Abstract
This retrospective case series study, using data obtained through questionnaires and histopathological diagnoses from 656 patients enrolled in the Department of Defense (DoD) Clinical Breast Care Project (CBCP), evaluated associations between hormonal contraceptive use and breast cancer pathology including benign breast pathologies. Three combination hormonal contraceptive agents (COCs) Lo Ovral (LO), Ortho Novum (ON), and Ortho Tri-Cyclen (OTC) were evaluated as they represented the most commonly used hormonal contraceptives in our cohort. The results of this study suggest that the ever use of LO + ON + OTC does not influence the overall incidence of benign breast condition or malignant disease compared to other COCs; however, patients that have used OTC had an association with a diagnosis of benign or luminal A pathologies whereas ON was associated with a diagnosis of benign and DCIS; LO showed no association with any diagnosis—benign or malignant. Patients that have used LO or ON were more likely to be diagnosed with breast cancer at age ≥ 40 years whereas patients that had ever used OTC were likely to be diagnosed before the age of 40. Caucasians were less likely to have used OTC and more likely to have used ON; however, use of either hormonal agent positively correlated with premenopausal status at diagnosis and having a benign condition. Age at diagnosis, ethnicity, BMI, family history, menstruation status, and duration of use were all independent predictors of different histopathological subtypes. We conclude that patient-specific variables should be considered when deciding on which type of hormonal contraceptive to use to minimize the risk of developing breast cancer or a breast-related pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer estimates for 2017 in the USA show an estimated 252,710 new cases of invasive breast cancer, 63,410 new cases of breast carcinoma in situ, and 40,610 breast cancer deaths [1]. This makes breast cancer the most common form of cancer and second leading cause of cancer-related deaths among women [1, 2]. Therefore, it is imperative to identify and understand relevant risk factors associated with breast cancer development in order to decrease the risk of its development and mortality.
To date, various patient factors have been identified for their correlation with breast cancer, and these include family history, age, ethnicity, breast density, age at menarche, contraceptive use, diet, obesity, and physical inactivity among others [3,4,5,6,7,8,9,10,11]. Given that many forms of breast cancer are hormonally dependent, the use of hormonal contraceptives has therefore gained much attention [7, 12]. Hormonal contraceptives are the most widely prescribed form of birth control and represent 13% of the 140 million users worldwide [13, 14]. The Center for Disease Control reported that between 2011 and 2013, 61.7% of women aged 15–44 were using some form of contraception, noting that 23.7% of those were hormonal in nature [15]. There are two general classes of hormonal contraceptives available: combination contraceptives (estrogen plus progestogen) and progestin only contraceptives. The mechanism of action of hormonal contraceptives is to prevent pregnancy by suppressing ovulation through feedback inhibition on the hypothalamic-pituitary axis [16]. In addition to contraception, many formulations also offer non-contraceptive benefits, including decreased incidence of anemia, decreased dysfunctional bleeding, decreased dysmenorrhea, reduced bone mineral density loss, and treatment of vasomotor symptoms [17,18,19,20,21].
Controversy exists as to whether the ever use of hormonal contraceptives increases the risk of breast cancer development [9, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Currently, all hormonal contraceptives carry warnings and precautions for increased breast cancer risk. In addition, the International Agency for Research on Cancer (ISDA) has classified exogenous estrogen and progestogen as a group I carcinogen due to estrogen and progestin’s mitogenic actions and estrogen’s carcinogenic metabolites [12, 38].
Hormonal contraceptive-associated breast cancer appears to be dependent upon patient factors, formulations, and duration of use [3,4,5,6,7,8,9,10,11, 23, 39]. According to the North America Menopause Society, breast cancer incidence increased with estrogen/progestin therapy use beyond 3–5 years in menopausal women; breast cancer mortality was higher in women assigned to estrogen-progestogen therapy versus placebo during the first 5 years of menopause; and these breast cancer risks declined 3 years after cessation of therapy [39]. Several studies also suggest that there is an increased risk of breast cancer when using therapies that contain combination estrogens and progestins [13, 40] and this may be dependent upon the type of estrogen [40] and progestin [12, 38, 41,42,43,44,45,46]. Even though myriad studies have reported on the potential association of breast cancer risk and molecular subtypes with hormonal contraceptive use, the findings are still not clear [34, 47,48,49,50,51,52]. Also, many of these studies did not assess relationships between the development of benign condition or malignant neoplasms following the ever use of specific hormonal contraceptive formulations and patient risk factors. In the present study, we determined the association of specific hormonal contraceptive formulations, specifically Lo Ovral (LO), Ortho Tri-Cyclen (OTC), and Ortho Novum (ON), with breast cancer in general and with the development of histopathological subtypes. Furthermore, we assessed documented risk/protection factors utilizing multinomial logistic regression models to evaluate the likelihood that the ever use of a specific hormonal contraceptive agent combined with specific patient factors can lead to the development of specific breast pathologies—both benign and malignant.
Methods
Ethics Statement
Appropriate IRB approval was obtained for the Clinical Breast Care Project (CBCP) from the study sites at the Walter Reed National Military Medical Center and Windber Research Institute/Windber Medical Center. IRB approval was also obtained from Duquesne University School of Pharmacy and Division of Pharmaceutical, Administrative and Social Sciences. All study subjects were duly consented patients enrolled into the Clinical Breast Care Project (CBCP) protocols at the study sites. They were assigned unique research numbers and all samples (blood and solid tissue) were also appropriately coded to maintain patient confidentiality.
