Abstract
Zinc bioleaching from sphalerite associated with pyrite ore using Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidant was studied. The ore containing 3.4 wt% zinc and in some experiments its combination with sphalerite concentrate was prepared. The effect of culture media, pH, Fe2+ iron concentration, and the addition of different materials including shredded newspaper, starch, and sugar as a catalyst on the zinc bioleaching were evaluated. It was found that 9 K media, pH of 1.8, and 10 g L−1 Fe2+ iron concentration were optimum conditions. The catalysts acted as an electron acceptor for Fe3+ iron reduction. The amount of zinc bioleaching was obtained 88% for the ore and 95% for the second sample at the optimum pH of 1.8 in 18 days. The addition of starch and shredded newspaper increased the bioleaching rate of zinc. Also, the bioleaching time was decreased from 18 days to 10 and 13 days in the presence of shredded newspaper and starch, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mineral bioleaching is one of the most important methods to improve the performance of different minerals processing in the mining industry. Recently, mineral bioleaching has been commonly used from laboratory to industrial scales because of the benefits such as the low cost and environmental friendliness, and mostly appropriate for the low-grade and complex ores [1, 2].
Zinc is one of the important non-ferrous metals that enjoys high importance owing to its wide application in the different industries such as galvanization industries and alloy production [3]. It holds fourth rank after stainless steel, aluminium, and copper in terms of demand and consumption [4]. Currently, zinc is produced mainly from zinc sulphidic minerals especially sphalerite [5] because it is easy to separate the sulphides from the gangue minerals using traditional flotation methods. It is required to separate sulphur from zinc concentrate before extraction of zinc from the ore using hydrometallurgical or pyrometallurgical techniques [6]. The zinc concentrate contains 25–30 wt% sulphur [7]. Roasting or sintering methods are used for separation of sulphur from the zinc concentrate. Due to zinc consumption increasing and zinc high-grade ores decreasing, the supply gap becomes a tremendous issue for the zinc industry. So, the development of novel technologies in order to produce zinc from low-grade resources has become an important research field in recent years [6]. Nowadays, more than 80–85% of the world’s zinc production is through the hydrometallurgical process [8, 9]. Furthermore, formation of a passive layer on the surface of sphalerite minerals limits the diffusion of the oxidizing agent to the mineral surface; therefore, it is highly important to find a way to overcome this problem in zinc (bio)leaching [10,11,12,13,14]. Using microorganisms has become attractive to many researchers to conduct research studies in order to improve the efficiency of hydrometallurgical methods for the extraction of copper or zinc [15, 16].
The microorganisms such as Leptospirillum ferrooxidans, Acidithiobacillus ferrooxidans, and Acidithiobacillus thiooxidans are mostly used in bioleaching. The microbes need reduced sulphur and reduced iron for their growth as they are either iron oxidizers (Leptospirillum ferriphilum/Leptospirillum ferrooxidans), sulphur oxidizers (Acoithiobacillus thiooxidans) or both iron and sulphur oxidizer (Acithiobacillus ferrooxidans). They break down the sulphide ore matrices using oxidization of sulphidic minerals and releasing different metals such as zinc, copper, nickel into leaching solution [17]. There are two mechanisms including thiosulphate and polysulphide pathways for bioleaching of metals. In thiosulphate pathway, Fe3+ irons attack the mineral surface and produce Fe2+ irons and oxidized metal as reaction products. In terms of polysulphide pathway, the combination of Fe3+ irons and proton attack results in oxidization of sulphidic ores such as sphalerite [18].
However, bioleaching of sulphide minerals such as chalcocite or chalcopyrite faces a number of issues mainly the slow rate of sulphide mineral bioleaching, which causes long residence times or requirement of a large volume of the reactor when it is used in tank leaching [17].
A number of methods have been used to improve zinc dissolution rate. Castro and Donati used thermoacidophilic archaeon Acidianus copahuensis to enhance zinc bioleaching and it was found that the copahuensis was able to extract about 100% of zinc when tetrathionate was used in the system [19]. Slow dissolution rate could be enhanced through the addition of a catalyst. Different metal ions including Ag+, Hg2+, Bi3+, Cu2+, and Co2+ could be successfully used as catalyst during bioleaching [20]. Low-temperature thermal pretreatment at 400 °C led to the enhancement of zinc bioleaching from 72% to nearly 100% [21]. Using different catalysts is common in chalcopyrite bioleaching [22,23,24]; however, there are few research studies to investigate the effect of catalyst on the zinc bioleaching from sphalerite.
An appropriate catalyst should be able to increase the dissolution rate and having the ability to optimize oxidation–reduction reactions. Also, it should be possible to use it in large scale such as heap bioleaching. Panda et al. [25] used waste newspaper to enhance chalcopyrite bioleaching. It was found that the waste newspaper acted as a reductant and a low-cost reagent in bioleaching and increased copper bioleaching. Addition of 2 g L−1 waste newspaper increased the copper recovery by 80% [25]. Sugar acids were used to remove heavy metals from soil [26]. Bioleaching of Mn(II) from manganese nodules was performed using Bacillus sp in the presence of starch as carbon source and energy for the microbial growth [27].
To the best of our knowledge, the effect of different catalysts on the bioleaching of zinc from low-grade zinc ore has not been extensively studied. Therefore, the current research study focuses on the bioleaching of sphalerite and addition of various catalysts including sugar, starch, and shredded newspaper in order to increase the dissolution rate. Furthermore, the optimum conditions in terms of pH, iron concentration, and culture media were determined.
2 Materials and Method
2.1 Ore Preparation
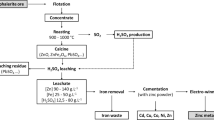
A bulk sample of low-grade sphalerite ore was obtained from the Goushfil Zinc mine in the Isfahan Province, Iran. A low-grade sphalerite ore containing high amount of pyrite was used for the bioleaching experiments. The minerals’ compositions of the low-grade sphalerite ore were obtained using XRD analysis, and it is shown in Fig. 1 and Table 1. As it can be seen from Table 1, major minerals identified by XRD analysis were sphalerite, pyrite, dolomite, pyrolusite, and barite. First, a jaw crusher was applied to comminute the Goushfil zinc ore. Then, a roll crusher was used to grind the ore to the smaller particle size distribution (PSD). Finally, the ore was ground by a rod mill to a certain PSD. The particle size distribution of the ore used in the bioleaching experiments is provided in Fig. 2. In some experiments, the low-grade ore was combined with sphalerite concentrate (Zn = 45 wt%, ratio of 2/3) to obtain a ore with 11 wt% zinc.
2.2 Catalysts Preparation
The amount of 100 g tailing newspaper was cut to small pieces (less than 1 cm2), then, it was dissolved in a certain amount of sulphuric acid. The zinc concentration in tailing newspaper was considered as 21. 8 ppm [28]. In terms of the starch and sugar, 2 g starch or sugar was dissolved in the sulphuric acid and it was placed in an incubator with rotation speed of 140 rpm for 30 min.
2.3 Microorganisms and Culture Conditions
Bacteria were cultured in 9 K culture medium, which consisted of 0.5 g L−1 MgSO4·7H2O, 3.0 g L−1 NH4SO4, 0.1 g L−1 KCl, 0.01 g L−1 Ca(NO3)2·H2O, 0.5 g L−1 K2HPO4, and 44.22 g L−1 FeSO4.7H2O. The pH of the culture medium was adjusted to 2.3 using dilute sulphuric acid. A constant-temperature shaker with the temperature of 40 °C and rotating speed of 150 rpm was employed to culture bacteria. A Norris medium consisting of 0.5 g L−1 (NH4)2SO4, 0.4 g L−1 K2HPO4, and 0.4 g L−1 MgSO4.7H2O was prepared and the pH was adjusted to 1.5 by adding H2SO4. The mixed culture including (both iron and sulphur oxidizer/Acidithiobacillus ferrooxidans), (sulphur oxidizers/Acidithiobacillus thiooxidans), and (iron oxidizer/Leptospirillum ferrooxidans) was used in this study. All microorganisms were mesophile.
2.4 Analysis
The experiments were monitored daily for pH using a Metrohm 827 pH meter. Also, the redox potential was measured with a reference to a saturated Ag/AgCl electrode using a Pt electrode attached to a Metrohm 827 pH meter. The concentration of zinc in the leaching media at the end of each day was analysed by atomic absorption spectroscopy (Perkin ElmerAA-400 model).
2.5 Shake Flask Experiments
Shake flask culture experiments were conducted to examine the effect of the different operating parameters and catalysts on the zinc recovery. All experiments were conducted in 500 mL Erlenmeyer flasks with 180 ml Norris or 9 K medium, placed in orbital shaking incubators operated at 150 rpm and 35 °C. 10 g of ore was added into Erlenmeyer flasks. Then, the flasks were inoculated with 20 ml solution containing active microorganisms. The initial pH was adjusted to 1.6–1.8 using H2SO4 and NaOH. The flasks were monitored for redox potential, pH, and zinc concentration at appropriate intervals.
3 Results and Discussion
3.1 Preliminary Bioleaching Experiments
The change in pH (a), redox potential (mV) (b), and zinc bioleaching (c) during bioleaching experiment is shown in Fig. 3. Figure 3a shows that in the initial leaching period (first day), the pH values increases because of the acid leaching or consumption of acid by gangue minerals, then it gradually decreases as the bioleaching goes on to the third day, afterwards, there is a sharp decrease in the pH on the fourth day. It can be attributed to the activity of microorganisms in the bioleaching solution and the formation of H+ in the solution. After day 4, the pH remains constant to around 1.8 during rest of the bioleaching experiment. From Fig. 3b, it can be seen that there is a sharp increase in redox potential on the day 4 from 400 to 480 mV. It means that the lag phase is 4 days for this system and after that microbial activities are observed. Then, a more gradual increase in the redox potential in the sphalerite bioleaching experiments from 480 to 700 mV between day 4 and day 18 is observed. The zinc bioleaching is a bit linear during whole bioleaching experiment. It means that there is ample sphalerite for bio-oxidation in the whole experiment and the rate controlling parameter is reaction kinetic, not diffusion. Reaction-controlled and diffusion-controlled models are provided in Fig. 4. As it can be seen, that the reaction-controlled model has better fitted the results based on R-square value than diffusion-controlled model.
3.2 Effect of Culture Media
The culture media plays an important role in the bioleaching of metals because it provides nutrients for the microorganisms. The effects of two different culture media including 9 K and Norris media on the bioleaching of zinc from the low-grade sphalerite ore are shown in Fig. 5. It is found that the zinc bioleaching reaches 70% during the 10 days for the Norris media while 9 K media require 14 days to extract 70% of zinc from the low-grade zinc ore. The zinc recovery is 75% and 85% for the Norris and 9 K media after 18 days bioleaching experiment, respectively. These results are consistent with findings of the other literature. It is found that there is a limitation of the extraction of metals in the Norris medium when the zinc concentration is high [29, 30]. The leaching rate is high after lag phase, but it decreases by the enhancement of zinc concentration in the solution. Thus, use of 9 K medium is suggested for next bioleaching experiments.
3.3 Effect of Mixing the Low-Grade Ore with the Zinc Concentrate
Using pyrometallurgical methods for the extraction of zinc concentrates is limited due to high smelting and transport costs and also strict environmental regulations [31]. However, the bioleaching can be used to extract zinc from a concentrate when it is mixed with a low-grade ore containing high amount of iron. Iron oxidizing macroorganisms oxidize iron minerals such as pyrite and subsequently produced Fe3+ irons attack the zinc concentrate to extract it into solution. Figure 6a, b, and c shows the effect of low-grade ore mixing with zinc concentrate on the pH, redox potential, and zinc leaching in the bioleaching experiment, respectively. As it can be seen, the mixed ore performance is a bit more better than the low-grade ore in terms of zinc recovery. This can be used to take the advantage of both leaching of zinc from concentrate using environmentally hydrometallurgical method as well as increasing zinc recovery from the low-grade ore. The zinc bioleaching is obtained as 95.7% and 88.3% for the mixed and low-grade ores, respectively. The amount of pH and redox potential is higher at initial stage in the low-grade ore. The higher pH value can be due to the presence of more acid consumable minerals in the low-grade ore. The percentage of sphalerite is higher in the mixed ore which can increase chemical leaching during the lag phase and subsequently, increase the amount of ferrous iron in the solution. The higher ferrous iron concentration means lower redox potential value during the initial stage (Fig. 6b).
3.4 Effect of pH on the Zinc Bioleaching
The effect of pH on the zinc bioleaching from the low-grade and mixed ores is shown in Fig. 7. There is an increase in the zinc recovery from 56 to 86.4% and 51% to 95.5% for the low-grade and mixed ores, when pH increases from 1 to 1.8. But, a decrease in the zinc recovery by 8.4% and 13.5% with the increasing pH from 1.8 to 2.2 is observed. Therefore, the pH value of 1.8 is the optimum value for the zinc bioleaching. The change in the zinc bioleaching is because there is a pH range for the microorganisms to be active for the growth and mineral oxidation. It has been found that the higher amount of acid which is related to the pH value can have negative impact on the growth and metabolic activity of the leaching microorganisms [32]. The decrease in the zinc bioleaching at higher pH (higher than 1.8) may be due to the precipitation of ferric iron as ferric hydroxide.
3.5 Effect of Ferrous Iron Concentration
In order to determine the optimum iron concentration as energy source in the culture, in terms of zinc bioleaching, its concentration was changes in the system. Ferrous iron concentration effect on the zinc bioleaching for two different ores is given in Fig. 8. For the low-grade ore, increasing Fe2+ iron concentration from 0 to 10 g L−1 has led to the enhancement of zinc bioleaching by about 30%, but it remains constant when the Fe2+ iron concentration increases from 10 g L−1 to 44 g L−1. It is not needed to add much more Fe2+ iron concentration in the solution for the increasing zinc recovery because the ore has higher amount of pyrite which provides enough Fe2+ iron concentration in the solution for the growth and metabolic activity of microorganisms and subsequently the zinc bioleaching. For the mixed ore, there is an increase in the zinc bioleaching from 43% to about 96% with increasing Fe2+ iron concentration from 0 to 44 g L−1. The amount of zinc in the mixed ore is high (11 wt%) and pyrite has been removed from the ore during the flotation process. It means that the amount Fe2+ iron concentration as energy source is lower for the bioleaching of this high amount of zinc. Although, it should be paid attention that there is an optimum value for Fe2+ iron concentration because increasing its concentration can have a negative impact and cause inhibition for the microorganisms’ growth and activity.
3.6 Effect of Catalyst on the Zinc Bioleaching
It has been found that a number of materials such as starch, shredded newspaper, and sugar can act as a catalyst and low-cost reagent for the reduction of ferric iron. Also, these cellulose materials react with acid and act as electron acceptor based on the following mechanisms [25]:
The action of the microorganisms on the pyrite dissolution in the presence of these materials can be written as follows [25]:
The Fe3+ produced in Eq. 3 and the oxidation of ferrous iron acts on the pyrite moiety for further production of the protons and Fe2+ according to the following equation:
The ferric sulphate generated in the reaction 3 will provide an oxidative attack to the mineral moiety. Then, it will oxidize the sphalerite based on Eq. 5:
The effect of addition of three different catalysts including starch, shredded newspaper, and sugar on the pH, redox potential, and zinc bioleaching is shown in Fig. 9. It is observed that the maximum zinc recovery is obtained in 10 days in the presence of 2 g L−1 shredded newspaper and it reaches 88.4%. Also, the highest zinc bioleaching is obtained to be about 88% after 13 days, when starch is used as catalyst in the solution. However, the presence of sugar did not have effect on bioleaching time of zinc. The shredded newspaper acts as the best catalyst because it decreases the time of bioleaching from 18 days to 10 days in comparison with the zinc bioleaching without any catalyst. The results of the redox potential which is related to the ratio of ferric iron to ferrous iron confirms the higher activity and growth of microorganisms in the presence of shredded newspaper in terms of converting produced ferrous iron into ferric iron.
4 Conclusion
Bioleaching is considered as environmental friendly and economic alternative. The current study was conducted to improve zinc recovery from a low-grade zinc ore containing high amount of pyrite. It was done to optimize the shake flask bioleaching process. The amount of pH, addition of Fe2+ iron as energy source, combining with zinc concentrate, and culture media parameters were evaluated and optimized. The optimum operating conditions were found to be pH of 1.8, ferrous iron concentration of 10 g L−1, 9 K culture medium, and temperature of 35 °C. In addition, it was concluded that combination of low-grade ore with a zinc concentrate could be used to extract zinc from a concentrate and also increase zinc recovery from the low-grade ore. Finally, the zinc bioleaching time improved by addition of shredded newspaper and starch into the leaching solution by 8 and 5 days respectively.
References
Ehrlich H L, Hydrometallurgy, 59 (2001) 127. https://doi.org/10.1016/S0304-386X(00)00165-1.
Shi S-Y, Fang Z-H, and Ni J-R, Process Biochem. 41 (2006) 438 https://doi.org/10.1016/j.procbio.2005.07.008.
Ejtemaei M, Gharabaghi M, and Irannajad M, Adv. Colloid Interface Sci. 206 (2014) 68 https://doi.org/10.1016/j.cis.2013.02.003.
Feng Z C Handbook of zinc oxide and related materials, CRC press, Boca Raton (2012).
Xu H, Wei C, Li C, Fan G, Deng Z, Zhou X, and Qiu S, Sep. Purifi. Technol. 85 (2012) 206. https://doi.org/10.1016/j.seppur.2011.10.012.
Abkhoshk E, Jorjani E, Al-Harahsheh M S, Rashchi F, and Naazeri M, Hydrometallurgy, 149 (2014) 153. https://doi.org/10.1016/j.hydromet.2014.08.001.
M. Muravyov, J. Biotechnol. 305 (2019) S51. https://doi.org/10.1016/j.jbiotec.2019.05.180.
Tang L, Tang C, Xiao J, Zeng P, and Tang M, J.f Cleaner Prod. 201 (2018) 764. https://doi.org/10.1016/j.jclepro.2018.08.096.
Sundramurthy, V P, Rajoo, B, Srinivasan N R, and Kavitha R, App. Biolo. Chem. 63 (2020), 44. https://doi.org/10.1186/s13765-020-00528-8.
Ahmadi A, and Mousavi S J, Int. J. Miner. Process. 135 (2015) 32. https://doi.org/10.1016/j.minpro.2015.01.002.
Schippers A, Tanne C, Stummeyer J, and Graupner T, Miner. Eng. 132 (2019) 251. https://doi.org/10.1016/j.mineng.2018.12.007.
Rodrı X, Guez Y, Ballester A, Blázquez M L, González F, and Muñoz J A, Hydrometallurgy, 71 (2003) 57. https://doi.org/10.1016/S0304-386X(03)00174-9.
Nikkhou F, Xia F, and Deditius A P, Hydrometallurgy, 188 (2019) 201 https://doi.org/10.1016/j.hydromet.2019.06.017.
Lorenzo-Tallafigo J, Iglesias-Gonzalez N, Romero R, Mazuelos A, and Carranza F, Miner. Eng. 125 (2018) 50. https://doi.org/10.1016/j.mineng.2018.05.026.
Brierley J A, Hydrometallurgy, 94 (2008) 2. https://doi.org/10.1016/j.hydromet.2008.05.014.
Natarajan K A, in Biotechnology of Metals, (Ed: K. A. Natarajan), Elsevier, Amsterdam, (2018), 151–177.
Nemati M, and Harrison S T L, J. Chem. Technol. Biotechnol. 75 (2000) 526. https://doi.org/10.1002/1097-4660(200007)75:7%3c526::aid-jctb249%3e3.0.co;2-4.
Rohwerder T, Gehrke T, Kinzler K, and Sand W, App. