Abstract
Rice (Oryza sativa L.) production is always threatened by biotic and abiotic stresses. Both stresses can reduce rice productivity and quality. Yellow stem borer (YSB; Scirpophaga incertulas Walker) is one of the biotic stresses and is reported as the most destructive pest of tropical rice insects. The application of pesticides is less effective since the insect larvae live and feed inside the stem, thus inhibiting pesticides to reach the larvae. Planting YSB-resistant cultivars is a good strategy besides environmentally friendly. However, no sufficient level of resistance to YSB has been identified among rice germplasm collection, making the use of conventional breeding methods is difficult. Bacillus thuringiensis (Bt) is a Gram-positive bacterium produces insecticidal proteins, Cry toxins that are toxic to YSB. The introduction of cry genes from Bt into rice plants by using genetic engineering has been widely and successfully carried the out. A number of strategies have been conducted to increase the expression level and to prolong the effectiveness of Bt toxins in transgenic rice, including plant codon usage-optimized genes, the use of strong promoters and wound-inducible promoters and gene stacking. Insect bioassays under laboratory, greenhouse and field conditions showed that transgenic rice plants harboring and expressing cry genes are highly resistant to YSB compared to the original cultivars from which transgenic plants were developed. Thus, the development of transgenic rice plants harboring and expressing cry genes from Bt is a good strategy to build plant resistance against YSB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is an important food crop for more than half of the world’s population with globally providing approximately 20% of the calorie intake. Most of the population growth occurs in Asia where people make rice as their staple food (Kubo and Purevdorj 2004; Chatterjee and Mondal 2014). The International Rice Research Institute predicted that 800 million tons of rice will be required in 2025 (Purevdorj and Kubo 2005). Thus, increasing human population has to be in line with rice production to meet food security. Rice supply and demand must be balanced. However, rice production faces many problems that come from biotic and abiotic stresses. Among biotic stresses, insect pests contribute to the decline in rice productivity and quality, among which is yellow stem borer (Scirpophaga incertulas Walker).
Yellow stem borer (YSB) which is one of Lepidopteran, is a dominant pest in Asia (Renuka et al. 2017). YSB is a pest of deepwater rice and is reported as the most destructive tropical insect pest in paddy fields, since their chronic pattern of infestation and widespread distribution (Cohen et al. 2008; Pathak and Khan 1994; Ghareyazie et al. 1997; Deka and Barthakur 2010). YSB is a monophagous pest and there are no reports that YSB has successfully completed its life cycle in any other plants outside the Oryza species (Renuka et al. 2017).
YSB attacks the rice plant from seedling to grain filling stage, resulting in different symptoms (Devasena et al. 2018). The symptoms of deadhearts are produced when insect attacks at vegetative stage, meanwhile the symptoms called whiteheads occur when insect attacks at generative stage of rice plant growth (Cohen et al. 2008; Deka and Barthakur 2010; Ho et al. 2013). The production losses also vary. Every 1% of deadhearts or whiteheads cause production losses of 2.5% and 4%, respectively (Renuka et al. 2017), even when no chemical control is applied, YSB attacks can destroy the whole crop (Breitler et al. 2004).
The application of pesticides that commonly used to control insect pests is ineffective since larvae of YSB burrow deep into the rice stem and feed inside the stem thus inhibiting pesticides to reach the larvae (Manikandan et al. 2016; Kumar et al. 2010). Moreover, the excessive use of pesticides, beside increasing the production costs also cause deleterious effects to the environment and human health and lead to the emergence of pesticide-resistant insects (Nayak et al. 1997; Breitler et al. 2004; Ho et al. 2006).
Planting cultivars with built-in resistance to YSB is a good strategy (Ho et al. 2006; Hoa and Nhu 2011). One of the important requirements for conducting a plant breeding program is the availability of resistant genes in the plant. However, The International Rice Research Institute (IRRI) has screened more than 30,000 germplasm collection of rice and no sufficient level of resistance to YSB among germplasm has been identified, making the option to use the conventional breeding methods is ineffective. Therefore, another strategies need to be considered (Wu et al. 1997; Chen et al. 2005; Kumar et al. 2010; Yong-Mei et al. 2014; Breitler et al. 2000; Hoa and Nhu 2011).
Bacillus thuringiensis (Bt) has been identified as a reservoir of resistant genes against YSB. Bt is a Gram-positive bacterium produces proteins with entomopathogenic properties, known as Cry toxins (Bravo et al. 2007; Bravo and Soberón 2008). The development of insect-resistant transgenic rice lines by introducing cry genes from Bt with different versions has been widely carried out (Ye et al. 2001; Cheng et al. 1998; Gao et al. 2011; Manikandan et al. 2016). Bioassays against YSB showed that the transgenic rice plants expressing Bt insecticidal proteins exhibited resistance against YSB under laboratory, greenhouse and field conditions (Tu et al. 2000; Datta et al. 2002; Kumar et al. 2010; Chakraborty et al. 2016). In this article the overview of YSB, mode of action of Cry toxins and the development of YSB -resistant transgenic rice lines expressing Cry toxins will be reported.