Subject Selection
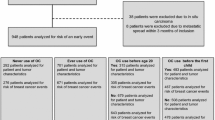
This study drew from patients enrolled in the CBCP protocols between December 2000 and September 2015. Patients were stratified based on their use of hormonal contraceptive agents, pathology (benign, atypical, in situ, invasive, and pathological subtypes [luminal A, luminal B, HER2, triple negative-TN]), demography, and medical/family history. Questionnaires were utilized for the collection of all required information and pathological data was documented by a licensed pathologist. Using these questionnaires, patients were deemed unqualified if they lacked information on the formulation name or lacked consistent hormonal contraceptive prescription identification. Patients lacking complete pathology data for the breast cancer subtype were also disqualified. From a total of 4299 patients with information on contraceptive utilization, only 656 had adequate hormonal contraceptive and pathology data to qualify for the study. Patient selection for study inclusion is illustrated in Fig. 1.
Data Query
All patient information associated with the questionnaire and pathology diagnosis was then queried within the CBCP Data Warehouse for Translational Research and the results exported to Excel spreadsheets for data analysis.
Patient Variables
This study includes patients with benign breast conditions, carcinoma in situ, specifically ductal carcinoma in situ (DCIS) and invasive breast cancer. Immunohistochemistry was performed for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor 2 (HER2), and Ki-67 on all of the invasive BC cases by a Clinical Laboratory Improvement Association (CLIA) certified laboratory. A tumor was considered to be positive for (1) ER or PR when ≥ 5% of the cells were positive, (2) HER2 + if IHC staining intensity was a 3+ or fluorescence in situ hybridization (FISH) indicated a ratio of ≥ 2.2 for copies of the HER2 gene to the centromere of chromosome 17, (3) Ki-67 + when ≥ 15% of the cells were positive. Determination of the histopathological subtype is summarized in Table 1 and is the response variable in this study [53].
Statistics
To determine the likelihood of the presence of an observed variable within and between the selected HC groups and the correlation between the use of a particular HC agent and the different histopathological subtypes, contingency analysis was performed (Pearson’s chi-squared two-tailed test). For each analysis, the categorical outcome was defined as one of two possibilities (a specific subtype vs. another). A multivariate analysis (logistic regression) was conducted to evaluate the association of predictor variables with the likelihood of having benign pathology (no cancer) or other cancers (luminal A, luminal B, HER2, or triple negative). Calculations were set at a 95% confidence interval where p < 0.05 was considered statistically significant.
Results
Characteristics of Study Population
Sixty-five percent of the patients in the study used hormonal contraceptive containing either of the following three agents LO, OTC, or ON, while 28% of the patients used an alternative single agent. Based on this, we narrowed our focus to the three specific agents (Fig. 2, Table 2). Additionally, seven cancer predisposing factors were selected for this study based on published literature: (1) ethnicity, (2) reproductive status (pre- or postmenopausal), (3) body mass index (BMI), (4) family history, (5) length of hormonal contraceptive utilization, (6) age at diagnosis, and (7) time between last dose and diagnosis [3,4,5,6,7,8,9,10,11, 34, 47,48,49,50,51,52]. The details of the patient demographics, clinical and pathology data, and hormonal contraceptive use are listed in Table 3. Histopathological evaluation resulted in two thirds of the cases having benign pathology and one third of the cases being malignant. Mean duration of use of hormonal contraceptive agents was less than 1–5 years. The ethnicity was predominantly white; however, African Americans represented nearly 30% of the cohort analyzed. Over 74% of the cohort was premenopausal at diagnosis (Table 3).
Patients were first stratified by hormonal contraceptive (COC) agent into subpopulations of the total number of included patients (n = 656). The number of patients who had ever used a specific agent (e.g., OTC) was then compared against the populations that used any other formulations (e.g., ON, LO, and others). From a total of 4299 CBCP participants with both a completed patient questionnaire and a complete pathological diagnosis, 656 met the predetermined inclusion criteria for eligibility in this study (Fig. 1). Within the group of selected patients, over 50 different hormonal contraceptive agents were identified. The top three formulations observed were LO (n = 87; 13.26%), OTC (n = 87; 13.26%), and ON (n = 252; 38.41%), constituting approximately 65% of the selected patients (Fig. 2). Importantly, each of these three top formulations—two of which were triphasic formulations (OTC and ON) and one was a monophasic formulation (LO)—was found to contain 30 (LO) or 35 μg (OTC, ON) ethinyl estradiol in addition to different progestins (LO = 0.3 mg norgestrel; OTC = 0.18/0.215/0.25 mg norgestimate; ON = 0.5/0.75/1 mg norethindrone; Table 2).
Patient Pathology Subtypes with Hormonal Contraceptive Use
The correlation between pathological subtypes and different hormonal contraceptive agents was determined and no significant difference was identified for the histopathological subtype in patients who utilized LO compared to the patients using any of the remaining agents (Table 4). However, OTC use showed a statistically significant correlation with benign (p < 0.001) and luminal A (p = 0.0470) lesions, and ON use showed a statistically significant correlation with benign lesions (p = 0.0051) and DCIS (p = 0.0202; Table 4). Trends for a negative association between DCIS in patients following OTC use (p = 0.0754) and a positive association of ON use with HER2 (p = 0.0828) and triple negative breast cancers (p = 0.0643; Table 4) were observed although not significant.
