses, sugar and tuber crops, vol 3. Springer Verlag, Berlin, pp 1–31
Miklas PN, Beaver JS, Grafton KF, Freytag GF (1994) Registration of TARS VCI-4B multilple disease resistant dry bean germplasm. Crop Sci 34:1415
Miklas PN, Stone EJ, Beaver J, Montoya C, Zapata M (1996) Selective mapping of QTL conditioning disease resistance in common bean. Crop Sci 36:1344–1351
Miklas PN, Delorme R, Stone V, Daly MJ, Stavely JR, Steadman JR, Bassett MJ, Beaver JS (2000a) Bacterial, fungal, virus disease loci mapped in a recombinant inbred common bean population (‘Dorado’/XAN176). J Am Soc Hortic Sci 125:476–481
Miklas PN, Smith JR, Riley R, Grafton KF, Singh SP, Jung G, Coyne DP (2000b) Marker-assisted breeding for pyramided resistance to common bacterial blight in common bean. Annu Rep Bean Improv Coop 43:39–40
Miklas PN, Coyne DP, Grafton KF, Mutlu N, Reiser J, Lindgren DT, Singh SP (2003) A major QTL for common bacterial blight resistance derives from the common bean great northern landrace cultivar Montana No. 5. Euphytica 131:137–146
Miklas PN, Kelly JD, Beebe SE, Blair MW (2006a) Common bean breeding for resistance against biotic and abiotic stresses: from classical to MAS breeding. Euphytica 147:105–131
Miklas PN, Smith JR, Singh SP (2006b) Registration of common bacterial blight resistant dark red kidney bean germplasm USDK-CBB-15. Crop Sci 46:1005–1007
Miklas PN, Smith JR, Singh SP (2006c) Registration of common bacterial blight resistant white kidney bean germplasm line USWK-CBB-17. Crop Sci 46:2338–2339
Mills LJ, Silbernagel MJ (1992) A rapid screening technique to combine resistance to halo blight and bean common mosaic virus in Phaseolus vulgaris L. Euphytica 58:201–208
Mkandawire AB, Mabagala RB, Guzman P, Gepts P, Gilbertson RL (2004) Genetic diversity and pathogenic variation of common blight bacteria (Xanthomonas campestris pv. phaseoli and X. campestris pv. phaseoli var. fuscans) suggests pathogen coevolution with the common bean. Phytopathology 94:593–603
Mohan ST (1982) Evaluation of Phaseolus coccineus Lam. germplasm for resistance to common bacterial blight on bean. Turrialba 32:489–490
Mutlu N, Vidaver AK, Coyne DP, Steadman JR, Lambrecht PA, Reiser J (2008) Differential pathogenicity of Xanthomonas campestris pv. phaseoli and X. fuscans subsp. fuscans strains on bean genotypes with common blight resistance. Plant Dis 92:546–554
O’Boyle PD, Kelly JD, Kirk WW (2007) Use of marker assisted selection to breed for resistance to common bacterial blight in common bean. J Am Soc Hortic Sci 132:381–386
Park SJ, Dhanvantari BN (1987) Transfer of common blight (Xanthomonas campestris pv. phaseoli) resistance from Phaseolus coccineus Lam. to P. vulgaris L. through interspecific hybridization. Can J Plant Sci 67:685–695
Pedraza F, Gallego G, Beebe SE, Tohme J (1997) Marcadores SCAR y RAPD para la resistencia a la bacteriosis común (CBB). In: Singh SP, Voysest O (eds) Taller de Mejoramiento de Frijol para el Siglo XXI: Bases para Una Estrategia para América Latina. CIAT, Cali, Colombia, pp 130–134
Saettler AW (1989) Common bacterial blight. In: Schwartz HF, Pastor-Corrales MA (eds) Bean production problems in the tropics. CIAT, Cali, pp 261–283
Schuster ML, Coyne DP (1981) Biology, epidemiology, genetics and breeding for resistance to bacterial pathogens of Phaseolus vulgaris L. Hortic Rev 3:28–57
Schuster ML, Coyne DP, Behre T, Leyna H (1983) Sources of Phaseolus species resistance and leaf and pod differential reactions to common blight. Hort Sci 18:901–903
Singh SP (1982) A key for identification of different growth habits of Phaseolus vulgaris L. Annu Rep Bean Improv Coop 25:92–95
