Abstract
In the era of personalized and genomic medicine, awareness of patients with rare diseases is increasing as new approaches to diagnosis and treatment are developed. This study examined perceived barriers experienced by families with rare diseases and explored possible differences between participants in Malaysia and California, USA. The study involved N = 108 participants recruited in genetics clinic appointments at the University of Malaya Medical Center and three sites in Southern California. Participants completed a survey involving multiple choice and Likert scale items pertaining to perceived barriers to access genetics-related healthcare. Results from this study provide evidence of similar perceived barriers, despite differences in the two populations. Participants selected the expansion of healthcare provider knowledge of rare diseases to be the most beneficial approach to overcome perceived barriers. In both locations, it was also noted that travel distance to clinic was not perceived as a large stress factor. Taking these observations together, a healthcare model with a central location of providers well-versed in medical genetics may be considered if further data support our findings. The data from this study support a need for improving healthcare provider knowledge of genetics. Future studies exploring how these perceived stress factors are impacting families as well as different methods of educating providers are suggested by findings from the study, as well as studies querying the opinions of those who are unable to access genetics services.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction and background information
Rare diseases affect approximately 25 to 30 million people in the United States (U.S.) alone (GARD 2016). Many of these conditions have a genetic cause and require lifelong care which can be costly for both the patients and the healthcare system (Orphanet 2017). Patients experience countless barriers when attempting to access genetics-related healthcare, such as cost of testing and lack of educated medical professionals (Valencia et al. 2015; Harvey et al. 2007). These two factors together, among others, cause a great number of patients and their families to go through what is known as the “diagnostic odyssey”, the search for a diagnosis and cause of a condition (Valencia et al. 2015; Shashi et al. 2013).
The cost of genetic testing has been proven to be a barrier for many families (Zayts et al. 2013). This is due to lack of coverage by insurance policies and the high cost of these tests, oftentimes costing thousands of U.S. dollars (USD), although some U.S. insurances have begun integrating genetic testing reimbursement policies (Cigna 2017). As with many new medical technologies, the cost-benefit balance of many genetic tests for the healthcare system has yet to be determined. The benefits of more established tests, such as cancer genetic tests, are now being recognized and covered by insurance policies. A number of insurance policies cover certain genetic tests when family history criteria and genetic counseling pre-requisites have been met. However, newer tests, such as whole genome sequencing (WGS) tests, are still considered “experimental” by many policies (Cigna 2017). These tests can have up to a 30% chance of ending the diagnostic odyssey for patients and can provide valuable information such as recurrence risks, mechanism of disease, and future outcomes for the patient (Yang et al. 2013).
Malaysia (MY) uses a two-tiered healthcare system consisting of a government-funded public sector and a private sector. The MY public health sector also includes the optional service of an orang kurang upaya (OKU) card for disabled individuals. Individuals with sensory impairments, physical disabilities, learning difficulties, mental illness, or multiple disabilities are eligible to apply (JKM 2016). The OKU card is able to facilitate access to services such as healthcare, education, employment, social, and rehabilitation services. However, funding for genetic testing in MY is mainly through charitable sources or research funds.
Technological improvements have helped drive the cost of genetic testing down significantly in the past 7 years (Wetterstrand 2016). The cost per raw megabase of DNA sequencing has dropped from about $1000 USD in 2005 to less than 10 cents today with the recent advancements of massively parallel sequencing and high throughput screening, which allow for several samples to be run at the same time. The cost of clinical genetic testing also includes the cost of storing and interpreting the data, which are current limitations of whole exome sequencing (WES) and WGS (Katsanis and Katsanis 2013). As the cost is driven down, the attention in more recent years has turned to the providers ordering these tests.
Many patients with conditions suspicious for genetic etiology are cared for by primary care physicians and specialists treating the manifesting symptoms (Taber et al. 2014; Harvey et al. 2007). The lack of appropriate genetics education among non-genetics healthcare providers remains a problem in both the U.S. and MY (Bowdin et al. 2016; Klitzman et al. 2013; Laedtke et al. 2012). There are shortages of trained geneticists both in the U.S. and in MY, with approximately 50% of medical genetics residency slots being filled each year in the U.S. Attempts of increasing recruitment into the field of medical genetics have not been fruitful (Korf et al., 2005). MY had four centers in 2013 offering genetic services, with a few outpatient clinics offered by those centers, where there is approximately one medical geneticist for every 3 million people (Zayts et al. 2013). In contrast, the University of California of Los Angeles health system currently employs approximately seven medical geneticists in its pediatric genetics practice (UCLA Health 2017), while Los Angeles county has a population of approximately 10 million as of 2016 (U.S. Census Bureau 2016). The medical geneticist service ratio provided by one hospital system alone already surpasses the service ratio in MY, and there are several other hospital systems employing medical geneticists in Southern California (CA). Non-genetics healthcare providers have also begun integrating genomic medicine into their practices, although they may not feel prepared to do so (Taber et al. 2014; Salari 2009; Harvey et al. 2007).
