Abstract
A green method using biological dependable processes has been established for the synthesis of CuO nanoparticles. Synthesis of CuO nanoparticles were done using an environmental friendly method by taking aqueous solution of copper(II) sulfate and leaf extract of Ixoro coccinea. Characterization of the synthesized CuO nanoparticles was done using instruments such as UV-visible spectrophotometer, scanning electron microscopy, transmission electron microscopy, and Fourier-transform infrared spectroscopy. The existence of peaks obtained in FTIR confirms CuO nanoparticles. A visible change was observed in the solution prepared as it changed from light blue to light brown to dark brown once the extract was added to the copper sulfate solution. An average size of 300 nm was obtained during SEM analysis due to the formation of nanoparticle clusters. The TEM images of CuO nanoparticles separated after ultrasonication of the dispersion show that they possess an average size of 80–110 nm. It was found that the ultrasonication increases the distribution of nanoparticles in a fluid by preventing the formation of clusters. It was found that Ixiro coccinea plant leaves are a suitable alternative for the easy and green synthesis of CuO nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over the last few years, interest has grown for the synthesis of metal oxide nanoparticles due to its wide range of uses ranging from optoelectronics, nanodevices, nanoelectronics, nanosensors, information storage, to catalysis. Generally, various metal oxides are available in the nature but some of them having a wide range of applications in a technological aspect. Typically, oxides of transition metals such as copper oxide, zinc oxide, iron oxide etc., had potential interest due to their ability to adapt as nanofluid coolant in several heat transfer applications. Electrically, CuO is a semiconducting material due to its narrow band gap and which can be used in photoconductive applications [1]. Copper being the starting material for the synthesis of copper oxide (CuO), nanoparticles are abundant in nature as present in various salts (sulfates, chlorides etc.). Hence, CuO has attracted much attention because of its low cost. They have found application in various fields. It has excellent super conducting properties [1], and it found wide applications in dye-sensitized solar cells [2], li-ion battery [3], ink-jet-printed electronics [4], Gas sensing [5], antimicrobial activity [6], dye removal [7], and anticancer activity [8].
CuO nanoparticles have been synthesized by a variety of physical and chemical methods. The chemical methods are solid state thermal decomposition [9], electrochemical reduction [10], sonochemical methods [11], chemical precipitation method [12], solution plasma [13], thermal decomposition method [14], microwave irradiation method [15], pulsed wire explosion method [11], and sol-gel method [16]. Likewise, biological methods include leaf extracts of various plants [2, 8, 17], bacterial synthesis [18], and so on. Biological synthesis methods are favored over chemical synthesis methods because of its rising environmental issues. Chemical methods tend to produce more waste products that can cause harm to the environment. Further, biological synthesis method using plant leaf extracts are advantageous over other biological methods as it eliminates the use of cell culture and which results large-scale nanoparticle synthesis. The leaves of Ixoro coccinea plant which belongs to Rubiaceae family native to southern India and Sri Lanka were selected as the raw material. The leaves of the plant tend to have wound healing properties [19] and anti-inflammatory [20] and antimicrobial activities [21].
In this paper, biological synthesis method have been done for synthesizing copper oxide nanoparticles from the leaf extract of the Ixoro coccinea plant. The characterization studies were also done using various instrumental methods.
2 Materials and Methods
Bio-reduction with the help of a plant extract involves mixing the aqueous extract with an aqueous solution of the appropriate metal salt. The synthesis of nanoparticle occurs at room temperature.
2.1 Materials Used
The CuSO4.5H2O used for synthesis of CuO nanoparticles was purchased from TCI chemicals, Chennai. This was used without further purification. The water used was deionized and distilled in the laboratory. The leaves were collected from the university campus.
2.2 Preparation of Plant Extract
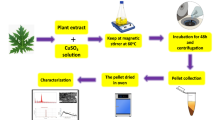
Ixoro coccinea leaf extract was used to synthesize CuO nanoparticles. Leaves of the plant were collected from the university campus. Collected leaves were rinsed under tap water initially and after grating them into small pieces, they were washed with distilled water prepared in the laboratory. About 40 g of leaves were taken in a 500-ml conical flask, and 200 ml of water was added to the flask (leaves:water = 1:5). The flask was kept in a heating mantle and heated at 70–80 °C until the water turned to boil which in turn lead to the solution turning brown. The extract was cooled down and filtered using Whatman filter paper.
