Abstract
Copper Oxide Nanoparticles (NPs) have been successfully synthesized through the green synthesis route using Catharanthus roseus leaf extract that acted as an efficient stabilizer and capping agent of the NPs. The as-prepared CuO is then calcined at 400 °C, 500 °C, and 600 °C in order to gain better crystalline CuO NPs. The X-ray Diffraction (XRD) analysis of calcined samples showed that the particles were crystalline with hexagonal and monoclinic structure and the crystallite size was found to be about (30.77–13.25)nm for CuO. The surface morphology of the NPs was investigated by Scanning Electron Microscopy and Field Emission Scanning Electron Microscopy (FESEM) which showed that NPs were spherical in shape with uniform size distribution. Elemental analysis of the NPs was carried out with Energy Dispersive X-ray (EDX) Spectroscopy and it indicated the elemental signature of the presence of Copper, Oxygen, and Carbon in the CuO NPs. The Fourier Transform Infrared Spectroscopy (FTIR) analysis showed that the capping agents of the NPs contained the functional groups alcohol, alkene, ketone, terpenoid, and organic acid. The thermal stability of CuO NPs was investigated using Differential Scanning Calorimetry (DSC) and Thermo Gravimetric Analysis (TGA). DSC showed one exothermic peak. The heat enthalpy of CuO NPs is 2326 j/g and 242.7 μVs/mg, respectively. The percentage of weight loss was about 13.33% for CuO as found from TGA. The CuO NPs were paramagnetic in nature with zero coercivity and zero remanence magnetization which was observed using a Vibrating Sample Magnetometer (VSM). The average crystallite size was determined by the Scherer formula which showed that the crystallite size of calcined CuO NPs decreases with an increase in calcination temperature. But the crystallite size increases with calcination temperature, which supported the results of XRD and VSM.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The CuO Nanoparticles from Catharanthus Roseus leaf extract is an innovative idea [1,2,3,4]. Although it has been conducted MnO2 NPs from Bryophillim pinnatum leaf extract by our research group previously[5,6,7,8,9,10] It has been conducted the different characterizations like XRD, GC–MS, SEM, FESEM, FTIR, EDX, DSC, VSM, TGA, etc. The CuO nanoparticles are stable, robust, and have a longer shelf life compared to organic, antimicrobial agents [11,12,13]. There are a few strategies for the union of CuO NPs, which incorporate sol–gel process, co-precipitation, sonochemical method, hydrothermal techniques, non-aqueous synthesis, ultrasound irradiation, micro-emulsion method, electrochemical and microwave irradiation synthetic route, and so on[14,15,16]. The main aim of our research work on CuO Nanoparticles (NPs) is to use for electricity generation system by using electrochemical cell. The Catharanthus Roseus leaf is also a medicinal leaf [17,18,19,20,21]. It has a great medicinal value. There are some countries where it is used as a medicinal value. The biogenic amalgamation includes the blend of NPs utilizing plants and microorganisms or their concentrates [22, 23]. The microbial combination is a tedious and to some degree dangerous strategy, since it includes the upkeep of microbial societies under advanced, septic research center conditions. Developing a facile and green method for synthesizing CuO Nanoparticles (NPs) is of importance and still a challenge for materials scientists [24, 25]. The Catharanthus roseus leaves possess biomolecules such as terpenoid, carotenoid, sterol, alcohol, and organic acid, which could be used as reducing agent to react with Cu2+ ions as scaffolds to direct the formation of the CuO Nanoparticles (NPs) in a solution that have been extensively explored for possible medical applications [26]. The CuO Nanoparticles (NPs) from Catharanthus roseus leaves provide a particularly useful platform and demonstrate unique properties with potentially wide-ranging therapeutic applications [27].