Recent studies have found that lack of knowledge of the non-genetics health professional is still a barrier with up to 25% of primary care providers feeling that they are not equipped to discuss genetic test results (Mikat-Stevens et al. 2015; Delikurt et al. 2015; Salm et al. 2014; Mainous III et al. 2013). Interpreting test results and conveying the information to patients are difficult with a limited understanding of medical genetics. For example, variants of uncertain significance may be incorrectly interpreted as positive results which could cause unnecessary distress for the patient and lead to inappropriate management decisions. This demonstrates the need for all physicians to have adequate knowledge of genetic testing. Approaches to increase the genetic knowledge base of existing providers and improvements in medical school education have been studied, such as webinars and workshops offering continuing education credits (Carroll et al. 2009). These approaches have been implemented in North America since the early 2000s, when genetic testing was becoming more widely used. However, there are few studies that seek the patient’s perspective on this matter, and few studies regarding access to genetics services other than cancer genetics (Delikurt et al. 2015).
This study aimed to investigate the perceptions of patients in MY, a country that has few geneticists and less funding for genetic tests, and of patients in the U.S., a country that has implemented increased genomic healthcare education programs and lowered costs of genetic testing. It explored perceived barriers in these unique and diverse sets of populations. MY and Southern CA are both ethnically diverse areas and were chosen in an attempt to provide a broader understanding of existing commonalities or differences among patients. The shortage of studies of healthcare perceptions among patients in Southeast Asia and Asian Americans demonstrates a need for exploratory research in the area for a better understanding of their opinions. As a starting point, insight may be gained by involving families who have surpassed the obstacles of obtaining and attending a genetics clinic, as well as soliciting what types of improvements these patients see as most beneficial.
It is anticipated that the CA and MY patients will differ in their perception of the barriers faced in accessing adequate genetics-related healthcare. It is expected that the respondents will perceive the lack of care centers and the travel time and costs for care to be larger burdens due to the lack of genetics health professionals in MY. Participants in both countries are anticipated to respond similarly in regards to hoping for improvements to healthcare provider knowledge regarding the rare disease conditions. The goal of this study was to explore what areas of the current healthcare system are perceived as needing improvement by querying patients in genetics clinics.
Methods
Participants
Participants in this study were required to be at least 18 years of age and must either have a diagnosis of or be suspected of having a rare disease, or have a family member who fits this description (see Table 1 for demographic characteristics). Participants were required to be attending or have attended a genetics or metabolic clinic appointment. Participants with limited eyesight who wished to participate were able to have an onsite researcher read the survey aloud. Participants were required to understand either English, Malay, or Mandarin.
Recruitment
Participants were recruited through the genetics and metabolic outpatient clinics and pediatric inpatient consultations at University Malaya Medical Centre in Kuala Lumpur, Malaysia (UMMC) and from the various genetics, metabolic, and specialty genetics clinics at Long Beach Memorial Medical Center (LBMMC), Children’s Hospital of Orange County (CHOC), and University of California Irvine Medical Center (UCIMC) in CA, U.S. This study was reviewed and classified as exempt research by the Institutional Review Board of the University of California, Irvine and was approved by the UMMC Medical Ethics Committee. Patients were given a study information sheet prior to proceeding with the study. The recruitment period was between July 2015 and March 2016. Participants were approached during their clinic appointments by the researchers, and clinician judgment regarding amount of time available and emotional stability of the patient was used to determine whether a patient could or should not be approached regarding the study. A response rate could not be calculated due to the process of the recruitment.
Data collection
A total of 111 study participants began the survey, all of whom were eligible to participate based on prior mentioned criteria. Of these participants, three surveys were not included in the analyses due to less than half the survey being completed because of time constraints, leaving 108 (N = 108) participant responses used for analyses.
Instrumentation
The survey was a paper questionnaire available in English and Malay. Malay translations were produced by native speakers. Response translations were performed by a native speaker of Malay, and then translated back to Malay by another native speaker for comparison, to minimize misinterpretations in the translation process. It consisted of 18 total multiple-choice questions and 26 Likert scale questions about perceived barriers to healthcare. Ten of the multiple-choice questions also included a free-response section for those who selected “other”. Topics included diagnosis status of the patient, familial genetic testing, number of providers seen, provider specialties making correct and incorrect diagnoses, transportation, and patient treatment and therapy status. Participants were also asked to select the largest difficulty faced when receiving satisfactory healthcare from the following: lack of funds, lack of healthcare provider knowledge, lack of public knowledge, lack of time, lack of transportation, patient’s fear of burden on family, and prior negative experiences in healthcare. Participants were also asked to choose what they believed would be most beneficial for those with the patient’s condition, in addition to what would be considered least helpful. The survey topics and selections were developed from preliminary results from a previous survey performed by the Malaysian Rare Disorders Society (MRDS) in 2013, guidance from clinical geneticists and genetic counselors, and previously studied barriers and perceptions in healthcare (Hennekam 2011). Please refer to Appendix A (supplementary material) for the complete survey. All questions were created by the researchers and piloted with 15 random participants prior to data collection. Participants were able to ask the researcher questions during the questionnaire for clarification.