2.3 Synthesis of CuO Nanoparticles
In this process, 3 μM solution of copper sulfate was prepared and made up to 500 ml using standard flask. Varying volumes (10, 20, 30, 40, 50, 60, 70 ml) of the leaf extract were added to 50 ml CuSO4.5H2O solution. The prepared solutions were then kept overnight at room temperature so that the bio reductions may take place, and the copper salts are converted to copper oxide. The solution was then centrifuged at 10,000 rpm using 50-ml falcon tubes for 20 min and washed several times with DI water. The supernatant solution was removed, and the nanoparticle was transferred onto a crucible and dried in a hot air oven at 120 °C. The solid CuO nanoparticle obtained was characterized.
2.4 Characterization of CuO Nanoparticles
UV-Vis spectral analysis was done using PerkinElmer Lambda 650 at wavelengths ranging from 200 to 800 nm. FTIR was recorded by Frontier FTIR, PerkinElmer, USA. The particle size and surface morphology was measured using Jeol/JEM 2100 transmission electron microscope using LaB6 as the source operated at 200 V. Morphological features were studied by using Hitachi SU6600 variable pressure field emission scanning electron microscope (FESEM).
3 Results and Discussion
3.1 Effect of Initial Concentration of Leaf Extract
The initial volume of the leaf extract taken with 50 ml of CuSO4.5H2O were varied from 5 to 60 ml, and its effect on concentration of CuO nanoparticles produced was studied. The UV spectrum of CuO nanofluid synthesized at various initial concentrations of the leaf extract is shown in Fig. 1. The concentration of CuO present in the solution is represented by the absorbance value [17]. It reveals that the concentration of the CuO in the solution is greater if the concentration of the leaf extract was more.
3.2 UV-Vis Spectroscopy
The UV-visible spectrum of the synthesized CuO nanoparticle dispersed in water for a wide range of wavelength is represented in Fig. 1. The spectrum shows that there is a strong absorbance of the UV rays between the wavelength 200–300 nm [17]. This reveals the presence of CuO nanoparticle in the sample. The peak formed at 191 nm is due to surface plasmon absorption of CuO nanoparticles. The combined oscillation of the free conduction band electrons present in the metal oxides which is excited by the incident UV radiation is the reason for surface plasmon absorption. This type of resonance is seen when the wavelength of the incident light far exceeds the particle diameter [22].
3.3 Scanning Electron Microscopy
The SEM images of CuO nanoparticles synthesized by green sol-gel method are shown in Fig. 2. The images reveal that CuO nanoparticles have a great tendency to agglomerate. Hence, the SEM images show an average size of 300 nm for the synthesized CuO nanoparticles [23,24,25]. Random size distribution of the synthesized raw nanoparticles can be estimated using the obtained SEM images.
3.4 Transmission Electron Microscopy
Transmission electron microscopy images are useful tools for the morphological analysis of nanoparticles. It gives us information about the topography of the sample species. TEM image of the synthesized CuO nanoparticles after 30 min sonication using acetone as the base fluid is shown in Fig. 3. Spherical morphology of the CuO nanoparticles can be estimated using the images. The images tell us that the average size of the nanoparticles were lesser than 5 nm, and size of the particles were evenly distributed [2]. After analyzing the TEM images, we can find that the CuO nanoparticles are concentrated at particular positions which show its tendency to aggregate [24].
By comparing the SEM results and TEM results, it was found that the CuO nanoparticles will form aggregates by absorbing moisture and ultrasonication of the CuO nanofluid can make them disaggregate to form low size nanoparticles.
3.5 Fourier-Transform Infrared Spectroscopy
FTIR analysis of the green synthesized CuO nanoparticle is shown in Fig. 4. It reveals that the peaks obtained at 488 and 658 cm−1 were contributed by Cu–O and Cu2–O bond vibrational frequencies respectively [17]. The strong vibrational peak at 1026 cm−1 was attributed to H–OH bond stretches found in alcohols [26]. Peaks at 1479 and 1524 cm−1 lie in the range of 1380–1640 cm−1 which denote the O–H bending [26]. Peaks close to 700 and 1300 cm−1 have been attributed to alcohol and phenolic groups, C–N stretching in amines [27]. The existence of the peaks at 2930 and 2815 cm−1 are due to the C–H stretching [10, 27]. Overall, it is seen that there is a presence of alcohols and phenolic group stuck on the nanoparticles surface even though the leaves are washed repeatedly.
4 Conclusion
The green synthesis of copper oxide nanoparticles was successfully done using the leaf extract of Ixoro coccinea. Characterization of the synthesized nanoparticle was done by using various techniques such as FTIR, SEM, TEM, and UV-visible spectrophotometer. UV spectrum of the CuO nanoparticle shows the abnormal capacity of the CuO nanoparticle to absorb UV rays at a wavelength region from 200 to 300 nm. The SEM results reveal the aggregation ability of the CuO nanoparticles whereas the TEM results reveal the average nanoparticle size has reduced to 5 nm by sonicating the nanoparticle in acetone base fluid. The FTIR peaks obtained at show the bonding vibrations such as Cu–O, O–H, etc., which is present in the CuO materials. It was concluded that the green synthesis of CuO nanoparticles form the Ixoro coccinea leaf extract was an eco-friendly promising technique. This is a low-cost synthesis method and it does not require any toxic chemicals for the synthesis. It is summarized that the present method of synthesis of CuO nanoparticles provides a strong potential for future development of green development of nanomaterials.
References
Lv, J. J., Li, M. Y., & Zeng, Q. X. (2011). Preparation and characterization of copper oxide and copper nanoparticles. Advanced Materials Research, 308, 715–721.
Sharma, J. K., Akhtar, M. S., Ameen, S., Srivastava, P., & Singh, G. (2015). Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. Journal of Alloys and Compounds, 632, 321–325.
Waser, O., Hess, M., Güntner, A., Novák, P., & Pratsinis, S. E. (2013). Size controlled CuO nanoparticles for Li-ion batteries. Journal of Power Sources, 241, 415–422.
Lee, Y., Choi, J.-R., Lee, K. J., Stott, N. E., & Kim, D. (2008). Large-scale synthesis of copper nanoparticles by chemically controlled reduction for applications of inkjet-printed electronics. Nanotechnology, 19, 598–604.
L., X., Fang Wang, H. L., Li, H., Yuan, Z., Sun, Y., Chang, F., & Deng, H. (2016). A highly sensitive gas sensor based on CuO nanoparticles synthetized via a sol–gel method. RSC Advances, 6, 79343–79349.
Sutradhar, P., Saha, M., & Maiti, D. (2014). Microwave synthesis of copper oxide nanoparticles using tea leaf and coffee powder extracts and its antibacterial activity. Journal of Nanostructure in Chemistry, 4, 86–91.
Ghulam, M., Hajira, T., Muhammad, S., & Nasir, A. (2013). Synthesis and characterization of cupric oxide (CuO) nanoparticles and their application for the removal of dyes. African Journal of Biotechnology, 12, 6650–6660.
Sankar, R., Maheswari, R., Karthik, S., Shivashangari, K. S., & Ravikumar, V. (2014). Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Materials Science and Engineering. C, 44, 234–239.
Shahsavani, E., Feizi, N., & Khalaji, A. D. (2016). Copper oxide nanoparticles prepared by solid state thermal decomposition: synthesis and characterization. Journal of Ultrafine Grained and Nanostructured Materials, 49, 48–50.
Jadhav, S., Gaikwad, S., Nimse, M., & Rajbhoj, A. (2011). Copper oxide nanoparticles: synthesis, characterization and their antibacterial activity. Journal of Cluster Science, 22, 121–129.