Yellow stem borer and its impact on rice plants
The moths lay eggs in masses and egg masses are covered with brownish hairs. The newly emerged larvae crawl upward to the tip of the leaf to stay for only short periods. Some larvae form threads and swing to land on other plants or fall on the water (Fig. 1). The larvae that remaining on the tip will climb down to the base and crawl between the leaf sheath and stem. The larvae feed on the leaf sheath tissues for about 1 week and then bore into the stem, staying in the pith and feed on the inner surface of the stem walls, severance the apical parts of the plant from the base. This causes the central leaf does not open, the color changes to brown and dries out and easy to pull out although the remain leaves still green and healthy. The symptoms called deadhearts (Fig. 2a). If the larvae of YSB feeding above the primordia and no further damage occurs, the dead central leaves are pushed out by the new growth, so the plants can compensate for the damage caused by YSB by producing new shoot (Pathak and Khan 1994).
Infestation of YSB during the reproductive stage or after panicle initiation causes the severance of growing plant parts from the base dries the panicles and panicles failed to emerge completely. However, when panicles have emerged, the damage blocks the transport of nutrients from the stem to the grains results in the production of panicles without grains. The affected panicles are being whitish and remain straight. The symptoms are known as whiteheads (Fig. 2b) (Ghareyazie et al. 1997; Pathak and Khan 1994; Deka and Barthakur 2010).
Since the insect larvae live and feed inside the stem and remain there for the rest of their lives as larvae and pupae, thus protecting them completely from the application of chemical pesticides (Nayak et al. 1997; Ho et al. 2006).
Mode of action of Cry toxins in Lepidopteran
Bacillus thuringiensis (Bt) is a Gram-positive soil bacterium produces crystalline inclusions during sporulation (Höfte and Whiteley 1989; Bravo et al. 2018). The crystal inclusions consist of δ-endotoxins, that are encoded by a number of cry genes (Tu et al. 2000; Riudavets et al. 2006; Bravo et al. 2007, 2012, 2018; Sharma et al. 2000). The various δ-endotoxins have been classified into classes (Cry 1, 2, 3, 4, etc.) based on amino acids similarities. These classes are then composed into subclasses (Cry1A, Cry1B, Cry1C, etc.) and each subclass is divided into subfamilies or variants (Cry1Aa, Cry1Ab, Cry1Ac, etc.) (Sanchis and Bourguet 2008).
Cry proteins with entomopathogenic properties are grouped into five types based on the specificity of the target insect. Cry1 proteins are specific to Lepidopteran larvae, Cry2 proteins are specific to Lepidopteran and Dipteran larvae, Cry3 proteins are specific to Coleopteran larvae, Cry4 proteins are specific to Dipteran larvae and Cry5 proteins are specific to Lepidopteran and Coleopteran larvae (Sharma et al. 2000; Bravo et al. 2007).
The main target of Bt toxins is insect midgut. When susceptible larvae ingest the crystalline inclusions, the crystals are solubilized under alkaline condition (high pH) which is typical of Lepidopteran insect midgut, releasing proteins called δ-endotoxins. The δ-endotoxins are in fact prototoxins. Further, they are cleaved by midgut proteases to produce mature toxins which exhibit a highly specific insecticidal activity. The region within the N-terminal toxin is composed of at least 6 α-helices which play an important role in penetrating the peritrophic membrane (Sharma et al. 2000; Pardo-López et al. 2013; Palma et al. 2014; Bravo et al. 2015, 2018).
The activated toxins bind to the specific receptors, on the brush border membrane of the midgut epithelial cells of susceptible larvae before penetrating into the target cell membrane. Further, the α-helices penetrate the membrane irreversibly, forming pores. The formation of toxin-induced pores in the membrane, destroying the cells and lead to the death of the susceptible insect larvae (Tu et al. 2000; Bravo et al. 2018; Kumar et al. 2008; Sharma et al. 2000; Schünemann et al. 2014).
Cry proteins are highly specific to a limited number of target insect species and they are harmless to non-target insects including natural enemies, vertebrates, plants and humans (Riudavets et al. 2006; Pardo-López et al. 2013; Bravo et al. 2012, 2018).
The improvement of rice plant resistance to YSB by introducing the cry genes
Since no sufficient level of resistance to YSB has been identified among rice germplasm collection, the development of YSB-resistant cultivars through conventional breeding methods is difficult (Kumar et al. 2010; Ho et al. 2006). Therefore, an alternative strategy is to employ genetic engineering that allows breeders to introduce desirable genes across taxa. Genetic engineering can overcome the crossing by producing new shoot barriers between unrelated organisms and this technology complements traditional methods in crop improvement (Nayak et al. 1997; Ramesh et al. 2004; Ho et al. 2006).
Insecticidal proteins from Bt, also known as Cry proteins have been reported can control the major Lepidopteran pests of rice including YSB (Frutos et al. 1999; Tabashnik et al. 2015; Zhou et al. 2014; Breitler et al. 2000). By using genetic engineering, it is possible to introduce cry gene from Bt into rice plant to build resistance toward YSB. Several gene transformation techniques can be used to transfer the desirable genes into plants and the most widely used is Agrobacterium-mediated transformation (Deka and Barthakur 2010). Transformation of cry genes into rice genome has been succesfully conducted and since 1995 Bt crops have been grown increasingly over the years (Breitler et al. 2004; Liu et al. 2016).
