Abstract
Ethanol induces massive neuroapoptosis in the developing brain. However, whether ethanol-induced neuroapoptosis also involves GABA interneurons remains unknown. Here, we addressed this question in the postnatal days (P) P3-P24 GAD67-GFP mouse hippocampus using cleaved caspase-3 staining, a sensitive measure of ethanol-induced apoptotic neurodegeneration combined with DAPI staining to monitor the apoptotic nuclear degradation. We observed that 8 h following ethanol treatment (6 g/kg, intraperiotoneally), significant proportion of GAD67-GFP expressing hippocampal interneurons was stained with cleaved caspase-3 antibodies and displayed chromatin condensation with a formation of the DAPI-stained apoptotic bodies. Maximal number of the cleaved caspase-3 stained interneurons (16.6 % of the total number of GFP expressing neurons and 21.6 % of the total number of caspase-3 stained cells) was observed in the hippocampal slices from P6-9 mice, and minimal damage to interneurons was observed in P3–4 and > P11 mice. While the apoptotic interneurons were found in all hippocampal regions and layers, their highest density was observed in the CA1 region and hilus. Thus, ethanol-induced neuroapoptosis involves hippocampal interneurons that may contribute to the life-long neurobehavioral deficits, increased excitability, and higher incidence of seizures characteristic of the fetal alcohol spectrum disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ethanol induces massive neuroapoptosis in the developing brain [1, 2], that is considered as the main pathophysiological mechanism of the neurobehavioral abnormalities in the fetal alcohol spectrum disorders [3]. Different brain structures and neuronal populations display age-dependent vulnerability to the neuroapoptotic ethanol action, which is typically characterized by the bell-shaped developmental profile [1, 2]. Whether ethanol-induced cortical neuroapoptosis also involves GABA interneurons, which are the main inhibitory neurons in cortex remains unknown, however. Here, we addressed this question in the hippocampus, which is one the most vulnerable to the neuroapoptotic ethanol actions brain structures [1, 4] using cleaved caspase-3 labeling in GAD67-GFP mouse line, in which interneurons express GFP under control of the GABA synthesizing enzyme GAD67.
2 Material and Methods
This work has been carried out in accordance with EU Directive 2010/63/EU for animal experiments and all the protocols were approved by INSERM (N007.08.01) and KSMU (N9-2013). GAD67-GFP mice [5] of both sexes from postnatal days (P, P0 = day of birth) P3–24 were used. Ethanol (Carlo Erba, Val de Reuil, France) was administered intraperitoneally at a dose of 6 g/kg (20 % in normal saline solution). The control animals received the same volume of saline. After the treatment, both the ethanol-treated and the control pups were kept in a thermostated box at 36 °C. Eight hours after ethanol administration (6 g/kg, 20 % solution in normal saline intraperitoneally), mice were deeply anesthetized with urethane (2 g/kg, intraperitoneally; Sigma-Aldrich, St. Louis, MO) and perfused through the left cardiac ventricle with 4 % paraformaldehyde (Diapath, Martinengo, Italy). The brains were removed and left for fixation in 4 % paraformaldehyde for 12–14 h at room temperature. Then, the brains were rinsed in PBS (3 × 30 min) and cut in agar blocks into 50-μm-thick coronal sections using a Vibratome (Leica, Germany). After 1–2-h incubation in blocking solution (PBS, 0,3 % Triton, 5 % Normal Goat Serum; Sigma-Aldrich, St. Louis, MO), sections were incubated at 4°C with primary antibodies against Cleaved Caspase-3(ASP 175) (Cell Signalling Technology, Beverly, MA) diluted at 1:1000 in a solution containing PBS, 0.3 % Triton, 1 % Normal Goat Serum. Then, sections were rinsed in PBS (4 × 5 min) and incubated for 1 h at room temperature with the secondary fluorescent antibodies Cy3 AffiniPure Donkey Anti-Rabbit IgG (H + L) (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted at 1:1000 in PBS. Then, sections were rinsed in water and mounted with a DAPI containing Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
3 Results and Discussion
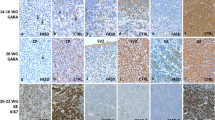