2 Methodology
CuO NPs were synthesized via a facile green synthesis route where (Cu(CH3COO)2-H2O) were used as a precursor and Catharanthus roseus leaf extract was used as a source of reducing and capping agents. To synthesis CuO NPs, the Catharanthus roseus leaf extract was added to an aqueous mixture of Cu2+ acetate at a 1:1 M ratio. The chemical reactions for CuO NPs are given by the following:
Catharanthus roseus + H2O + Cu2+(aq) Stirring
 [Cathararanthus roseus/Cu2+]
[Cathararanthus roseus/Cu2+]
 [Cathararanthus roseus/Cu2+] [Cathararanthus roseus/CuO] ↓(s) + H2O (aq)
[Cathararanthus roseus/Cu2+] [Cathararanthus roseus/CuO] ↓(s) + H2O (aq)
The method of Catharanthus Roseus leaf extract preparation for getting CuO Nanoparticles (NPs) is shown in Fig. 1. From Fig. 1, it is found that the finished product was Catharanthus Roseus leaf extract (C.R.L. extract).
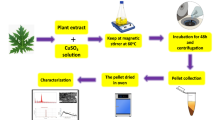
The method of CuO Nanoparticles (NPs) preparation using Catharanthus Roseus leaf extract is shown in Fig. 2. Finally, it is found (from Fig. 2) that the finished product was CuO Nanoparticles (NPs) using Catharanthus Roseus leaf extract (C.R.L. extract).
3 Results and Discussion
Table 1 shows the amount inpercentage (%) of functional group of Catharanthus roseus leaf extracts. It is found that the maximum functional group present in Catharanthus roseus leaf extract was Alcohol (42.64%) and the minimumfFunctional group present in Catharanthus roseus leaf extract was Carotenoid (1.82%).
3.1 Structural Analysis Using XRD
It is shown (in Fig. 3) that the diffraction peaks of synthesized CuO NPs are assigned to the crystal planes of (110), (−111), (111); (−202), (020), (202); (−113), (−311), and (220), respectively. The analyzed diffraction peaks were matched well with the standard magnetite XRD patterns with JCPDS file no: 05-0661, which declared the crystallographic system of the monoclinic structure.
3.2 Structural Parameter of CuO NPs
The structural parameter for different calcination temperatures at 400 °C, 500 °C, and 600 °C is shown in Table 2. The maximum crystallite size, D(nm) was 30.77 nm at 400 °C and the minimum crystallite size, D(nm) was 13.25 nm at 500 °C. The maximum dislocation density, δ(line/nm2 × 10–3) was 5.70 at 500 °C and the minimum dislocation density, δ(line/nm2 × 10–3) was 1.06 at 400 °C. The maximum microstrain, Ɛ(×10–3) was 1.34 at 500 °C and the minimum microstrain, Ɛ(×10–3) was 0.85 at 400 °C.
It is shown (in Fig. 4) that the average crystallite size decreases from 400 °C to 500 °C with increasing calcination temperature and then increases with temperature. It is shown (in Fig. 5) that the dislocation density increases with increasing calcination temperature up to 500 °C and then decreases with temperature.
3.3 Williamson–Hall Method
Williamson–Hall X-ray line broadening analysis provides a method of finding an average size of coherently diffracting domains and strain. Strain-induced peak broadening arises due to crystal imperfection; and distortion, which had been calculated using the \(\mathrm{relation}:\)
To estimate microstrain from the X-ray diffraction pattern, Williamson and Hall [9] proposed a modified Scherrer’s formula as follows:
The equation represents the linear plot of βhklcosθ_ against 4 sinθ for the samples calcined at (a) 400 °C, (b) 500 °C, and (c) 600 °C temperatures displayed in Fig. 6. The slope of the plots provides the values of strain (ε) [9].
It is shown (in Fig. 6) that the plots provide the value of the strain, which varies with temperatures. The value of the slope of the strain was 3.53 × 10–3, 2.24 × 10–3, and 3.23 × 10–3 at 400’C, 500’C, and 600’C, respectively. It is found that the maximum value of the slope of the strain was 3.53 × 10–3 at 400 °C and the minimum value of the slope of the strain was 2.24 × 10–3 at 500 °C.