Patient Demographics and Hormonal Contraceptive Use
Patient variables were assessed between groups to determine if they impacted on the effects of LO, OTC, or ON on breast pathology development in our cohort. Age, ethnicity, BMI, menstruation status, duration of hormonal contraceptive use, time between last hormonal contraceptive dose, and diagnosis and initial diagnosis all showed statistically significant differences between the three hormonal contraceptive agents (Table 5). Most notably, patient age differed significantly between agents in that ever use of LO or ON strongly correlated with a diagnosis at the age of ≥ 40 years (p = 0.0004 and p < 0.0001, respectively), whereas use of OTC strongly correlated with a diagnosis at the age of < 40 years (p < 0.0001). When assessing patient ethnicity, Caucasians were less likely to have used OTC (p = 0.0093) and more likely to have used ON (p = 0.0048; Table 5). However, use of either formulation positively correlated with a premenopausal diagnosis (p = 0.0015 and p = < 0.0001, respectively; Table 5) as well as the occurrence of benign condition (p < 0.0001 and p = 0.0051, respectively; Table 5). Significant differences were observed for BMI across all three agents with each showing statistical difference within different BMI ranges: LO use was associated with a BMI of 18.5–24.9 (p = 0.022), OTC use was associated with a BMI of 25–29.9 (p = 0.029), and ON use was associated with a BMI of ≥ 30 (p = 0.020) (Table 5).
Duration of Hormonal Contraceptive Use and Breast Cancer Diagnosis
Examination of duration of LO, OTC, or ON use and time between the last dose and breast pathology diagnosis revealed that these factors played significant roles in the diagnosis of breast pathologies. For example, LO use for 11–15 years was associated with breast pathology diagnosis and time between discontinuation of LO (i.e., < 1 or 11–20 years) was also associated with diagnosis of breast cancer pathology (Table 5). For OTC, less than 1 year and up to 10 years of usage was associated with breast cancer diagnosis and 2 to > 20 years of time between last OTC use and diagnosis were also observed (Table 5). For ON, longer durations of use (i.e., 6–10 or 16–20 years) and less than 1 year or 11 to > 20 years of time between last ON dose and diagnosis were also observed (Table 5).
Hormonal Contraceptive Agent and Specific Pathological Subtype
Given the fact that we identified differences in both pathological subtypes and patient variables between hormonal contraceptive agents (Table 4), we asked whether we could identify associations between patient factors across all hormonal contraceptive agents that contribute to a specific subtype of breast cancer. Table 6 outlines the results for all patients. When considering the ever use of any hormonal contraceptive agent, age at diagnosis was found to be statistically significant for benign (p < 0.0001), DCIS (p = 0.0026), luminal A (p < 0.0001), luminal B (p = 0.0006), and TN (p = 0.0146) pathologies. Specifically, women less than 40 years of age were more likely to have benign pathology compared to women 40 years or older. Women 40 or older were more likely to develop DCIS, luminal A, luminal B, and TN breast cancer. Ethnicity, specifically Caucasian and African Americans but not Hispanics, were significantly associated with luminal A and DCIS. Body mass index (BMI) was significantly associated with luminal A and HER2 breast cancer. A negative family history was associated with a benign diagnosis and a positive family history of breast cancer was associated with a diagnosis of luminal B breast cancer. Menstruation status at time of diagnosis was associated with luminal A, luminal B, and DCIS indicating that those diagnosed with benign, DCIS, luminal A, and luminal B pathologies were premenopausal. Time between the last dose and diagnosis was significant for luminal A, DCIS, and triple negative breast cancers; diagnosis of luminal A and DCIS occurred even after 20 years of discontinuation of these hormonal contraceptive agents and 2–20 years for triple negative breast cancer diagnosis. Duration of use was associated with luminal A and DCIS. Specifically, 1–5 or > 25 years of usage was associated with a diagnosis of luminal A breast cancer and 21–25 years of usage was associated with a diagnosis of DCIS (Table 6).
Patient Variables, Type of Hormonal Contraceptive, and Breast Cancer Pathologies
Using the results from Table 5 as a baseline, contingency analyses were performed for LO, OTC, and ON to determine whether patient variables factored into the effect of LO, OTC, and ON on breast cancer pathologies as reported in Tables 7–9. For the subpopulation that had used LO (Table 7), the cohort that chose “other” (i.e., patients that did not identify as Caucasian, African American, or Hispanic), or patients with a BMI ≥ 30 or being postmenopausal at time of diagnosis or use of LO for > 25 years were more likely to develop DCIS. A HER2-positive subtype was more prominent in patients using LO for 21–25 years and who were diagnosed 2–10 years following the last LO dose (Table 7).