The expression of single cry gene in transgenic rice plants to confer full rice plant protection from YSB has been reported in both Japonica and Indica rice. Transgenic Indica Basmati rice (B-370) expressing cry1Ac and cry2A genes separately and each driven by the maize ubiquitin promoter and CaMV35S promoter respectively, exhibited a high level of resistance to YSB under field studies for two consecutive years. Five transgenic rice lines of B-370 showed resistance against YSB with the percentage of deadhearts and whiteheads ranged from 16.2 to 36.6% and 13.3 to 18.1%, respectively, compared to 100% of deadhearts and whiteheads in untransformed B-370 control. Nevertheless, the level of resistance of transgenic rice harboring cry2A gene was lower compared to transgenic rice containing cry1Ac. These data might be correlated with the protein accumulation in the plants. The content of Cry1Ac in four transgenic lines within the range of 0.97–1.13% of total leaf soluble protein. Meanwhile, the content of Cry 2A in one transgenic line was lower and calculated within the range of 0.12–0.95% of total leaf soluble protein. The low expression of Cry2A may be due to the gene was driven by the CaMV35S promoter, thus the use of strong promoters such as ubiquitin promoter should be considered (Bashir et al. 2004). The maize ubiquitin promoter is a constitutive promoter that can enhance the expression of foreign genes in monocots and is more active than CaMV 35S promoter in transgenic rice plants (Zhou et al. 2013; Breitler et al. 2004; Bashir et al. 2005).
There are many approaches to increase the expression of cry gene in transgenic rice plants, in addition to the use of strong promoters. Most of published studies reported that plant codon usage-optimized genes and the use of green tissue-specific promoters are the options (Bano-Maqbool et al. 1998; Wu et al. 1997; Breitler et al. 2004; Chakraborty et al. 2016).
The plant codon-optimized cry1C* gene with GC content of 44.65% and shares 84% nucleotide sequence homology with the original cry1Ca5 gene and a modified cry2A* gene with GC content of 59.04% and shares 69.45% homology in nucleotide sequence with the original cry2Aa, and each gene was driven by a maize ubiquitin promoter had been introduced into Indica rice cv. Minghui 63, separately (Tang et al. 2006; Chen et al. 2005). Evaluation of resistance under field conditions in one homozygous transgenic line, T1c-19 line harboring cry1C* with the highest Cry1C* protein content (1.38 µg g−1 fresh leaf) showed that no deadhearts and whiteheads symptoms were observed in T1c-19 (0%), whereas the percentage of deadhearts and whiteheads in Minghui 63 as a control of susceptible cultivar was 17.3% and 5.6%, respectively (Tang et al. 2006). Moreover, no pesticides for YSB were applied during the experiment.
Similar expression of cry2A* gene in transgenic rice lines against YSB was obtained. Laboratory assay to determine the efficacy of cry2A* gene showed that the Cry2A* content in four homozygous transgenic lines within the range of 9.65 -12.11 µg gˉ1 fresh leaf was able to kill 100% larvae of YSB within 5 days after infestation. On the contrary, in original susceptible Minghui 63, the percentage of dead larvae was only 10.1% and the remaining larvae grew normally. Plant resistance performance in the field showed that the percentage of deadhearts and whiteheads of four homozygous transgenic lines ranged from 0.98 to 4.18% and 0.00 to 0.50%, respectively, whereas in original susceptible Minghui 63 reached 17.24% and 5.57% (Chen et al. 2005). These data explained that transgenic rice lines with codon optimized, cry1C* and cry2A* genes under the control of a maize ubiquitin promoter were highly significantly resistant against YSB compared to the original susceptible Minghui 63 under laboratory and field conditions.
Breitler et al. (2000) reported that a novel monocot codon-optimized cry1B synthetic gene with a GC content of 58% and driven by the maize ubiquitin promoter had been transferred into highly susceptible cultivar to Striped Stem Borer (SSB; Chilo suppressalis Walker), Ariete cultivar and confers full protection in one homozygous transgenic event (A64) against SSB under phytotron conditions. The accumulation of Cry1B at 0.4% of the total soluble protein was sufficient to protect transgenic plants from SSB attacks and this was demonstrated by 100% insect mortality within 7 days after infestation. On the contrary, no dead larvae (0%) were found in original susceptible Ariete cultivar. Similar to YSB, SSB is a rice stem borer and belongs to the Lepidopteran group. SSB is most abundant in temperate countries and is reported as an important pest in Asia and Europe and no sufficient level of resistance to SSB in rice germplasm has been identified (Breitler et al. 2000, 2004).
The use of a green tissue-specific promoter of rbcS gene to target protein to the chloroplast is another alternative. The use of a Chrysanthemum rbcS1 promoter to drive a chimeric cry2AX1 to the chloroplast, resulted in higher expression and better stability of Cry2AX1 in green tissues. A chimeric cry2AX1 with a total of 633 amino acids composed of the N-terminal residues, 1–585 are from Cry2Aa and the C-terminal residues, 586–633 are corresponded to 576–623 of Cry2Ac (Manikandan et al. 2016; Chakraborty et al. 2016). A chimeric cry2AX1 was then introduced into Indica rice line, JK1044R. The highest expression level of Cry2AX1 among five homozygous transgenic lines was detected in BtE15 line. The level of Cry2AX1 in the leaf of BtE15 line was 0.68 µg g−1 fresh leaf at vegetative stage and 1.18 µg g−1 fresh leaf at the generative stage, whereas in leaf-sheath reached 0.81 and 1.34 µg g−1, respectively. Evaluation of transgenic BtE15 line against YSB had been conducted using in vitro and in planta bioassays. In vitro bioassay on BtE15 line showed that the percentage of dead larvae was 88% at vegetative stage and 93% at the generative stage, meanwhile in non-transgenic control plants were 7% and 8%, respectively. In planta bioassay on BtE15 line showed that the percentage of deadhearts and whiteheads were 1.74% and 0%, respectively, and in non-transgenic control plants were 22.08% and 15.74%. These data indicated that the accumulation of Cry2AX1 in green tissues of BtE15 line exhibited significantly resistant against YSB (Chakraborty et al. 2016). In addition to have a better stability and expression of Cry2AX1, the use of rbcS1 promoter can avoid the accumulation of Cry in rice seed endosperm (Chakraborty et al. 2016; Ye et al. 2009).