The Likert scale questions reported in this study consisted of the rating of five items (time and distance to travel to care center, cost of travel, cost of treatment or medical care, amount of in home assistance needed for the patient, knowledge of the inheritance of the disease) regarding burden and amount of stress for the participant’s family. The scale ranged from 1 to 5 and included not applicable (1 = no burden, 2 = low burden, 3 = moderate burden, 4 = high burden, 5 = severe high burden, N/A = not applicable). This section also asked the participant to choose one item from the section that is perceived as the largest burden or stress factor for the participant and their family. The results of the remaining 21 Likert scale questions are not reported in this study. These questions explored possible causes of delay in diagnosis and perception of satisfaction with the patient’s healthcare.
Data analysis
Descriptive statistics consisted of counts for categorical variables (multiple choice questions) and means and standard deviations of the Likert scale variables. Responses with low counts were grouped with similar responses to increase the statistical power, as well as to fulfill assumptions required for performing statistical tests. Analyses of the categorical variables were performed using a two-tailed Pearson Chi-squared (Χ2) test or a Fisher’s exact test with a significance level of p < 0.05 to determine a statistical difference of responses between groups. The significance levels were adjusted for Table 1 using Bonferroni correction with an alpha of 0.05 and five statistical tests and were adjusted for analyses in Table 2 with an alpha of 0.05 and four statistical tests, to correct for multiple comparisons.
Survey analysis of the Likert scale variables was performed using independent sample t tests to determine if there was a statistically significant difference in the mean value between two groups, using a significance level of p < 0.05. One-way analysis of variance (ANOVA) was used for analysis of the Likert scale variables when determining if there was a statistically significant difference in the mean values between more than two groups, using a significance level of p < 0.05. If significance was detected, a Tukey post hoc test was then performed to determine which comparisons between groups contributed to the significance and corrects for family-wise error rate. These tests were conducted using the statistical software, IBM Statistical Package for Social Sciences 23 (IBM, Armonk, NY).
Results
Participant demographic information is displayed in Table 1. The number of participants (n) refers to those who responded to the questionnaire items. Participants were allowed to skip questions, therefore not all totals add up to N = 108. The participant demographics in the two countries differed most in religious belief and ethnicity. A higher proportion of participants in CA identified as patients. Of the 21 patient participants, 4 were recruited in MY (mean age 25.3 years) and 17 were recruited in CA (mean age 45.6 years). Of the 87 caretaker participants, 50 were recruited in MY (mean age 35.9 years) and 37 were recruited in CA (mean age 39.4 years). The overall mean age of patient participants was 41.7 years, with a range between 18 and 77 years. The overall mean age of caretaker participants was 37.5 years, with a range between 22 and 82 years. The mean age of patients reported by the caretaker participants was 8.27 years. Proportions based on age, gender, income level, and educational background were similar between the two countries. For statistical analyses, education levels were grouped into “Up to high school”, “Some college or bachelor’s degree”, and “graduate/professional degree” for analysis. There was no statistical difference observed in education levels reported by participants in the two countries when using a Chi-square test, X2 (2 df, N = 103) = 4.977, p = .083; however, it was noted that there were more MY participants reporting “up to high school” education than CA participants, and more CA participants reporting “some college or bachelor’s degree”.
There was no observed statistical difference in responses to items regarding genetic testing status and disclosure when comparing the two countries’ participants (see Appendix A: questions 7, 8 and 9a).
Participants were asked what type of transportation the patient usually uses to go to the hospital, shown in Table 2. There was no significant difference between the proportions of responses chosen by participants recruited in MY and CA using the Fisher’s exact test (p = 0.421), where 91% of participants (n = 97) selected “car/motorcycle” as the type of transportation used by the patient to go to the hospital.
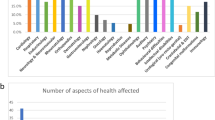
Participants were asked to choose the largest difficulty faced to receive satisfactory healthcare for the patient’s condition, which are displayed in Fig. 1 and Table 2. There was not an observed statistical difference after Bonferroni correction (p = 0.042) using the Fisher’s exact test comparing the proportions of responses chosen by participants recruited in MY and CA. A majority of participants in MY selected “lack of healthcare provider knowledge” (40%) and “lack of funds” (22%) as the largest difficulty faced, while participants in CA selected “lack of healthcare provider knowledge” (58%) and “lack of public knowledge” (19%) as the largest difficulty. There were no significant observed associations between the response chosen as the largest difficulty faced to receiving satisfactory healthcare and the following demographic characteristics: family insurance type (p = 0.228), family income level (p = 0.231), participant education level (p = 0.502), participant sex (p = 0.157), or participants’ patient or caretaker status (p = 0.123).