For the subpopulation that had used OTC (Table 8) patients less than 40 years or with a negative family history of breast cancer was associated with a diagnosis of a benign neoplasm while patients with an age > 40 years at time of diagnosis, a menstruation status of being postmenopausal status at time of diagnosis and over 25 years of OTC usage, were associated with luminal A breast cancer. A BMI ≥ 30, a positive family history of breast cancer as well as long-term (> 25 years) usage of OTC was associated with luminal B breast cancer. An age of diagnosis > 40 years, Caucasian and “other” ethnicity (i.e., non-African American or non-Hispanic), a high BMI (≥ 30), a positive family history of breast cancer and long-term (> 25 years) use of OTC were associated with triple negative breast cancer (Table 8).
For those who used ON, ethnicity (i.e., Caucasian and African American) and 21–25 years of taking ON was associated with DCIS. For luminal A breast cancer, age at diagnosis of > 40 years, a BMI ≥ 30, and > 20 years between the last dose of ON and time of diagnosis were associated. For luminal B breast cancer, age at diagnosis ≥ 40 years, > 20 years between the last ON dose and diagnosis, and > 25 years of usage were associated. Premenopausal status and women ≥ 40 years were associated with a benign diagnosis (Table 9).
Multivariate analysis revealed that women above the age of 40 years are 3.9 times (p < 0.001) more likely to have breast cancer pathology than women below 40 years of age. Women with a BMI of ≥ 30 have 2.1 higher odds (p = 0.017) of having cancer than women with BMI between 18.5 and 24.9. Women with negative family history of cancer were found to have 0.61 times lower odds (p = 0.04) of a breast cancer pathology than women with positive family history of breast cancer. Women who used contraceptives for longer than 25 years were 4.5 times (p = 0.019) more likely to have breast cancer pathology than women who used it for less than 1–5 years. Type of birth control, menopausal status, and ethnicity did not significantly predict breast cancer pathology (Table 10).
Discussion
Given the fact that one in eight women will develop breast cancer in their lifetime, it is imperative to further understand patient factors associated with an increased risk of breast cancer development so that these risks can be minimized. Use of hormonal therapies has been associated with increased risk of breast cancer [13, 40] and the type of estrogen or progestin may play significant roles [12, 38, 40,41,42,43,44,45,46]. Inconsistent findings have been reported on the risk of breast cancer and hormonal contraceptive use confounded, perhaps, by the fact that many of the studies were conducted in postmenopausal women, which may have underestimated the risk of breast cancer in younger premenopausal women [13, 54,55,56].
This study takes a multilevel approach to evaluate both hormonal contraceptive agents and patient demographic factors to identify potential risks associated with breast pathology diagnosis—both benign and malignant. Our study determined that age at diagnosis, family history, menopausal status, duration of hormonal contraceptive use and time between the last dose of hormonal contraceptive taken, and diagnosis played significant roles. With respect to duration of use and time between the last hormonal contraceptive use and breast pathology diagnosis, our findings are consistent with recent findings [13] demonstrating that risk of breast cancer increased from 1.09 with < 1 year of usage to 1.38 with > 10 years of use and that this risk remained high for those women taking hormonal contraceptives for ≥ 5 years. In our study, use of hormonal contraceptives in older women (≥ 40 years) may increase one’s risk of DCIS, luminal A, luminal B, and triple negative breast cancers and being premenopausal may increase one’s risk of DCIS, luminal A, and luminal B breast cancers. Patient variables may predict a specific subtyping between different hormonal contraceptive agents. For example, postmenopausal women taking LO had greater association with developing DCIS. Luminal A, luminal B, and triple negative breast cancers may be more associated with use of OTC along with older age, postmenopausal status, and positive family history. ON usage was more associated with luminal A and B breast cancers and this was dependent upon age and menopausal status. Given the fact that ON is also indicated for controlling vasomotor symptoms associated with postmenopausal women and prophylaxis for postmenopausal osteoporosis, it is not surprising that more postmenopausal women and women ≥ 40 years of age were diagnosed with luminal A and B conditions [57]. Considering that LO, OTC, and ON contain similar doses of ethinyl estradiol (30–35 μg) but different types of formulations (monophasic vs triphasic) and progestins (norgestimate, norethindrone, norgestrel) raises the possibility that the formulation type and progestin component may have some role in the observed pathological diagnosis in other studies [12, 38, 41,42,43,44,45,46].
With respect to estrogen, low-dose estrogen (i.e., 20 μg) oral contraceptives were not associated with an increase in breast cancer risk whereas moderate (30–35 μg) and high dose (50 μg) were associated with a 1.6× or 2.7× elevations in breast cancer risk, respectively [34]. All of the oral contraceptive agents examined in our study would be classified as moderate dose because they contained between 30 and 35 μg ethinyl estradiol.
Our findings that LO use was associated with the least amount of diagnoses draw attention to the type of formulation since it was the only monophasic formulation compared to OTC and ON, which are triphasic formulations. Triphasic formulations containing levonorgestrel showed a relative risk (RR) of 3.3 in the Nurses’ Health Study II [35] and a RR of 1.8 in the study by Beaber et al., [34]. Earlier studies have shown that norgestimate (OR, 1.2; 95% CI, 0.6–2.2) was not associated with an increased risk of breast cancer but levonorgestrel oral contraceptives were (OR 1.5; 95% CI 1.0–2.3) [34]. Monophasic moderate-dose estrogen + 0.5 mg norethindrone demonstrated no increase in risk (0.8 or decrease in risk) whereas moderate dose estrogen + 1.5 mg norethindrone was associated with a 2.1 increase in breast cancer risk suggesting that risk increased with increasing norethindrone dose (1.0 vs 0.5 mg) [34]. Triphasic oral contraceptives with an average dose of norethindrone (0.75 mg) had the greatest risk of 3.1×; however, this formulation was uniquely different. In the Nurses’ Health Study II, they demonstrated that breast cancer risk increased in women less than 55 years who were current oral contraceptive users and one triphasic formulation containing levonorgestrel accounted for much of the risk (3.1 ×) [35]. In the Beaber study [34], moderate estrogen dose and 0.09 mg levonorgestrel demonstrated a 1.8 × increase in risk.