The continuous exposure of transgenic crops expressing a single Bt toxin may lead to the breakdown of plant resistance to YSB in the field. This is due to the pests have the potential to evolve to breakdown the effectiveness of Bt toxins. Many strategies to prevent or to delay the emergence of pest resistance in the stem borer populations to Bt toxins have been proposed (Chen et al. 2005; Kumar et al. 2010; Manikandan et al. 2016). The concept of gene stacking by combining two or more cry genes with different modes of action in one plant has been recommended as an efective strategy (Chen et al. 2005; Bravo and Soberón 2008; Kumar et al. 2010; Manikandan et al. 2016). This strategy based on the assumption that the emergence of insect resistant to a Bt toxin is due to a mutation in one of the insect’s genes and single mutation does not confer simultaneous resistance to other toxins. Multiple mutations are required to break the effectiveness of two or more Bt toxins. Thus, the combination of two cry genes should have different modes of action, otherwise a single mutation of insect may confer a cross resistance to both Cry toxins (Bashir et al. 2004, 2005; Chen et al. 2005; Tang et al. 2006).
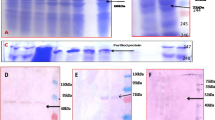
The translational fusion gene cry1B-cry1Ab driven by the maize ubiquitin promoter had been introduced into two popular cultivars in Vietnam i.e. Nang Huong Cho Dao (NHCD) and Mot Bui (MB). The cry1B and cry1Ab genes were fused at 28th codon downstream from Cry1B activation-site codons and 29th codon upstream from Cry1Ab activation-site codons. This fusion gene encodes a single Cry1B-Cry1Ab fusion protein with molecular weight of 66-kDa. Western blot analysis showed that the presence of a single band of 66-kDa in two transgenic rice lines, Nab4 and Mab2 indicated the expression of cry1B-1Ab with the level of protein ranged from 0.8 to 1.3% of the total soluble protein. Insect bioassay under laboratory conditions using the cut-stem method showed that the percentage of dead larvae in transgenic rice lines tested reached 100% at 4 days after infestation. Meanwhile, the percentage of dead larvae in untransformed plants within the range of 0–16.6%. These data indicated that the protein content with a range of 0.8–1.3% of the total soluble protein was able to protect plants from YSB attacks. However, the efficacy of the translational fusion gene in transgenic rice lines should be conducted in the field under natural conditions (Ho et al. 2006).
Another strategy to prevent or to delay the emergence of resistance among the target insects to Bt toxin is the use of wound-inducible promoters (Breitler et al. 2004; Breitler et al. 2001). One of the wound-inducible genes is the maize proteinase inhibitor (mpi) gene encoding for MPI protein. The accumulation of MPI was observed in response to mechanical wounding or insect feeding (Tamayo et al. 2000). A monocot codon-optimized cry1B synthetic gene driven by a wound-inducible promoter of mpi gene had been transformed into widely grown Mediterranean Japonica rice cultivar, Ariete. The homozygous transgenic line harboring cry1B gene under the control of mpi promoter, A9.1 line was protected from SSB attacks compared to the original Ariete cultivar under greenhouse and field conditions (Breitler et al. 2001, 2004). Moreover, A9.1 line has the same level of resistance as A64.1 line against YSB and the accumulation of Cry1B in insect-wounded leaves of A.9 line was comparable to the A.64.1 line i.e. 0.2%–0.4% of total soluble protein. A64.1 is a transgenic rice line expressing the cry1B gene under the control of the constitutive maize ubiquitin promoter and show a highly resistant against SSB (Breitler et al. 2000, 2004).
To study the role of a wound-inducible promoter of mpi gene in relation to Cry1B accumulation in transgenic rice plants, Western analyses on both unwounded and wounded of leaf blade, leaf sheath, stem and root tissues had been conducted. Cry1B was not detected in unwounded tissues but can be found adjacent the insect-wounded tissues of transgenic rice line (A9.1) harboring cry1B gene under the control of mpi promoter (pMpi-cry1B). On the contrary, Cry1B was detected even in unwounded tissues of transgenic rice line, A64.1 expressing the same gene driven by the constitutive maize ubiquitin promoter (pUbi-cry1B). The results indicated that the activity of mpi promoter was triggered by wounding (Breitler et al. 2004).
Moreover, the accumulation of Cry1B was not detected in seed parts (embryo, mature endosperm, polished grain and cooked polished grain) of A9.1 line whereas the toxin was found in comparable tissues except in cooked rice of A64.1 line (Breitler et al. 2004). This finding supports the previous statement that the activity of the mpi promoter was triggered under wound induced conditions.