Participants were asked to select what they believed would be the most helpful for people with the diagnosed genetic condition, which are displayed in Fig. 2 and Table 2. The proportions of MY participant responses significantly differed from CA participant responses (p = 0.006), with 53% of CA participants selecting to expand healthcare provider knowledge, while the proportions of MY participant responses had a wider spread of responses, with the most being centered on newborn screening (24%), support groups (18%), healthcare provider knowledge (16%), and public awareness of the rare disease (16%). The most beneficial tool selected by the greatest proportion of total participants (34%) was “expanding healthcare provider knowledge”. There were no observed associations between the response chosen as the most helpful for people with the diagnosed genetic condition and the following demographic characteristics: family insurance type (p = 0.426), family income level (p = 0.320), participant education level (p = 0.220), participant sex (p = 0.195), or participants’ patient or caretaker status (p = 0.230). There was no observed difference in response proportions for those with or without diagnoses of genetic conditions (p = 0.087) or with the number of doctors the patient has seen (p = 0.389).
Participants were also asked to select which medical professional made the correct diagnosis, which are displayed in Table 2. Of those with diagnoses (n = 88), a statistical difference was observed after Bonferroni correction when using a two-sided Fisher’s exact test (p = 0.001) where participants recruited in MY largely had correct diagnoses made by geneticists or metabolic specialists (n = 31) while a majority of the participants recruited in CA reported that specialists (n = 22) made correct diagnoses, closely followed by geneticists or metabolic specialists (n = 17).
Further, Likert scale results have been omitted from reporting due to several confounding variables between the two cohorts such as reported ethnicity, religion, and other cultural differences. We have chosen to report the findings that remain similar between the two cohorts despite these differences to demonstrate the similarities experienced by participants of all reported backgrounds.
Discussion
Developed countries have moved their focus away from infectious diseases and towards chronic illness and preventative care. As healthcare is moving towards personalized medicine and integrating genomic medicine into medical care, individuals with rare disease have become an increasingly important part of the healthcare system. There is a lack of studies comparing global patient perceptions of healthcare, particularly for the field of clinical genetics. The goals of this study were to explore the barriers being perceived by individuals with rare disease when accessing general genetic services in two very different countries and to determine whether these perceived barriers differ or remain similar. This study found that despite the plethora of differences between the two participant groups, a common need for better healthcare provider knowledge was discovered. A better understanding of these factors may direct appropriate actions and interventions on improving access and satisfaction of these services. Even though a response rate was unable to be calculated for this study, the exploratory nature of this study provided valuable insights into the assessed populations.
Practice implications
Participants in the study largely did not consider travel time and travel costs to be an important factor of stress and burden for the family. In concordance with this finding, the study also found that participants in both MY and CA did not perceive the lack of care centers to be a priority. In fact, increasing the number of care centers was chosen more often as being the least beneficial for patients with rare diseases. In MY, although there are significantly fewer genetics clinics (Lee and Thong 2013; Zayts et al. 2013), the patients traveling to these clinics preferred to increase provider knowledge as opposed to increasing the amount of clinics available. This could suggest that dissatisfaction with the current centralized healthcare model is not primarily stemming from the distance to travel to clinics, or that travel is not perceived to be as burdensome as other barriers in middle- and high-income countries. It is also possible that these families have adjusted their lifestyles and finances to the logistics and travel costs needed for the patient’s continuing care in tertiary centers in urban locations. At this time, there is still a lack of data querying patients who are unable to attain genetics services and further research is needed to determine where resources would be best allocated. For example, a study in the United Kingdom found that approximately 26% of patients referred to genetics services in a 3-month period did not create an appointment or failed to attend their appointment, but was unable to assess the reasons why these patients did not move forward with their referrals (Benjamin et al. 2015).
The aspect of healthcare perceived as causing the greatest difficulty and requiring the most improvement for patients with rare disease at both collection sites pointed towards improving healthcare provider knowledge of genetic conditions. This was ascertained by asking what the participant perceived to be the greatest difficulty experienced during their experience with healthcare, as well as what improvement of the healthcare system would be the most beneficial. This could be interpreted as patients wanting to increase knowledge of the existing providers, or wanting to increase the number of more knowledgeable providers in their geographic area. In addition, participants also self-reported the medical professionals who they believed had made the correct diagnosis, with the majority selecting a geneticist or metabolic specialist (55%), while pediatricians and general practitioners had only made the correct diagnosis for approximately 7% of the participants. It is important to keep in mind that participants in this study were ascertained at a genetics clinic, and therefore do not reflect the true number of cases with genetic etiologies and their diagnostic rates.