Besides the formulation, the type of progestin contained within the oral contraceptive may play a role. Medroxy progesterone acetate (MPA) has been shown to increase breast cancer risk in the Million Women Study and the Women’s Health Initiative [41] and so progestins with specific characteristics (e.g., pharmacological, metabolic, structural) like MPA may increase breast cancer risk [58]. Using rodent models, MPA has the characteristics of possessing high progestogenic properties (++), medium glucocorticoid properties (+), and low to no androgenic properties (±) with no anti-androgenic (−) or anti-mineralocorticoid properties (−) [58]. Norethindrone (the progestin contained within ON) possesses similar characteristics as MPA with respect to progestogenic (++), anti-androgenic (−), and anti-mineralocorticoid (−) properties but differs from MPA by possessing androgenic (+) but no glucocorticoid (−) properties. For levonorgestrel (the progestin contained within LO), it is similar to MPA with respect to progestogenic properties (++) and anti-androgenic properties (−) but differs from MPA with respect to displaying androgenic (+) properties, no glucocorticoid (−) properties, and low to none (±) anti-mineralocorticoid properties [58].
OTC and LO contain progestins classified as gonanes and ON contains a progestin classified as an estrane [59]. Estrane progestin contraceptives have been shown to increase breast cancer risk by 60% while gonane progestin oral contraceptives demonstrated a 40% increase in breast cancer risk [34]. Norgestimate did not appear to be associated with an increase in breast cancer risk (20% increase) but levonorgestrel oral contraceptives were associated with a 50% increase in risk although neither odds ratio was statistically different than using other progestin types [34].
Although all progestins are structurally related to testosterone, the progestin contained within OTC (norgestimate) is more progestogenic compared to ON and LO, which contain progestins that are more androgenic in nature [58]. The rank order of potency (from most potent to least potent) for the progestins based on ovulation inhibition potency is levonorgestrel (LO) > norgestimate (OTC) > norethindrone (ON) [59]. With respect to potency to affect the endometrium in postmenopausal women, levonorgestrel (LO) was the most potent followed by norethindrone (ON) while MPA fell in between [59], which is consistent with their binding profile to PRs (from most potent to least potent: levonorgestrel > MPA > norethindrone > progesterone) [58]. Although it has been suggested that the androgenic component may underlie the cancer-inducing effects of progestins, studies demonstrate that levonorgestrel and norethindrone, like MPA, increases factors such as cancer diagnosis, processes of angiogenesis, VEGF expression via PR/PREs, vascular inflammation by inducing adhesion molecule-1 (ICAM-1) and vascular adhesion molecule 1 (VCAM-1) via PRs [58].
The pharmacokinetic parameters of OTC, ON, and LO differ, which may contribute to the pathologies observed in response to the oral contraceptives. Like MPA, both norethindrone and norgestrel, the progestins contained within ON and LO, respectively, undergo A-ring reduction forming both sulfated and glucuronidated versions that can be converted back to their active forms by sulfatases or metabolized to ethinyl estradiol [59]. This may impact on their half-lives in the body which are t1/2 = 5–13 h for ON and t1/2 = 10–13 h for LO [59]. OTC is metabolized to levonorgestrel and levonorgestrel-3-oxide [59].
Current literature provides mixed data regarding oral contraceptive use and type of breast cancer diagnosed (ER+ vs ER−). Our study, together with that of Beaber et al., 2014 [34] and Huang et al., 2000 [60] showed strong association of ER+ breast cancer with oral contraceptive use. However, Althuis et al. 2003 [25] and Dolle et al., 2009 [48] did not find such an association with ER+ breast cancer but did find an association with ER− breast cancer. Rosenberg et al., 2010 [33] and Sweeney et al., 2007 [61] also demonstrated a greater risk for ER− breast cancer. Also, Ma et al., 2010 [62] found no association with luminal A and triple negative breast cancer contrary to our observations with both pre- and postmenopausal women.
In our study, age, family history of breast cancer, BMI (≥ 30) and duration of oral contraceptive use were observed to increase the risk of breast cancer. With respect to BMI, women taking LO had a lower BMI compared to women taking OTC (BMI range 25–29.9) and ON (BMI > 30) and women taking LO had the least amount of breast cancer pathologies. Duration of oral contraceptive use (> 25 years) was also associated with breast cancer pathology and this is in line with earlier findings that found risk of overall breast cancer and ER+ cancer increased with increased number of pills dispensed over the prior year [34].