The absence of Cry1B toxin in cooked polished grain of both transgenic rice lines, A9.1 and A64.1 is due to the boiling process. Heating can destroy the heat-sensitive Cry1B (Breitler et al. 2004). Although Cry toxins have been reported to be safe for human consumption (Bashir et al. 2004), additional evidence related to the absence of Cry1B in cooked rice provides an added value in increasing consumer confidence in the acceptance of Bt rice (Breitler et al. 2004).
The development of Javanica rice cultivar, Rojolele expressing the cry1B gene had been conducted in our laboratory. The goal of our research is to build the resistance of susceptible Rojolele cultivar against YSB. Rojolele cultivar was chosen since it has a good quality in taste and texture and aromatic as well, which makes this cultivar popular in Indonesia. The construct of cry1B gene driven by a wound-inducible promoter of mpi gene, was provided by Dr. Emmanuel Guiderdoni (CIRAD, France).
Agrobacterium-mediated transformation had been chosen to introduce cry1B gene from Bt into a local Javanica rice cultivar, Rojolele. Four homozygous transgenic rice lines harboring cry1B gene under the control of mpi promoter have been obtained. The efficacy of cry1B gene in four homozygous transgenic rice lines was examined using in vitro and in planta bioassays under laboratory and greenhouse conditions. Both assays were conducted at the vegetative stage. In vitro bioassay using the cut-leaf method showed that the percentage larval mortality of YSB in four homozygous transgenic rice lines ranged from 93.62 to 100% within 3 days after infestation. Meanwhile, the number of mortality of YSB larvae in non-transgenic Rojolele cultivar only reached 11.11% and the remaining larvae still alive (Estiati et al. 2020). Due to the very few surviving larvae in transgenic tissues, it is suggested that the toxin level of Cry1B in four homozygous transgenic lines was sufficient to kill YSB larvae (Fig. 3).
In planta bioassay using the whole plant showed that the percentage of deadhearts in four homozygous transgenic rice lines ranged from 0 to 9.83%. Conversely, in non-transgenic Rojolele and IR64 cultivars as susceptible checks reached 93.5% and 98.18%, respectively. The contrasting appearance between transgenic rice lines and untransformed control cultivars can also be seen (Fig. 4). Based on the scale from 0 to 9 (IRRI 2013), the four homozygous transgenic rice lines have score of 0 and 1 and they are categorized as resistant lines, whereas the non-transgenic Rojolele and IR64 cultivars have a score of 9 and they are categorized as highly susceptible cultivars against YSB (Estiati et al. 2020).

(Source: Estiati et al. 2020)
In vitro bioassay on homozygous transgenic rice lines harboring cry1B gene and non-transformed Rojolele as a control of susceptible cultivar. Each leaf section was infested with 10 larvae of YSB. a–d Homozygous transgenic rice lines; e non-transgenic Rojolele cultivar. The observations were conducted at 3 days after infestation. The leaves of four homozygous transgenic rice lines exhibited little detectable damage. Meanwhile, severely damaged leaves were seen in non-transgenic Rojolele plant as a result of YSB feeding

(Source: Estiati et al. 2020)
The appearance of transgenic rice lines and susceptible checks following infestation with the larvae of YSB at 14 days after inoculation. a Non-transgenc IR64 cultivar; b non-transgenic Rojolele cultivar; c–f homozygous transgenic rice lines. The deadhearts symptoms were visible both in non-transgenic Rojolele and IR64 cultivars but were absent in four homozygous transgenic rice lines
From insect bioassays under in vitro and in planta conditions exhibited that the expression of cry1B gene in transgenic rice provides satisfactory plant protection against YSB and SSB (Estiati et al. 2020; Breitler et al. 2004).
Conclusion
YSB is difficult to control by using pesticides since the insect larvae stay only for a short period outside the plant before enter the plant and live in the stem (Pathak and Khan 1994). Planting the YSB-resistant cultivars in the field is the right approach. Since no rice germplasm with sufficient level of resistance to YSB has been identified, plant improvement by utilizing the resistance genes from rice plants is less effective (Breitler et al. 2000; Deka and Barthakur 2010). Genetic engineering is an alternative. Genetic engineering overcomes the problem of sexual incompatibilities thus it is possible to transfer or to introduce the desirable genes between any two unrelated species of organisms (Ye et al. 2009; Kumar et al. 2010). Introducing cry genes from Bt into various rice cultivars has been widely carried out. The cry genes have been introduced into rice cultivars adapted to local growth conditions and consumer requirements. The efficacy of the genes in transgenic rice plants under laboratory, greenhouse and field conditions exhibited a high level of protection from damages by YSB (Breitler et al. 2000). It has been reported that the higher concentration of Cry protein in transgenic plants correlates with the higher mortality of insect larvae (Manikandan et al. 2016; Kumar et al. 2010; Breiler et al. 2000). Thus, the level of Cry protein correlates with the level of insect resistance.
A number of strategies have been conducted to increase the expression of cry genes in transgenic rice and to prevent or to delay the emergence of insect resistance in the stem borer populations to Bt toxins. These including the use of strong promoters such as the constitutive ubiquitin (ubi) promoter from maize, a green tissue-specific promoter (rbcS) to target protein to the chloroplast, optimized codon usage to match codon preference in plants, discovery of novel insecticidal genes with novel modes of action, gene stacking and the use of wound-inducible promoters (Breitler et al. 2000; 2004; Cheng et al. 1998; Chakraborty et al. 2016; Manikandan et al. 2016).