Singh SP, Muñoz CG (1999) Resistance to common bacterial blight among Phaseolus species and common bean improvement. Crop Sci 39:80–89
Singh SP, Schwartz HF (2010) Breeding common bean for resistance to diseases: a review. Crop Sci 50:2199–2223
Singh SP, Muñoz CG, Terán H (2001) Registration of common bacterial blight resistant dry bean germplasm VAX 1, VAX 3, and VAX 4. Crop Sci 41:275–276
Steel RGD, Torrie JH (1980) Principles and Procedures of Statistics, 2nd edn. McGraw-Hill, New York, p 633
Strausbaugh CA, Myers JR, Forster RL, McClean PE (2003) A quantitative method to screen common bean plants for resistance to Bean common mosaic necrosis virus. Phytopathology 93:1430–1436
Tar’an B, Michaels TE, Pauls KP (2001) Mapping genetic factors affecting the reaction to Xanthomonas axonopodis pv. phaseoli in Phaseolus vulgaris L. under field conditions. Genome 44:1046–1056
Terán H, Jara C, Mahuku G, Beebe S, Singh SP (2013) Simultaneous selection for resistance to five bacterial, fungal, and viral diseases in three Andean × Middle American inter-gene pool common bean populations. Euphytica 189:283–292
Urrea CA, Miklas PN, Beaver JS (1999) Inheritance of resistance to common bacterial blight in four tepary lines. J Am Soc Hortic Sci 124:24–27
Vandemark GJ, Fourie D, Miklas PN (2008) Genotyping with real-time PCR reveals recessive epistasis between independent QTL conferring resistance to common bacterial blight in dry bean. Theor Appl Genet 117:513–522
Vandemark G, Fourie JD, Larsen RC, Miklas PN (2009) Interaction between QTL SAP6 and SU91 on resistance to common bacterial blight in red kidney and pinto bean populations. Euphytica 170:371–381
Viteri DM, Cregan PB, Trapp J, Miklas PN, Singh SP (2014) A new common bacterial blight resistance QTL in VAX 1 common bean and interaction of the new QTL, SAP6, and SU91 with bacterial strains. Crop Sci 54 (in press)
Wallen VR, Jackson HR (1975) Model for yield loss determination of bacterial blight of field beans utilizing aerial infrared photography combined with field plot studies. Phytopathology 65:942–948
Welsh MM, Grafton KF (2001) Resistance to common bacterial blight of bean introgressed from Phaseolus coccineus. Hort Sci 36:750–751
Yoshii K, Galvez E, Alvarez AG (1976) Estimation of yield losses in beans caused by common blight. Proc Am Phytopathol Soc 3:298
Yu K, Park SJ, Poysa V (2000) Marker-assisted selection of common beans for resistance to common bacterial blight: efficacy and economics. Plant Breed 119:411–415
Zapata M, Freytag GF, Wilkinson RE (1985) Evaluation for bacterial blight resistance in beans. Phytopathology 75:1032–1039
Zapata M, Beaver JS, Porch TG (2011) Dominant gene for common bean resistance to common bacterial blight caused by Xanthomonas axonopodis pv. phaseoli. Euphytica 179:373–382
Acknowledgments
We thank Drs. Phillip Miklas for supplying seed of USDK-CBB-15 and USWK-CBB-17, Tim Porch for seed of 08SH840 and CXR 1, and M. Carmen-Asensio-Manzanera-S. for seed of the RCS Andean common bean breeding lines. We also thank Drs. Phillip Miklas and Howard Schwartz for their valuable comments and edits. The greenhouse and laboratory support from the Idaho Agricultural Experiment Station at Kimberly to carry out this research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viteri, D.M., Singh, S.P. Response of 21 common beans of diverse origins to two strains of the common bacterial blight pathogen, Xanthomonas campestris pv. phaseoli . Euphytica 200, 379–388 (2014). https://doi.org/10.1007/s10681-014-1161-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-014-1161-x