While utilizing patient questionnaires and pathological diagnoses from patients enrolled in the CBCP provided an adequate sample size for this retrospective study, there were however limitations. First, the accuracy of information documented in the core questionnaire is dependent upon patient self-report and appropriate documentation by the interviewing health care provider. Additionally, the large exclusion group was precipitated by inconsistencies in spelling and documentation of the formulation’s name or lack thereof. These inconsistencies may have implications on the information we have regarding the true number of agents the patient had ever used along with the duration of each agent. Pertinent information, such as dose and compliance, was not among the data captured in the questionnaire and therefore could not be considered a predictor in this study. The required exclusions due to inadequate information reduced our total sample size.
Overall, the findings from this study provide valuable information on the association between patient factors (such as family history of breast cancer, BMI, age, and duration of use) and effects of the combination of oral contraceptive on breast pathology—benign and malignant. These factors along with duration of use should be considered when choosing a combined hormonal contraceptive for use in women to reduce their risk of developing breast cancer.
References
American Cancer Society. Cancer facts & figures 2016. 2016. Journal. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc047079.pdf
Centers for Disease Control and Prevention: Cancer among women. 2015. Journal . http://www.cdc.gov/cancer/dcpc/data/women.htm
Amadou A, Fabre A, Torres-Mejía G, Ortega-Olvera C, Angeles-Llerenas A, McKenzie F, Biessy C, Hainaut P, Romieu I (2013) Hormonal therapy and risk of breast cancer in Mexican women. PLoS One 8(11):e79695
Boyd NF, Melnichouk O, Martin LJ, Hislop G, Chiarelli AM, Yaffe MJ, Minkin S (2011) Mammographic density, response to hormones, and breast cancer risk. J Clin Oncol 29(22):2985–2992
Brynhildsen (2014) Combined hormonal contraceptives: prescribing patterns, compliance, and benefits versus risks. Therapeutic advances in drug safety 5(5):201–213
Bui KT, Wakefield CE, Kasparian NA, Tyler J, Abbott J, Tucker K (2013) Oral contraceptive use in women at increased risk of breast/ovarian cancer: knowledge and attitudes. Psycho-Oncology 22(1):228–232
Dorgan JF, Klifa C, Deshmukh S, Egleston BL, Shepherd JA, Kwiterovich PO, van Horn L, Snetselaar LG, Stevens VJ, Robson AM, Lasser NL, Hylton NM (2013) Menstrual and reproductive characteristics and breast density in young women. Cancer Causes Control 24(11):1973–1983
Imkampe A-K, Bates T (2012) Correlation of age at oral contraceptive pill start with age at breast cancer diagnosis. Breast J 18(1):35–40
Lu, Yani, Ma, Huiyan, Malone, Kathleen E, Norman, Sandra A, et al. (2011) Oral contraceptive use and survival in women with invasive breast cancer. Cancer Epidemiology and Prevention Biomarkers
Moorman PG, Havrilesky LJ, Gierisch JM, Coeytaux RR, Lowery WJ, Peragallo Urrutia R, Dinan M, McBroom AJ, Hasselblad V, Sanders GD, Myers ER (2013) Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol 31(33):4188–4198
Assi HA, Khoury KE, Dbouk H, Khalil LE et al (2013) Epidemiology and prognosis of breast cancer in young women. Journal of thoracic disease 5(Suppl 1):S2
Thorbjarnardottir T, Olafsdottir EJ, Valdimarsdottir UA, Olafsson O, Tryggvadottir L (2014) Oral contraceptives, hormone replacement therapy and breast cancer risk: a cohort study of 16 928 women 48 years and older. Acta Oncol 53(6):752–758
Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø (2017) Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med 377(23):2228–2239
World contraceptive patterns 2013. New York: United Nations, Department of Economic and Social Affairs Journal .(http://www.un.org/en/development/desa/population/publications/family/contraceptive-wallchart-2013.shtml)
Current contraceptive status among women aged 15–44: United States, 2011–2013. 2014. Journal (Issue)
Schindler AE (2013) Non-contraceptive benefits of oral hormonal contraceptives. International journal of endocrinology and metabolism 11(1):41–47
Christopher LA, Miller L (2007) Women in war: operational issues of menstruation and unintended pregnancy. Mil Med 172(1):9–16
Evans G, Sutton EL (2015) Oral contraception. Medical Clinics 99(3):479–503
Gambacciani M, Levancini M (2014) Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Przeglad menopauzalny = Menopause review 13(4):213–220
Powell-Dunford NC, Cuda AS, Moore JL, Crago MS, Kelly AM, Deuster PA (2011) Menstrual suppression for combat operations: advantages of oral contraceptive pills. Womens Health Issues 21(1):86–91
Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JAV, Santen RJ (2015) Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism 100(11):3975–4011
Hoften V, Carlijn B, Huibert P, Petra HM, Grobbee DE et al (2000) Long-term oral contraceptive use increases breast cancer risk in women over 55 years of age: the DOM cohort. Int J Cancer 87(4):591–594