The advantages of using a green tissue-specific rbcS1 promoter from Chrysanthemum morifolium and a wound-inducible mpi promoter from maize in addition to increase the expression of cry genes, also prevent the accumulation of Cry toxins in the rice seed parts as well (Chakraborty et al. 2016; Breitler et al. 2004). The absence of Cry accumulation in the rice seed parts gives a positive value in increasing consumer confidence in the acceptance of Bt rice.
Gene stacking or combining two or more cry genes with different modes of action in one plant is a strategy to prevent or to delay the insect pest from evolving to become resistant to Bt toxins. Insect has the ability to overcome the efffectiveness of a Bt toxin with mutation in one of insect’s gene. Thus, in order to survive, multiple mutations are required by the insect to break the effectiveness of multiple Bt toxins and this will not be realized in a short time (Bashir et al. 2005; Chen et al. 2005; Tang et al. 2006; Ho et al. 2006; Bravo and Soberón 2008).
The level of insect resistance against YSB is depends on the accumulation of Cry protein in plant (Tang et al. 2006). The concentration of Cry in transgenic rice plants varied within the range of 0.1–0.5% of total soluble protein, even in some transgenic rice plants reached 1% of total soluble protein. These levels of Cry are sufficient to protect plants from YSB attacks. However, high toxin accumulation at a level of 3–5% of total soluble protein have been reported but the plants showed phenotypically abnormal leading to stunting, leaf chlorophyll bleaching, sterility and decrease of yield (Bano-Maqbool et al. 1998; Breitler et al. 2000; Chen et al. 2005; Chakraborty et al. 2016).
Although the accumulation of Cry toxins with different gene constructs are different among independent transgenic rice lines, the toxins are still providing full-plant protection against YSB compared to the non-transformed control cultivars. Transgenic rice plants expressing Cry toxins resulted in 90–100% insect mortality and showed almost zero percentage of deadhearts and whiteheads. Meanwhile, in non-transgenic rice plants that do not contain Cry toxins, the insect larvae grow normally and the dead larvae is only less than 10%. The non-transformed plants also produce a high percentage of deadhearts and whiteheads. Moreover, Bt toxins are highly specific to target insect thus they are harmless to non-target organisms especially for mammals (Tu et al. 2000; Kumar et al. 2008). Thus, the development of environmentally friendly YSB-resistant transgenic rice plants by introducing and expressing the cry genes from Bt is a good strategy to overcome yield losses due to YSB attacks in paddy field.
References
Bano-Maqbool S, Husnain T, Riazuddin S, Masson L, Christou P (1998) Effective control of yellow stem borer and rice leaf folder in transgenic indica rice varieties Basmati 370 and M7 using the novel δ-endotoxin cry2A Bacillus thuringiensis gene. Mol Breed 4:501–507
Bashir K, Husnain T, Fatima T, Latif Z, Mehdi SA, Riazuddin S (2004) Field evaluation and risk assessment of transgenic indica basmati rice. Mol Breed 13:301–312
Bashir K, Hussain T, Fatima T, Riaz N, Makhdoom R, Riazuddin S (2005) Novel indica basmati line (B-370) expressing two unrelated genes of Bacillus thuringiensis is highly resistant to two lepidopteran insects in the field. Crop Prot 24:870–879
Bravo A, Soberón M (2008) How to cope with insect resistance to Bt toxins? Trends Biotechnol 26(10):573–579
Bravo A, Gill SS, Soberón M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49(4):423–435
Bravo A, Gómez I, Porta H, Garcia- Gómez BI, Rodriguez-Almazan C, Pardo L, Soberón M (2012) Evolution of Bacillus thuringiensis Cry toxins insecticidal activity. Microb Biotechnol 6:17–26
Bravo A, de Castro DLM, Sánchez J, Cantón PE, Mendoza G, Gómez I, Pacheco S, Garcia- Gómez BI, Onofre J, Ocelotl J, Soberón M (2015) Mechanism of action of Bacillus thuringiensis insecticidal toxins and their use in the control of insect pests. In: Alouf J, Ladant D, Popoff MR (eds) The comprehensive sourcebook of bacterial protein toxins, 4th edn. Academic Press, Oxford, pp 858–873. https://doi.org/10.1016/B978-0-12-800188-2.00030-6
Bravo A, Gill SS, Soberón M (2018) Bacillus thuringiensis: mechanisms and use. Refer Mod Life Sci. https://doi.org/10.1016/b978-0-12-809633-8.04071-1
Breitler JC, Marfà V, Royer M, Meynard D, Vassal JM, Vercambre B, Frutos R, Messeguer J, Gabarra R, Guiderdoni E (2000) Expression of Bacillus thuringiensis cry1B synthetic gene protecs Mediterranean rice against the striped stem borer. Plant Cell Rep 19:1195–1202