Another significant difference between CA and MY was the perception that there were lack of funds and physician’s time for patients in MY. This was confirmed to be true as the healthcare expenditures (% of gross domestic product) for MY and the U.S. in 2015 were 4% and 16.8%, respectively (World Health Organization 2015). Thus, inadequate funding for healthcare and capacity-building for genetic services was a main concern in MY. The credentialing of medical and clinical geneticists is relatively standard globally; however, basic genetics background of physicians in all other specialties may be lacking (Hyland et al. 2016; Skirton et al. 2010). Improvement of the standardization of medical student courses and core competencies on a global level may help (Skirton et al. 2010). The National Coalition for Health Professional Education in Genetics and European Society of Human Genetics have both developed core competencies of genetics education for health professionals, regardless of medical specialty (Jenkins et al. 2001; Skirton et al. 2010). However, coursework continues to be highly variable within and between countries. For example, a medical student’s genetics course in Thailand could be anywhere from a week to a month long (Shotelersuk et al. 2014). At the growing pace of the utilization of genetic testing services, medical school curricula around the world are being pushed to expand and include proper education regarding genetic and genomic medicine (Perry et al. 2016; Skirton et al. 2010). The Association of Professors of Human and Molecular Genetics have developed medical school curricula, which were updated in 2013 (APHMG 2018). Automated family health tools may provide direction and alert healthcare providers when a genetic evaluation is needed, although proper referral education is necessary to effectively use the tool (Buchanan et al. 2015).
Furthermore, a majority of the participants with diagnoses in CA reported having the correct diagnosis made by a specialist (54%), compared to 17% of the participants in MY. In many developing countries, medical education was focused on communicable diseases such as infections and malnutrition. It is possible that increasing awareness and instituting preventive health strategies for non-communicable diseases such as genetic conditions and rare diseases are not given sufficient priority. It is also possible that this finding may be due to a difference in training during fellowships and specialization. This study did not collect information regarding specialties, and this is an area that could be further explored in the future to target which specialty areas would benefit from additional training in genetics.
Oftentimes, geneticists and genetic counselors educate other medical providers during residency programs, grand rounds, and continuing medical education programs (Carroll et al. 2009; Kemper et al. 2010). In the U.S., genetic counseling training programs are increasing enrollment numbers and new programs are slated to open as well; however, there are limits to expansions due to the need for clinical training sites (Reiser et al. 2015). U.S. genetic counseling program directors consider clinical training site availability the main barrier to expanding the size of genetic counseling programs (Pan et al. 2016; Reiser et al. 2015). With very few genetic counseling programs in Southeast Asia and lack of government recognition of the genetic counseling profession, increasing the number of trained genetic counselors quickly may not be currently feasible in the region. Setting guidelines for recognition and credentialing of genetic counseling programs in the Southeast Asia region may help with raising awareness and increasing career opportunities in genetic counseling (Laurino et al. 2018). Another possible solution would be to increase international enrollment numbers of Southeast Asian applicants in the U.S. training programs, although possible challenges include language barriers and cultural differences.
While it may be possible to increase the number of medical geneticists, another possible route is to integrate a more comprehensive genetics and genomic medicine course into continuing medical education of existing providers in all specialties. This may include increasing continuing medical education for existing practicing physicians; thus, this would allow them to become more comfortable and knowledgeable with ordering genetic testing and discussing results, as well as helping to educate providers when a referral is needed. Guidelines are becoming accepted among several organizations, such as the recent clinical WES guidelines accepted by the American Academy of Neurology (Fogel et al. 2016). Offering more education modules and continuing medical education (CME) credits may be an effective delivery method. U.S. physicians can maintain their licensure and certification by taking courses or lectures for CME credits; however, studies have found that these short-term programs are not resulting in behavioral changes (Wilkes et al. 2017). Studies suggest that 10% of primary care patients have a condition with a genetic component, which highlight the need for primary care physicians and general practitioners to have an increased understanding of genetics integration in medical care (Global Genes 2017; Mikat-Stevens et al. 2015; Rahimzadeh and Bartlett 2014). Despite knowing and addressing this for at least a decade (Acheson et al. 2005), the results of this study show that patients are still dissatisfied, adding to the growing evidence that changes should be considered. During this information driven age, perhaps this study and others shed light on the importance of engaging and involving patients in their care as individuals are becoming increasingly more resourceful on their own (Mandl and Kohane 2016, Wilkes et al. 2017).
In the MY participant subset, “expanded newborn screening” (24%) was selected most often as the most beneficial tool. The current newborn screening program in MY is focused mainly on glucose-6-phosphate dehydrogenase and thyroid-stimulating hormone assays using cord blood. An expanded comprehensive newborn screening program using heel prick to obtain dried blood spots (DBS) would help to identify newborns at risk for certain inherited metabolic conditions that would benefit greatly from early treatment. However, this proposed shift from cord blood to DBS screening will entail a major transition of care and may present a heavy burden for maternal and child health services. As the country’s healthcare model moves towards preventative care, this tool would require increased provider knowledge of genetics in general providers (e.g., pediatricians, neonatologists). This tool was also the second most popular response among the U.S. participants (14%). Many U.S. families affected by rare diseases (not included on newborn screening) often wish they had known the diagnosis earlier (Duffner et al. 2009), and oftentimes support groups campaign to add conditions to the current screening recommendations and raise awareness of rare diseases (Shafie et al. 2016; Duffner et al. 2009). Fifteen percent of total respondents indicated that increasing the number of support groups would be the most beneficial tool. With social media and the Internet, support group members can maintain communication and support one another digitally. The U.S. has several hundred active support groups that focus on providing patient friendly resources, raising awareness, promoting research, and other aspects of improving care of rare diseases (GARD 2016). MY had fewer than ten rare disease support groups in 2016, the largest of which is the MRDS (Shafie et al. 2016). Perhaps providing more specialized support groups, globalizing the reaches of the existing groups, or increasing the number of members and maintaining an active social media presence would provide more support for patients with rare disease and their families.