3.4 Surface Morphology Analysis: SEM and FESEM
The SEM images of CuO NPs calcined at 400 °C and 500 °C are shown in Fig. 7a and Fig. 7b. The SEM image shows that the samples are in the nano range. The FESEM image of CuO Nanoparticles (NPs) calcined at 600 °C is shown in Fig. 7c. The image shows that the surface morphology of the Nanoparticles (NPs) is homogeneous and almost spherical in shape.
3.5 Functional Group Analysis of Nanoparticles Using FTIR Technique
Table 3 shows the functional group analysis of Nanoparticles (NPs) using the FTIR technique. It is also found from Table 3 that the maximum peak position(cm−1) of the functional group was 3460 for (-OH) and the minimum peak position(cm−1) of the functional group was 515 for (Cu–O).
It is shown (in Fig. 8) that the stretching vibration of –OH functional group is at thepPeak position of 3460 cm−1. Whereas, the antisymmetric stretching vibration of –CH2– is in naphthalic or aliphatic at the peak position of 2925 cm−1. Again, it is also shown that the stretching vibration of –OH functional group is in polyols at the peak position of 1416 cm−1, whereas, the stretching vibration of C-N is in amines at the peak position of 1050 cm−1. Finally, it is shown that the stretching vibration of Cu–O bond is at the peak position of 515 cm−1.
3.6 Elemental Analysis of CuO NPs
Table 4 shows the Elemental Analysis of CuO Nanoparticles (NPs). It is shown that the mass (%) of Copper (Cu), Oxygen (O), and Carbon (C) are 40.36%, 20.52%, and 39.12%, respectively.
It can be seen that (from Fig. 9) no coercivity or remanence could be observed for the sample, suggesting the diamagnetic properties of the magnetite nanoparticles. This can be ascribed to the small size of nanoparticles which were smaller than the diamagnetic critical size.
3.7 Elemental Analysis and Magnetization of CuO NPs
It is shown (in Fig. 10) that the saturation magnetization of the nanoparticles (NPs) was 0.00125 emu/g.
3.8 Thermal Analysis of Nanoparticles Using TGA
In the TGA plot (from Fig. 11), the total weight losses are observed 13.33% up to 1100 °C. The weight losses are observed 2.36% up to 700 °C and 10.81% up to 800–1000 °C flat line stabilizes the pure CuO NPs with no impurities.
3.9 Thermal Analysis of Nanoparticles Using DSC
The DSC curve shows (from Fig. 12) a broad exothermic dip in between room temperature and 500 °C. There is also a sharp dip observed at around 950 °C, which might be due to crystallization and condensation to the anatase phase.
4 Conclusions
The XRD pattern of the average crystallite size was estimated for the synthesized spherical monocyclic crystalline typed CuO NPs as 21.96 nm. NPs synthesized with various calcination temperature also confirmed their crystallinity by XRD analysis and it was found that all of them have pure crystal phase with a spherical monocyclic crystalline structure, where the crystallites size of the NPs decreased with the increase of calcination temperature 400 to 500 °C and crystallite size increases with 600 °C. EDX spectra confirmed the presence of elemental components of the CuO NPs. FTIR spectroscopy showed the involvement of biomolecules present in the extract of Cathranthus roseus, which were verified in the synthesizing process of CuO NPs. The formation of CuO NPs was confirmed due to the noticeable absorption peaks at 520 cm−1. The FESEM image showed the high degree of crystallinity of the synthesized CuO NPs in which surface morphology was found to be homogeneous and almost spherical in shape with the presence of capping agents that stabilize the CuO NPs. The thermal analyses were carried out using DSC and TGA techniques in which the synthesized CuO NPs were responded with temperature. The magnetic properties of prepared CuO NPs are examined by VSM which demonstrates that the synthesized CuO NPs showed slightly superparamagnetic characteristics with zero corrosivity and remanence magnetization property.