Breitler JC, Cordero MJ, Royer M, Meynard D, Segundo BS, Guiderdoni E (2001) The − 689/+ 197 region of the maize proteinase inhibitor gene directs high level, wound-inducible expression of the cry1B gene which protects transgenic rice plants from stem borer attack. Mol. Breed 7:259–274
Breitler JC, Vassal JM, del Mar Catala M, Meynard D, Marfà V, Melé E, Royer M, Murillo I, Segundo BS, Guiderdoni E, Messeguer J (2004) Bt rice harboring cry genes controlled by a constitutive or wound-inducible promoter: protection and transgene expression under Meditterranean field conditions. Plant Biotechnol J 2:417–430
Chakraborty M, Reddy PS, Mustafa G, Rajesh G, Narasu VML, Udayasuriyan V, Rana D (2016) Transgenic rice expressing the cry2AX1 gene confers resistance to multiple lepidopteran pests. Transgen Res. https://doi.org/10.1007/s11248-016-9954-4
Chatterjee S, Mondal P (2014) Management of rice yellow stem borer, Scirpophaga incertulas Walker using some biorational insecticides. J Biopest 7:143–147
Chen H, Tang W, Xu C, Li X, Lin Y, Zhang Q (2005) Transgenic indica rice plants harboring a synthetic cry2A* gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theor Appl Genet 111:1330–1337
Cheng X, Sardana R, Kaplan H, Altosaar I (1998) Agrobacterium-transformed rice plants expressing synthetic cryIA(b) and cryIA(c) genes are highly toxic to striped stem borer and yellow stem borer. Proc Natl Acad Sci 95:2767–2772
Cohen MB, Chen M, Bentur JS, Heon KL, Ye G (2008) Bt rice in Asia: potential benefits, impact, and sustainability. In: Romeis J, Shelton AM, Kennedy GG (eds) Integration of insect-resistant genetically modified crops within IPM programs. Springer, New York, pp 223–248
Datta K, Baisakh N, Thet KM, Tu J, Datta SK (2002) Pyramiding transgenes for multiple resistance in rice against bacterial blight, yellow stem borer and sheath blight. Theor Appl Genet 106:1–8
Deka S, Barthakur S (2010) Overview on current status of biotechnological interventions of yellow stem borer Scirpophaga incertulas (Lepidoptera: Crambidae) resistance in rice. Biotechnol Adv 28:70–81
Devasena N, Soundararajan RP, Reuolin SJ, Jeyaprakash P, Robin S (2018) Evaluation opf rice genotypes for resistance to yellow stem borer, Scirpophaga incertulas (Walker) through artificial screening methods. J Entomol Zool Stud 6(1):874–878
Estiati A, Astuti D, Nurhasanah AN, Nugroho S (2020) In vitro and in planta efficacy studies on T6 generation of transgenic Rojolele rice lines against rice yellow stem borer (Scirpophaga incertulas Walker). IOP Conf Ser Earth Environ Sci 439:012054
Frutos R, Rang C, Royer M (1999) Managing insect resistance to plants producing Bacillus thuringiensis toxins. Crit Rev Biotechnol 19(3):227–276
Gao J, Zhang Y, Zhao Q, Lin C, Xu X, Shen Z (2011) Transgenic rice expressing a fusion protein of Cry1Ab and Cry9Aa confers resistance to a broad spectrum of Lepidopteran pests. Crop Sci 51:2535–2543
Ghareyazie B, Alinia F, Menguito CA, Rubia LG, de Palma JM, Liwanag EA, Cohen MB, Khush GS, Bennett J (1997) Enhanced resistance to two stem borer in an aromatic rice containing a synthetic cry1A(b) gene. Mol Breed 3:401–414
Ho NH, Baisakh N, Oliva N, Datta K, Frutos R, Datta SK (2006) Translational fusion hybrid Bt genes confer resistance against yellow stem borer in transgenic elite Vietnamese rice (Oryza sativa L.) cultivars. Crop Sci 46:781–789
Ho GTT, Le CV, Nguyen TH, Ueno T, Nguyen DV (2013) Incidence of yellow stem borer Scirpophaga incertulas Walker in Haiphong, Vietnam and control efficacy of egg mass removal and insecticides. J Fac Agric Kyushu Univ 58(2):301–306
Hoa TTC, Nhu HTH (2011) Development of transgenic rice lines resistant to insect pests using Agrobacterium tumefaciens-mediated transformation and mannose selection system. Omonrice 18:1–10
Höfte H, Whiteley HR (1989) Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev 53(2):242–255
International Rice Research Institute (IRRI) (2013) Standard evaluation system for rice, 5th edn. International Rice Research Institute (IRRI), Manila, p 55
Kubo M, Purevdorj M (2004) The future of rice production and consumption. J Food Distrib Res 35(1):128–142
Kumar S, Chandra A, Pandey KC (2008) Bacillus thuringiensis (Bt) transgenic crop: an environment friendly insect-pest management strategy. J Environ Biol 29(5):641–653
Kumar S, Arul L, Talwar D (2010) Generation of marker- free Bt transgenic indica rice and evaluation of its yellow stem borer resistance. J Appl Genet 51(3):243–257
Liu Q, Hallerman E, Peng Y, Li Y (2016) Development of Bt rice and Bt maize in China and their efficacy in target pest control. Int J Mol Sci 17:1561. https://doi.org/10.3390/ijms17101561
Manikandan R, Balakhrishnan N, Sudhakar D, Udayasuriyan V (2016) Transgenic rice plants expressing synthetic cry2AX1 gene exhibits resistance to rice leaffolder (Cnaphalocrosis medinalis). 3 Biotech 6:10. https://doi.org/10.1007/s13205-015-0315-4