We explored the neuroapoptotic alcohol actions on interneurons in the hippocampus of GAD67-GFP mice through the postnatal period. To this aim, we used cleaved caspase-3 staining, a sensitive measure of ethanol-induced apoptotic neurodegeneration [1, 6] (red color code in the figures) combined with DAPI co-staining to monitor the apoptotic nuclear degradation (blue color code) in the P3-24 GAD67-GFP mice, in which GABAergic interneurons are expressing GFP (green color code). We assumed that the neurons co-expressing cleaved caspase-3 and GFP and displayed yellow represent interneurons undergoing the apoptosis. Slices were prepared 8 h after the ethanol administration (6 g/kg, i.p.) in vivo that is the optimal time to reveal active caspase-3 immunostaining [1, 2]. The ethanol-induced apoptosis in hippocampus was characterized by a bell-shaped developmental profile, with the maximal number of the active caspase-3 stained GAD67-GFP neurons observed at P5–10 and minimal damage to interneurons was observed in P3–4 and > P11 mice that was similar to the developmental profile of general neuroapoptosis in cerebral cortex [1, 2] (Fig. 1a). Cleaved caspase-3 stained GAD67-GFP neurons were characterized by various degrees of morphological degeneration and nuclear damage revealed by DAPI staining of the chromatin [7] as illustrated by the confocal images on Fig. 1b. Apoptotic interneurons were observed in all hippocampal subfields and dentate gyrus, with their highest density in the hilar region and CA1, the two subfields known to be particularly sensitive to the neurotoxic actions of ethanol [4] (Fig. 1a). Cell count in the hippocampal slices comprising all hippocampal subfields and dentate from three ethanol-treated P6, P7 and P9 mice revealed the following neuronal densities: GFP expressing neurons (without cleaved caspase-3 staining): 14955 ± 3454 cells/mm3; cleaved caspase-3 stained cells (without GFP signal): 10227 ± 2692 cells/mm3; and cells co-expressing GFP and stained cleaved caspase-3 antibodies: 2898 ± 1192 cells/mm3 (Fig. 1c). We further estimated that cells co-expressing GFP and stained with cleaved caspase-3 antibodies (apoptotic interneurons) account for 21.6 ± 0.5 % of the total number of hippocampal neurons undergoing apoptosis, and for 16.6 ± 0.4 % of the total number of GAD67 expressing hippocampal interneurons (Fig. 1d).
Ethanol-induced apoptosis of interneurons revealed with cleaved capase-3 immunochemistry in the hippocampus of GAD67-GFP mouse line. a Confocal images of the hippocampus of GAD67-GFP mice of different postnatal ages treated with 6 g/kg ethanol (20 % wv, i.p.). The sections were stained with antibodies against activated caspase-3 (red) and DAPI staining of the chromatin (blue). GFP fluorescence (green) and GFP + activated caspase-3 co-staining (yellow). Boxed areas are shown at higher magnification on the right panels. Six cells indicated by arrows at P5 and P6 are shown at higher magnification on panel (b). b Examples of the bodies of apoptotic interneurons (cells 1–4), non-apoptotic interneuron (cell 5) and apoptotic pyramidal cell (cell 6) indicated by arrows on the panel (a). Note DAPI-stained apoptotic bodies in the active caspase-3 stained, GAD67-GFP positive interneurons. Brains were fixed 8 h after ethanol administration. c Numerical density of the cleaved caspase-3 stained and GFP expressing neurons in the hippocampus of P6-9 mice treated with ethanol. Pooled data from three mice (P6, P7, and P9): 2087 GFP-expressing neurons without cleaved caspase-3 staining, 1416 neurons stained with cleaved caspase-3 antibodies and 409 GFP positive neurons with cleaved caspase-3 staining. d Proportion of the apoptotic interneurons among the cells undergoing apoptosis and among the interneurons
Our results indicate that the ethanol-induced neuroapoptosis in the neonatal mouse hippocampus involves GABAergic interneurons. To our best knowledge, this is the first direct demonstration of the neurotoxic actions of ethanol on the GABAergic cells in developing cerebral cortex. The apoptotic actions of ethanol on interneurons likely involve long-lasting (for several hours) profound inhibition of hippocampal activity during the developmental window of particular vulnerability of cortical neurons to the neuroapoptotic actions of ethanol [1–3] (see also Chernova et al., this issue, and [1, 8–10] for the comparable effects of general anesthetics). Consequences of such important loss of interneurons as described in the present study can be dramatic for the brain. Indeed, inhibitory GABAergic interneurons are the critical elements of the cortical networks [11], and their loss, due to the neurotoxic action of ethanol, may lead to the enhanced excitability, seizures and intellectual disabilities, that all are the hallmarks of the fetal alcohol spectrum disorders.