References
Dr. Khan, M.K.A.: An Introduction to Solar Energy. Mizan Library, Dhaka, Bangladesh (2006)
Rai, G.D.: Solar Energy Utilization. Khanna Publishers, Delhi-110006, India (2004)
Gunawan, et al.: Energy storage system from galvanic cell using electrolyte from a plant as an alternative renewable energy. IOP Conf. Ser.: Mater. Sci. Eng. 509, 012045 (2019)
Hamid, M.R.: Characterization of a battery cell fueled by bryophyllum pinnatum sap. Int. J. Sci. Eng. Res. 4(3), 1–4 (2013). ISSN 2229-5518
Hamid, M.R., Yusuf, A., Wadud, A.M.A., Rahaman, M.M.: Design and performance test of a prototype of a 12 Volt DC battery fueled by bryophyllum pinnatum sap and improvement of its characteristics, department of electrical and electronic engineering, Ahsanullah university of science and technology, Dhaka, Bangladesh. Int. J. Electron. Electr. Eng. 4(5), 398–402 (2016)
Elgammal, A., Sharaf, A.M.: Dynamic self adjusting FACTS-switched filter compensation, schemes for wind-smart grid interface systems Int. J. Renew. Energy Res. 2(1), 103–111 (2016)
Nedaei, M.: Wind energy potential assessment in Chalus county in Iran. Int. J. Renew. Energy Res. 2(2), 338–347 (2012)
Tzen, E., Papapetrou, M.: Promotion of renewable energy sources for water production through desalination. Desalination Water Treat. 39(1–3), 302–307 (2012)
Ying, C.Y., Dayou, J.: Modelling of the electricity generation from living plants. J. Teknol. 78(6), 29–33 (2016)
Chee, F.P., Chen, C.A., Chang, J.H.W., Choo, Y.Y., Dayou, J.: Data acquisition system for in situ monitoring of chemoelectrical potential in living plant fuel cells. J. Biophys. (2016)
Volkov, A.G., Foster, J.C., Jovanov, E., Markin, V.S.: Anisotropy and nonlinear properties of electrochemical circuits in leaves of Aloe vera L. Bioelectrochem. 81(1), 4–9 (2011)
Choo, Y.Y., Dayou, J., Surugau, N.: Origin of weak electrical energy production from living- plants Int. J. Renew. Energy Res. 4(1), 198–203 (2014)
Ghate, N.B., Hazra, B., Sarkar, R., Mandal, N.: vitro anticancer activity of Spondias pinnata bark on human lung and breast carcinoma. Cytotechnology 66(2), 209–218 (2014)
Kamal, S., Akhter, R., Tithi, N.A., Wadud, M.A., Narjish, S.N., Shahriar, M., Bhuiyan, M.A.: Biological investigations of the leaf extract of Spondias pinnata. Int. J. Pharm. Sci. Rev. Res. 6(8), 3351 (2015)
Bogdanski, S., Ashton, A., Fogg, A., Burns, D.T., Headridge, J., Sowerbutts, A., East, G., Bishop, E., Bark, L., Grime, J.: Research topics in analytical chemistry. Proc. Soc. Anal. Chem. 9(9), 182–211 (1972)
Lau, O.W., Luk, S.F.: A survey on the composition of mineral water and identification of natural mineral water. Int. J. Food Sci. Technol. 37(3), 309–317 (2002)
Khan, M.K.A., Paul, S., Rahman, M.S., Kundu, R.K., Hasan, M.M., Moniruzzaman, M., Mamun, M.A.: A study of performance analysis of PKL electricity generation parameters: (An experimental analysis on voltage regulation, capacity and energy efficiency of pathorkuchi leaf (PKL) electricity cell). In: Power India International Conference (PIICON), 2016 IEEE 7th, 2016, pp. 1–6. IEEE (2016)
Khan, M.K.A., Rahman, M.S., Das, T., Ahmed, M.N., Saha, K.N., Paul, S.: Investigation on parameters performance of Zn/Cu electrodes of PKL, AVL, tomato and lemon juice based electrochemical cells: a comparative study. In: Electrical Information and Communication Technology (EICT), 2015 3rd International Conference on, pp. 1–6. IEEE (2017)