Marfà V, Melé E, Vassal JM, Messeguer J (2002) In vitro insect feeding bioassay to determine the resistance of transgenic rice plants transformed with insect resistance genes against striped stem borer (Chilo suppressalis). Vitro Cell Dev Biol Plant 38(4):310–315
Nayak P, Basu D, Das S, Basu A, Ghosh D, Ramakrishnan NA, Ghosh M, Sen SK (1997) Transgenic elite indica rice plants expressing CryIAc δ-endotoxin of Bacillus thuringiensis are resistant against yellow stem borer (Scirpophaga insertulas). Proc Natl Acad Sci 94:2111–2116
Palma L, Munoz D, Berry C, Murillo J, Caballero P (2014) Bacillus thuringiensis Toxins: an overview of their biocidal activity. Toxins 6:3296–3325. https://doi.org/10.3390/toxins6123296
Pardo-López L, Soberón M, Bravo A (2013) Bacillus thuringiensis insecticidal three-domain Cry toxins: mode of action, insect resistance and consequences for crop protection. FEMS Microbiol Rev 37:3–22
Pathak MD, Khan ZR (1994) Insect pests of rice. International Rice Research Institute (IRRI), Los Banos, p 89
Purevdorj M, Kubo M (2005) The future of rice production, consumption and seaborne trade: synthetic prediction method. J Food Distrib Res 36(1):250–259
Ramesh S, Nagadhara D, Reddy VD, Rao KV (2004) Production of transgenic indica rice resistant to yellow stem borer and sap-sucking insects, using super-binary vectors of Agrobacterium tumefaciens. Plant Sci 166:1077–1085
Renuka P, Madhav MS, Padmakumari AP, Barbadikar KM, Mangrauthia SK, Rao KVS, Marla SS, Babu VR (2017) Genes genomes. Genetics 7:3031–3045
Riudavets J, Gabarra R, Pons MJ, Messeguer J (2006) Effect of transgenic Bt rice on the survival of three nontarget stored product insect pests. Environ Entomol 35(5):1432–1438
Sanchis V, Bourguet D (2008) Bacillus thuringiensis: applications in agriculture and insect resistance management. A review. Agron Sustain Dev 28:11–20
Schünemann R, Knaak N, Fiuza LM (2014) Mode of action and specificity of Bacillus thuringiensis toxins in the control of caterpillars and stink bugs in soybean culture. ISRN Microbiol. https://doi.org/10.1155/2014/135675
Sharma HC, Sharma KK, Seetharama N, Ortiz R (2000) Prospects for using transgenic resistance to insects in crop improvement. EJB Electron J Biotechnol 3(2):76–95
Tabashnik BE, Zhang M, Fabrick JA, Wu Y, Gao M et al (2015) Dual mode of action of Bt proteins: prototoxin efficacy against resistant insects. Sci Rep 5:15107. https://doi.org/10.1038/srep15107
Tamayo MC, Rufat M, Bravo JM, Segundo BS (2000) Accumulation of a maize proteinase inhibitor in response to wounding and insect feeding, and characterization of its activity toward digestive proteinases of Spodoptera littoralis larvae. Planta 211:62–71
Tang W, Chen H, Xu C, Li X, Lin Y, Zhang Q (2006) Development of insect-resistant transgenic indica rice with a synthetic cry1C* gene. Mol Breed 18:1–10
Tu J, Zhang G, Datta K, Xu C, He Y, Zhang Q, Khush GS, Datta SK (2000) Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis δ-endotoxin. Nat Biotechnol 18:1101–1104
Wu C, Fan Y, Zhang C, Oliva N, Datta SK (1997) Transgenic fertile japonica rice plants expressing a modified cry1A(b) gene resistant to yellow stem borer. Plant Cell Rep 17:129–132
Ye G, Tu J, Hu C, Datta K, Datta SK (2001) Transgenic IR72 with fused Bt gene cry1Ab/cry1Ac from Bacillus thuringiensis is resistant against four Lepidopteran species under field conditions. Plant Biotechnol. 18(2):125–133
Ye R, Huang H, Yang Z, Chen T, Liu L, Li X, Chen H, Lin Y (2009) Development of insect-resistant transgenic rice with Cry1C*-free endosperm. Pest Manag Sci 65:1015–1020
Yong-Mei J, Rui M, Zhijing Y, Ling W, Wenzhu J, Xiufeng L (2014) Development of lepidopteran pest-resistant transgenic japonica rice harboring a synthetic cry2A* gene. J Integr Agric. https://doi.org/10.1016/S2095-3119(14)60897-2
Zhao Q, Liu M, Tan M, Gao J, Shen Z (2014) Expression of cry1Ab and cry2Ab by a polycistronic transgene with a self-cleavage peptide in rice. PloS One 9(10). http://www.plosone.org
Zhou J, Yang Y, Wang X, Yu F, Yu C, Chen J, Cheng Y, Yan C (2013) Enhanced transgene expression in rice following selection controlled by weak promoters. BMC Biotechnol 13:29. http://www.biomedcentral.com/1472-6750/13/29
Zhou J, Xiao K, Wei B, Wang Z, Tian Y, Tian Y, Song Q (2014) Bioaccumulation of Cry1Ab protein from an herbivore reduces anti-oxidant enzyme activities in two spider species. PloS One 9(1). http://www.plosone.org
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Estiati, A. Development of Bt rice potential for yellow stem borer control. J. Crop Sci. Biotechnol. 23, 395–403 (2020). https://doi.org/10.1007/s12892-020-00025-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-020-00025-w