Patients with rare disease and their families often feel dissatisfied with the healthcare system, due to having complicated undiagnosed diseases with a lack of support and knowledge. The hope is that this study identified some of the most important needs from the patients’ perspective in a unique participant population.
Study limitations
While this study was aimed at collecting data regarding barriers to access genetics-related healthcare in the population attending genetics clinics, it was unable to reach those patients who have been lost to care or were unable to surpass these barriers to receive adequate healthcare. The participants surveyed in this study were only those who were able to successfully surpass barriers to access genetics-related healthcare, which largely included those with health insurance.
This survey was unsuitable for individuals who do not understand English, Malay, or Chinese Mandarin. Therefore, other populations were lacking such as Hispanic, Middle Eastern, and Asians who are not of Chinese, Malay, or Asian Indian background. The participants may not be representative of the U.S. or MY as a whole due to small sample size. Statistically significant differences between the two populations may be due to ethnicity or religion, rather than collection site. Furthermore, the results of this study may not be applicable to all populations of different demographics. This study only provided insights on a unique subset of patients and their caretakers at specific collection sites in MY and Southern CA.
This survey did not use an absolute measure of travel time and costs. Therefore, there is no evidence to link the perception of burden incurred by travel time and costs to the true travel time of the participant. In addition, the survey grouped related yet different constructs, such as time and distance, or stress and burden. These groupings were made to broadly survey the possible perceptions of hindrance to obtaining satisfactory healthcare; however, there may be barriers that were not assessed in this study. Due to the design, the survey is also unable to differentiate findings of grouped constructs, which could be explored in future studies. Furthermore, some of the questions did not allow free response answers, and therefore may have required participants to choose responses that did not fit with their true perceptions. There is also the possibility that participants wanted to select multiple answers for questions allowing only one selection.
Research recommendations
Further studies could involve implementing global medical education initiatives for undergraduate medical genetics education, automated genetics tools for healthcare providers, residency programs, and continuing education webinars and workshop events. As providers around the world are becoming increasingly aware of genetics services, it is important to study the most effective modes of education for different types of providers such as current primary care physicians, specialists, nurses, and other healthcare professionals.
While one aim of this study was to compare perceptions of Asians from Southeast Asian countries to Asian Americans, there were not enough Asian Americans recruited to reliably make a comparison. There is a paucity of literature exploring Asian Americans’ perceptions and views of genetic testing as well as other U.S. minority populations, suggesting that more research involving minority populations is needed in order to better understand the cultural aspects of genetic counseling. There is also a paucity of literature investigating perceptions in the Southeast Asian region, which is a very diverse geographical region. Future studies could expand to further regions outside of Southern CA and MY in order to compare responses across the U.S. and globally in other Southeast Asian countries.
Conclusion
This study examined the barriers to healthcare perceived by patients and their caretakers in genetics and metabolic clinics in both Southern CA and MY. The purpose of this study was to have a better understanding of what hardships the patients and their caretakers are experiencing that are perceived as most burdensome and what aspects of improvement to healthcare they believe would be most beneficial for those with rare diseases. Differences in perceptions of participants in MY and CA were also explored.
The majority of participants believed that expanding healthcare provider knowledge would be the most beneficial for those with rare conditions. Most participants in MY did not perceive travel times and costs of travel or treatment to be the largest obstacles blocking adequate access to satisfactory genetics-related healthcare. These results suggest that there is a need for more genetics healthcare providers or improvements in healthcare provider education of medical genetics knowledge in the two regions.