Khan, K.A., Rahman, A., Rahman, M.S., Tahsin, A., Jubyer, K.M.: Paul, S.: Performance analysis of electrical parameters of PKL electricity (An experimental analysis on discharge rates, capacity & discharge time, pulse performance and cycle life & deep discharge of Pathor Kuchi Leaf (PKL) electricity cell).In: Innovative Smart Grid Technologies-Asia (ISGT-Asia), 2016 IEEE, pp. 540–544. IEEE (2016)
Khan, M.K.A., Paul, S., Rahman, M.S., Kundu, R.K., Hasan, M.M., Moniruzzaman, M., Al Mamun, M.:.A study of performance analysis of PKL electricity generation parameters: (An experimental analysis on voltage regulation, capacity and energy efficiency of pathor kuchi leaf (PKL) electricity cell). In: Power India International Conference (PIICON), 2016 IEEE 7th, pp. 1–6. IEEE (2016)
Paul, S., Khan, K.A., Islam, K.A., Islam, B., Reza, M.A.: Modeling of a Biomass Energy based (BPL) Generating Power Plant and its features in comparison with other generating Plants. IPCBEE 44 (2012). IACSIT Press, Singapore.https://doi.org/10.7763/IPCBEE.2012.V44.3
Khan, K.A., Paul, S.: A analytical study on electrochemistry for PKL (Pathor Kuchi Leaf) electricity generation system. In: Publication date 2013/5/21, Conference- Energytech, 2013 IEEE, pp. 1–6. Publisher, IEEE (2013)
Hasan, M., Khan, K.A.: Bryophyllum pinnatum leaf fueled cell: an alternate way of supplying electricity at the off-grid areas in Bangladesh. In: Proceedings of 4th International Conference on the Developments in Renewable Energy Technology [ICDRET 2016], p. 01, (2016). https://doi.org/10.1109/ICDRET. 7421522
Hasan, M., Khan, K.A., Mamun, M.A.: An estimation of the extractable electrical energy from bryophyllum pinnatum leaf. Am. Int. J. Res. Sci-Ence, Technol. Eng. Math. (AIJRSTEM) 01(19), 100–106 (2017)
Khan, M.K.A., Rahman, M.S., Das, T., Ahmed, M.N., Saha, K.N., Paul, S.: Investigation on parameters performance of Zn/Cu electrodes of PKL, AVL, tomato and lemon juice based electrochemical cells: a comparative study. In: Electrical Information and Communication Technology (EICT), 2017 3rd International Conference on, pp. 1–6. IEEE (2017). https://doi.org/10.1109/EICT.2017.8275150, IEEE, Khulna, Bangladesh, Bangladesh, 7–9
Duffie, J.A., Beckman, W.A.: Solar Energy Thermal Process. John Wiley and Sons. Inc N.Y. USA
Sharma, B.K.: (1998) Electrochemistry, Krishna Prakashan Media (P) Ltd., 11, Shivaji Road, Meerut-250 001 (UP) India (1998)
Acknowledgements
The authors are grateful to the GARE (Grant of Advanced Research in Education) project, Ministry of Education, GoB for financing during the research work (Project/User ID: PS2019949).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Khan, K.A., Shaiful Islam, M., Awal, A., Khan, M.N.I., Ullah, A.K.M.A. (2021). Studies on Performances of Copper Oxide Nanoparticles from Catharanthus Roseus Leaf Extract. In: Biswas, A., Saxena, R., De, D. (eds) Microelectronics, Circuits and Systems. Lecture Notes in Electrical Engineering, vol 755. Springer, Singapore. https://doi.org/10.1007/978-981-16-1570-2_17
Download citation
DOI: https://doi.org/10.1007/978-981-16-1570-2_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1569-6
Online ISBN: 978-981-16-1570-2
eBook Packages: EngineeringEngineering (R0)