Marchbanks PA, Curtis KM, Mandel MG, Wilson HG, Jeng G, Folger SG, McDonald JA, Daling JR, Bernstein L, Malone KE, Wingo PA, Simon MS, Norman SA, Strom BL, Ursin G, Weiss LK, Burkman RT, Spirtas R (2012) Oral contraceptive formulation and risk of breast cancer. Contraception 85(4):342–350
Kumle M, Weiderpass E, Braaten T, Persson I et al (2002) Use of oral contraceptives and breast cancer risk. Cancer Epidemiology and Prevention Biomarkers 11(11):1375–1381
Althuis MD, Brogan DD, Coates RJ, Daling JR, Gammon MD, Malone KE, Schoenberg JB, Brinton LA (2003) Breast cancers among very young premenopausal women (United States). Cancer Causes Control 14(2):151–160
Dumeaux V, Alsaker E, Lund E (2003) Breast cancer and specific types of oral contraceptives: a large Norwegian cohort study. Int J Cancer 105(6):844–850
Dumeaux V, Fournier A, Lund E, Clavel-Chapelon F (2005) Previous oral contraceptive use and breast cancer risk according to hormone replacement therapy use among postmenopausal women. Cancer Causes Control 16(5):537–544
Lee SY, Kim MT, Kim SW, Song MS, Yoon SJ (2003) Effect of lifetime lactation on breast cancer risk: a Korean women's cohort study. Int J Cancer 105(3):390–393
Wu M-H, Chou Y-C, Yu J-C, Yu C-P, Wu CC, Chu CM, Yang T, Lai CH, Hsieh CY, You SL, Chen CJ, Sun CA (2006) Hormonal and body-size factors in relation to breast cancer risk: a prospective study of 11,889 women in a low-incidence area. Ann Epidemiol 16(3):223–229
Hannaford PC, Selvaraj S, Elliott AM, Angus V, Iversen L, Lee AJ (2007) Cancer risk among users of oral contraceptives: cohort data from the Royal College of General Practitioner’s oral contraception study. BMJ 335(7621):651
Shantakumar S, Terry MB, Paykin A, Teitelbaum SL, Britton JA, Moorman PG, Kritchevsky SB, Neugut AI, Gammon MD (2007) Age and menopausal effects of hormonal birth control and hormone replacement therapy in relation to breast cancer risk. Am J Epidemiol 165(10):1187–1198
Dorjgochoo T, Shu X-O, Li H-L, Qian H-Z, Yang G, Cai H, Gao Y-T, Zheng W (2009) Use of oral contraceptives, intrauterine devices and tubal sterilization and cancer risk in a large prospective study, from 1996 to 2006. Int J Cancer 124(10):2442–2449
Rosenberg L, Zhang Y, Coogan PF, Strom BL, Palmer JR (2008) A case-control study of oral contraceptive use and incident breast cancer. Am J Epidemiol 169(4):473–479
Beaber EF, Buist DSM, Barlow WE, Malone KE, Reed SD, Li CI (2014) Recent oral contraceptive use by formulation and breast cancer risk among women 20 to 49 years of age. Cancer Res 74(15):4078–4089
Hunter, David J, Colditz, Graham A, Hankinson, Susan E, Malspeis, Susan, et al., 2010, Oral contraceptive use and breast cancer: a prospective study of young women. Cancer Epidemiology and Prevention Biomarkers: p. cebp. 0747.2010
Kawai M, Minami Y, Kuriyama S, Kakizaki M, Kakugawa Y, Nishino Y, Ishida T, Fukao A, Tsuji I, Ohuchi N (2010) Reproductive factors, exogenous female hormone use and breast cancer risk in Japanese: the Miyagi cohort study. Cancer Causes Control 21(1):135–145
Vessey M, Yeates D (2013) Oral contraceptive use and cancer: final report from the Oxford–Family Planning Association contraceptive study. Contraception 88(6):678–683
Lanfranchi, Angela (2014) Normal breast physiology: the reasons hormonal contraceptives and induced abortion increase breast-cancer risk. Issues L. & Med. 29: p. 135
North American Menopause Society: The 2012 hormone therapy position statement of the North American Menopause Society. 2012. Journal. 19: p. 257
Pup D, Lino B, Massimiliano DF, Raffaele C, Carla, et al. (2014) Nomegestrol acetate/estradiol hormonal oral contraceptive and breast cancer risk. Anti-Cancer Drugs 25(7):745–750
Chlebowski RT, Anderson GL (2012) Changing concepts: menopausal hormone therapy and breast cancer. J Natl Cancer Inst 104(7):517–527
Anderson GL, Women’s Health Initiative steering committee (2004) Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291:1701–1712
Fournier A, Bernio F, Clavel-Chapelon F (2008) Unequal risks for BC associated with different hormone replacement therapies: results from the E3N cohort study. BC Res Treat 107(1):101–111
Lyytinen H, Pukkala E, Ylikorkala O (2009) Breast cancer risk in postmenopausal women using estradiol–progestogen therapy. Obstet Gynecol 113(1):65–73
Stefanick ML, Anderson GL, Margolis KL, Hendrix SL, Rodabough RJ, Paskett ED, Lane DS, Hubbell FA, Assaf AR, Sarto GE, Schenken RS, Yasmeen S, Lessin L, Chlebowski RT (2006) Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy. JAMA 295(14):1647–1657
Wood CE, Register TC, Lees CJ, Chen H, Kimrey S, Mark Cline J (2007) Effects of estradiol with micronized progesterone or medroxyprogesterone acetate on risk markers for breast cancer in postmenopausal monkeys. Breast Cancer Res Treat 101(2):125–134
Beaber, Elisabeth F, Malone, Kathleen E, Tang, Mei-Tzu Chen, Barlow, William E, et al. (2014) Oral contraceptives and breast cancer risk overall and by molecular subtype among young women. Cancer Epidemiology and Prevention Biomarkers