References
Acheson LS, Stange KC, Zyzanski S (2005) Clinical genetics issues encountered by family physicians. Genet Med 7(7):501–508
Association of Professors of Human and Molecular Genetics (APHMG, 2018) Medical school core curriculum in genetics 2013. https://www.aphmg.org/about-aphmg. Accessed 4 November 2018
Benjamin C, Houghton C, Foo C, Edgar C, Mannion G, Birch J, Ellis I, Weber A (2015) A prospective cohort study assessing clinical referral management & workforce allocation within a UK regional medical genetics service. Eur J Hum Genet 23(8):996–1003
Bowdin S, Gilbert A, Bedoukian E, Carew C, Adam MP, Belmont J, Bernhardt B, Biesecker L, Bjornsson HT, Blitzer M, D’Alessandro LC (2016) Recommendations for the integration of genomics into clinical practice. Genet Med 18:1075–1084
Buchanan AH, Christianson CA, Himmel T, Powell KP, Agbaje A, Ginsburg GS, Henrich VC, Orlando LA (2015) Use of a patient-entered family health history tool with decision support in primary care: impact of identification of increased risk patients on genetic counseling attendance. J Genet Couns 24(1):179–188
Carroll JC, Rideout AL, Wilson BJ, Allanson JM, Blaine SM, Esplen MJ, Farrell SA, Graham GE, MacKenzie J, Meschino W, Miller F (2009) Genetic education for primary care providers improving attitudes, knowledge, and confidence. Can Fam Physician 55(12):e92–e99
Cigna Medical Coverage Policy (Cigna). Whole Exome and Whole Genome Sequencing Coverage Policy. Retrieved August, 2017, from https://cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/medical/mm_0519_coveragepositioncriteria_exome_genome_sequence.pdf
Delikurt T, Williamson GR, Anastasiadou V, Skirton H (2015) A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet 23(6):739–745
Duffner PK, Caggana M, Orsini JJ, Wenger DA, Patterson MC, Crosley CJ, Kurtzberg J, Arnold GL, Escolar ML, Adams DJ, Andriola MR (2009) Newborn screening for Krabbe disease: the New York state model. Pediatr Neurol 40(4):245–252
Fogel BL, Satya-Murti S, Cohen BH (2016) Clinical exome sequencing in neurologic disease. Neurology: Clin Pract 6(2):164–176
Genetic and rare diseases information center (GARD). (2016) Retrieved March, 2016, from https://rarediseases.info.nih.gov/gard
Global Genes ™ (2017) Rare Diseases: Facts and Statistics https://globalgenes.org/rare-diseases-facts-statistics. Accessed 15 February 2017
Harvey EK, Fogel CE, Peyrot M, Christensen KD, Terry SF, McInerney JD (2007) Providers' knowledge of genetics: a survey of 5915 individuals and families with genetic conditions. Genet Med 9(5):259–267
Hennekam RC (2011) Care for patients with ultra-rare disorders. Eur J Med Genet 54(3):220–224
Hyland KM, Dasgupta S, Garber K, Gold JA, Toriello H, Weissbecker K, Waggoner D (2016) Medical school core curriculum in genetics 2013. Association of Professors of Human and Medical Genetics website. http://media.wix.com/ugd/3a7b87_7064376a9eb346cfa1b85bc2f137c48f.pdf.. Accessed 1 July 2018
Jabatan kebajikan masyarakat (JKM). Retrieved March, 2016, from http://www.jkm.simple.my/
Jenkins J, Blitzer M, Boehm K, Feetham S, Gettig E, Johnson A, Lapham EV, Patenaude AF, Reynolds PP, Guttmacher AE (2001) Recommendations of core competencies in genetics essential for all health professionals. Genet Med 3(2):155–159
Katsanis SH, Katsanis N (2013) Molecular genetic testing and the future of clinical genomics. Nat Rev Genet 14(6):415–426
Kemper AR, Trotter TL, Lloyd-Puryear MA, Kyler P, Feero WG, Howell RR (2010) A blueprint for maternal and child health primary care physician education in medical genetics and genomic medicine: recommendations of the United States secretary for health and human services advisory committee on heritable disorders in newborns and children. Genet Med 12(2):77–80
Klitzman R, Chung W, Marder K, Shanmugham A, Chin LJ, Stark M, Leu CS, Appelbaum PS (2013) Attitudes and practices among internists concerning genetic testing. J Genet Couns 22(1):90–100
Korf BR, Feldman G, Wiesner GL (2005) Report of Banbury summit meeting on training of physicians in medical genetics, October 20-22, 2004. Genet Med 7(6):433–438
Laedtke AL, O’Neill SM, Rubinstein WS, Vogel KJ (2012) Family physicians’ awareness and knowledge of the genetic information non-discrimination act (GINA). J Genet Couns 21(2):345–352
Laurino MY, Leppig KA, Abad PJ, Cham B, Chu YWY, Kejriwal S, Lee JMH, Sternen DL, Thompson JK, Burgess MJ, Chien S, Elackatt N, Lim JY, Sura T, Faradz S, Padilla C, Paz ECD, Nauphar D, Nguyen KN, Zayts O, Vu DC, Thong MK (2018) A report on ten Asia Pacific countries on current status and future directions of the genetic counseling profession: the establishment of the professional Society of Genetic Counselors in Asia. J Genet Couns 27(1):21–32
Lee JMH, Thong MK (2013) Genetic counseling services and development of training programs in Malaysia. J Genet Couns 22(6):911–916
Mainous AG III, Johnson SP, Chirina S, Baker R (2013) Academic family physicians’ perception of genetic testing and integration into practice. Fam Med 45(4):257–262
Mandl KD, Kohane IS (2016) Time for a patient-driven health information economy? N Engl J Med 374(3):205–208