Science, E., Universit, B. H., Premier, M., & Premier, M. (2013). Copper (II)-oxide nanostructures: synthesis, characterizations and their applications—review. Journal of Materials and Environmental Science, 4, 792–797.
Luna, I. Z., Hilary, L. N., Chowdhury, a. M. S., Gafur, M. a., Khan, N., & Khan, R. a. (2015). Preparation and characterization of copper oxide nanoparticles synthesized via chemical precipitation method. Open Acess Library, 2, 1–8.
Saito, G., Hosokai, S., Tsubota, M., & Akiyama, T. (2011). Synthesis of copper/copper oxide nanoparticles by solution plasma. Journal of Applied Physics, 110, 23–30.
Khalaji, A. D., & Das, D. (2016). Preparation of CuO nanoparticles by thermal decomposition of double-helical dinuclear copper (II) Schiff-base complexes. Journal of Ultrafined Grained and Nanostructured Materials, 48, 93–99.
Wang, H., Xu, J. Z., Zhu, J. J., & Chen, H. Y. (2002). Preparation of CuO nanoparticles by microwave irradiation. Journal of Crystal Growth, 244, 88–94.
Lee, A., & Nikraz, H. (2015). BOD: COD ratio as an indicator for river pollution. International Proceedings of Chemical, Biological & Environmental Engineering, 51, 139–142.
Sankar, R., Manikandan, P., Malarvizhi, V., Fathima, T., Shivashangari, K. S., & Ravikumar, V. (2014). Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 121, 746–750.
Hasan, S. S., et al. (2008). Bacterial synthesis of copper/copper oxide nanoparticles. Journal of Nanoscience and Nanotechnology, 8, 3191–3196.
Upadhyay, A., Chattopadhyay, P., Goyary, D., Mitra Mazumder, P., & Veer, V. (2014). Ixora coccinea enhances cutaneous wound healing by upregulating the expression of collagen and basic fibroblast growth factor. ISRN Pharmacoogy, 14, 751–824.
Ratnasooriya, W. D., Deraniyagala, S. A., Galhena, G., Liyanage, S. S. P., Bathige, S. D. N. K., & Jayakody, J. R. A. C. (2005). Anti-inflammatory activity of the aqueous leaf extract of Ixora coccinea. Pharmaceutical Biology, 43, 149–152.
Annapurna, J., Amarnath, P., Amar Kumar, D., Ramakrishna, S., & Raghavan, K. (2003). Antimicrobial activity of Ixora coccinea leaves. Fitoterapia, 74, 291–293.
Mulvaney, P. (1996). Surface plasmon spectroscopy of nanosized metal particles. Langmuir, 12, 788–800.
Geoprincy, G., Saravanan, P., Nagendra gandhi, N., & Renganathan, S. (2011). A novel approach for studying the combined antimicrobial effects of silver nanoparticles and antibiotics through agar over layer method and disk diffusion method. Digest Journal of Nanomaterials and Biostructures, 6, 1557–1565.
Dagher, S., Haik, Y., Ayesh, A. I., & Tit, N. (2014). Synthesis and optical properties of colloidal CuO nanoparticles. Journal of Luminescence, 151, 149–154.
Neha Topnani, A., Surendra Kushwaha, A., & Athar, T. (2009). Wet synthesis of copper oxide nanopowder. International Journal of Green Nanotechnology: Materials Science & Engineering, 1, 67–73.
Gunalan, S., Sivaraj, R., & Venckatesh, R. (2012). Aloe barbadensis Miller mediated green synthesis of mono-disperse copper oxide nanoparticles: optical properties. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 97, 1140–1144.
Phiwdang, K., Suphankij, S., Mekprasart, W., & Pecharapa, W. (2013). Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia, 34, 740–745.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vishveshvar, K., Aravind Krishnan, M., Haribabu, K. et al. Green Synthesis of Copper Oxide Nanoparticles Using Ixiro coccinea Plant Leaves and its Characterization. BioNanoSci. 8, 554–558 (2018). https://doi.org/10.1007/s12668-018-0508-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0508-5