4 Conclusions
We showed that the third trimester equivalent alcohol exposure in the GAD67-GFP mouse induces massive apoptosis in the hippocampal interneurons manifested by staining of apoptotic GAD67-GFP expressing interneurons with cleaved caspase-3 antibodies and apoptotic nuclear degradation revealed by DAPI staining, and with the maximal damage to GAD67-GFP interneurons observed in P6-9 mice. Ethanol-induced neuroapoptosis of hippocampal interneurons, that are the critical inhibitory and synchronizing elements of the cortical circuits, may contribute to the life-long neurobehavioral deficits, increased excitability (see a report by Lotfullina et al. in the current issue), and higher incidence of seizures characteristic of the fetal alcohol spectrum disorders. In the future studies, it would be of interest to determine vulnerability of various interneuron populations to ethanol, to explore the changes caused by a loss of interneurons in the network functions as well as compensatory mechanisms and the ways of protection of interneurons from the ethanol-induced apoptosis.
References
Ikonomidou, C., Bittigau, P., Ishimaru, M. J., Wozniak, D. F., Koch, C., Genz, K., et al. (2000). Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science, 287, 1056–1060.
Lebedeva, J., Zakharov, A., Ogievetsky, E., Minlebaeva, A., Kurbanov, R., Gerasimova, E., Sitdikova, G., and Khazipov, R. (2015). Inhibition of Cortical Activity and Apoptosis Caused by Ethanol in Neonatal Rats In Vivo. Cereb. Cortex, pii: bhv293 (Epub ahead of print).
Olney, J. W. (2014). Focus on apoptosis to decipher how alcohol and many other drugs disrupt brain development. Frontiers in Pediatrics, 2, 81. doi:10.3389/fped.2014.00081.
West, J. R., Hamre, K. M., Cassell, M. D. (1986). Effects of ethanol exposure during the third trimester equivalent on neuron number in rat hippocampus and dentate gyrus. Alcoholism, Clinical and Experimental Research, 10, 190–197.
Tamamaki, N., Yanagawa, Y., Tomioka, R., Miyazaki, J., Obata, K., Kaneko, T. (2003). Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. Journal of Comparative Neurology, 467, 60–79.
Olney, J. W., Tenkova, T., Dikranian, K., Muglia, L. J., Jermakowicz, W. J., D’Sa, C., et al. (2002). Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiology of Disease, 9, 205–219.
Willingham, M. C. (1999). Cytochemical methods for the detection of apoptosis. Journal of Histochemistry and Cytochemistry, 47, 1101–1110.
Lebedeva, Y. A., Zakharova, A. V., Sitdikova, G. F., Zefirov, A. L., Khazipov, R. N. (2016). Ketamine-midazolam anesthesia induces total inhibition of cortical activity in the brain of newborn rats. Bulletin of Experimental Biology and Medicine, 161, 15–19.
Sitdikova, G., Zakharov, A., Janackova, S., Gerasimova, E., Lebedeva, J., Inacio, A. R., et al. (2014). Isoflurane suppresses early cortical activity. Annals of Clinical Translational Neurology, 1, 15–26.
Jevtovic-Todorovic, V., Hartman, R. E., Izumi, Y., Benshoff, N. D., Dikranian, K., Zorumski, C. F., et al. (2003). Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. Journal of Neuroscience, 23, 876–882.
Freund, T., & Buzsaki, G. (1996). Interneurons of the hippocampus. Hippocampus, 6, 345–470.
Acknowledgments
This work was supported by INSERM (LIA to RK), the Program of Competitive Growth of Kazan Federal University, and the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Elena Ogievetsky and Nailya Lotfullina contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ogievetsky, E., Lotfullina, N., Minlebaeva, A. et al. Ethanol-Induced Apoptosis of Interneurons in the Neonatal GAD67-GFP Mouse Hippocampus. BioNanoSci. 7, 151–154 (2017). https://doi.org/10.1007/s12668-016-0334-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-016-0334-6