Mikat-Stevens NA, Larson IA, Tarini BA (2015) Primary-care providers' perceived barriers to integration of genetics services: a systematic review of the literature. Genetics in Medicine 17(3):169–176
Orphanet: an online rare disease and orphan drug data base. © INSERM 1997. Available on http://www.orpha.net. Accessed [August 2017]
Pan V, Yashar BM, Pothast R, Wicklund C (2016) Expanding the genetic counseling workforce: program directors' views on increasing the size of genetic counseling graduate programs. Genet Med 18:842–849
Perry CG, Maloney KA, Beitelshees AL, Jeng LJ, Ambulos NP, Shuldiner AR, Blitzer MG (2016) Educational innovations in clinical pharmacogenomics. Clin Pharmacol Ther 99(6):582–584
Rahimzadeh V, Bartlett G (2014) Genetics and primary care: where are we headed? J Transl Med 12(1):238
Reiser C, LeRoy B, Grubs R, Walton C (2015) Report on an investigation into an entry level clinical doctorate for the genetic counseling profession and a survey of the Association of Genetic Counseling Program Directors. J Genet Couns 24(5):689–701
Salari K (2009) The dawning era of personalized medicine exposes a gap in medical education. PLoS Med 6(8):e1000138
Salm M, Abbate K, Appelbaum P, Ottman R, Chung W, Marder K, Leu CS, Alcalay R, Goldman J, Curtis AM, Leech C (2014) Use of genetic tests among neurologists and psychiatrists: knowledge, attitudes, behaviors, and needs for training. J Genet Couns 23(2):156–163
Shafie AA, Chaiyakunapruk N, Supian A, Lim J, Zafra M, Hassali MAA (2016) State of rare disease management in Southeast Asia. Orphanet J Rare Dis 11(1):107
Shashi V, McConkie-Rosell A, Rosell B, Schoch K, Vellore K, McDonald M, Jiang YH, Xie P, Need A, Goldstein DB (2013) The utility of the traditional medical genetics diagnostic evaluation in the context of next-generation sequencing for undiagnosed genetic disorders. Genet Med 16(2):176–182
Shotelersuk V, Limwongse C, Mahasirimongkol S (2014) Genetics and genomics in Thailand: challenges and opportunities. Mol Genet Genomic Med 2(3):210–216
Skirton H, Lewis C, Kent A, Coviello DA (2010) Genetic education and the challenge of genomic medicine: development of core competences to support preparation of health professionals in Europe. Eur J Hum Genet 18(9):972–977
Taber KAJ, Dickinson BD, Wilson M (2014) The promise and challenges of next-generation genome sequencing for clinical care. JAMA Intern Med 174(2):275–280
UCLA Health. Accessed on August, 2017. https://www.uclahealth.org/Mattel/pediatric-genetics
U.S. Census Bureau. (2016). https://factfinder.census.gov/faces/nav/jsf/pages/community_facts.xhtml?src=bkmk. Accessed on August, 2017
Valencia CA, Husami A, Holle J, Johnson JA, Qian Y, Mathur A, Wei C, Indugula SR, Zou F, Meng H and Wang L (2015) Clinical impact and cost-effectiveness of whole exome sequencing as a diagnostic tool: a pediatric center’s experience. Front Pediatr 3
Wetterstrand KA (2016) DNA sequencing costs: Data from the NHGRI genome sequencing program (GSP). Retrieved Jan 25, 2016, from www.genome.gov/sequencingcosts
Wilkes MS, Day FC, Fancher TL, McDermott H, Lehman E, Bell RA, Green MJ (2017) Increasing confidence and changing behaviors in primary care providers engaged in genetic counselling. BMC Med Educ 17(1):163
World Health Organization. Global Health Observatory data. (2015) http://www.who.int/gho/health_financing/health_expenditure/en/ (accessed Sept 10th, 2018)
Yang Y, Muzny DM, Reid JG, Bainbridge MN, Willis A, Ward PA, Braxton A, Beuten J, Xia F, Niu Z, Hardison M (2013) Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med 369(16):1502–1511
Zayts O, Sarangi S, Thong MK, Chung BHY, Lo IFM, Kan ASY, Lee JMH, Padilla CD, Cutiongco-de la Paz EM, Faradz SM, Wasant P (2013) Genetic counseling/consultation in South-East Asia: a report from the workshop at the 10th Asia Pacific conference on human genetics. J Genet Couns 22(6):917–924
Acknowledgements
We would like to thank the translators for converting the documents and responses. We also thank the following genetic counselors and physicians who allowed us to recruit participants at their clinics: Dr. Premala Muthukumarasamy and Ms. Rifhan Azwani Mazlan at UMMC; Dr. Natalie Gallant, Dr. Valerie Watiker, Kathryn Singh, and Katherine Hall at LBMMC; Dr. Maureen Bocian, Dr. Virginia Kimonis, Dr. June Anne Gold, and Meredith Jones at UCI and CHOC. We would also like to thank the participants and their families who completed the surveys. This research was conducted in order to fulfill a master’s degree requirement at the University of California Irvine School of Medicine.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human studies and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 101 kb)
Rights and permissions
About this article
Cite this article
Qian, E., Thong, MK., Flodman, P. et al. A comparative study of patients’ perceptions of genetic and genomic medicine services in California and Malaysia. J Community Genet 10, 351–361 (2019). https://doi.org/10.1007/s12687-018-0399-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-018-0399-8