Dolle JM, Daling JR, White E, Brinton LA, Doody DR, Porter PL, Malone KE (2009) Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiology and Prevention Biomarkers. 18(4):1157–1166
Elebro K, Butt S, Dorkhan M, Jernström H, Borgquist S (2014) Age at first childbirth and oral contraceptive use are associated with risk of androgen receptor-negative breast cancer: the Malmö diet and cancer cohort. Cancer Causes Control 25(8):945–957
Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane D, Stefanick ML, Vitolins M, Kabat GC, Rohan TE, Li CI (2011) Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst 103(6):470–477
Ritte R, Tikk K, Lukanova A, Tjønneland A, Olsen A, Overvad K, Dossus L, Fournier A, Clavel-Chapelon F, Grote V, Boeing H, Aleksandrova K, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Berrino F, Mattiello A, Tumino R, Sacerdote C, Quirós JR, Buckland G, Molina-Montes E, Chirlaque MD, Ardanaz E, Amiano P, Bueno-de-Mesquita HB, van Gils CH, Peeters PHM, Wareham N, Khaw KT, Key TJ, Travis RC, Weiderpass E, Dumeaux V, Lund E, Sund M, Andersson A, Romieu I, Rinaldi S, Vineis P, Merritt MA, Riboli E, Kaaks R (2013) Reproductive factors and risk of hormone receptor positive and negative breast cancer: a cohort study. BMC Cancer 13(1):584
Work ME, John EM, Andrulis IL, Knight JA, Liao Y, Mulligan AM, Southey MC, Giles GG, Dite GS, Apicella C, Hibshoosh H, Hopper JL, Terry MB (2014) Reproductive risk factors and oestrogen/progesterone receptor-negative breast cancer in the Breast Cancer Family Registry. Br J Cancer 110(5):1367–1377
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn H-J, Panel members (2011) Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747
Lancet (1996) Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. 347: p. 1713–27
Jernström H, Loman N, Johannsson OT, Borg Å, Olsson H (2005) Impact of teenage oral contraceptive use in a population-based series of early-onset breast cancer cases who have undergone BRCA mutation testing. Eur J Cancer 41(15):2312–2320
Kotsopoulos J, Lubinski J, Moller P, Lynch HT et al (2014) Timing of oral contraceptive use and the risk of breast cancer in BRCA1 mutation carriers. Breast Cancer Res Treat 143(3):579–586
Janssen Pharmaceutical, Inc, 2015, Ortho Novum and Modicon (norethindrone and ethinyl estradiol) package insert
Africander D, Verhoog N, Hapgood J (2011) Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 76:636–652
Stanczyk F (2003) All progestins are not created equal. Steroids 68:879–890
Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG (2000) Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol 151:703–714
Sweeney C, Giuliano AR, Baumgartner KB, Byers T, Herrick JS, Edwards SL, Slattery ML (2007) Oral, injected and implanted contraceptives and breast cancer risk among U.S. Hispanic and non-Hispanic white women. Int J Cancer 121:2517–2523
Ma H, Wang Y, Sullivan-Halley J, Weiss L, Marchbanks PA, Spirtas R, Ursin G, Burkman RT, Simon MS, Malone KE, Strom BL, McDonald JA, Press MF, Bernstein L (2010) Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women’s contraceptive and reproductive experiences study. Cancer Res 70:575–587
Acknowledgements
We thank all patients for their participation in the CBCP. We thank Jianfang Liu, Nicholas Costantino, and Brad Mosteller for their guidance on statistical analysis and Deepika Rao and Khalid Kamal especially for their help on the multivariate analyses.
Author information
Authors and Affiliations
Contributions
Designed and researched topic, drafted IRB requests at Duquesne, evaluated and extracted data from CBCP query searches, and analyzed data: JAD, JLG. Drafted and wrote the manuscript: JAD, JLG, and PAW-E. Contributed to statistical analysis, specifically multinomial logistical regression: SM. Participated in CBCP query searches: BD. Marc Purazo: preliminary statistics. Critically reviewed the manuscript and provided input: SS, JI, HH, PAW-E, JC, CDS, AD. Supervisors for this study: SS, PAW-E.
Corresponding author
Ethics declarations
Appropriate IRB approval was obtained for the Clinical Breast Care Project (CBCP) from the study sites at the Walter Reed National Military Medical Center and Windber Research Institute/Windber Medical Center. IRB approval was also obtained from Duquesne University School of Pharmacy and Division of Pharmaceutical, Administrative and Social Sciences.
Disclaimer
The identification of specific products, scientific instrumentation, or organization is considered an integral part of the scientific endeavor and does not constitute endorsement or implied endorsement on the part of the authors, Department of Defense, or any component agency. The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US Government.
Rights and permissions
About this article
Cite this article
Dorchak, J.A., Maria, S., Guarinoni, J.L. et al. The Impact of Hormonal Contraceptives on Breast Cancer Pathology. HORM CANC 9, 240–253 (2018). https://doi.org/10.1007/s12672-018-0332-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-018-0332-y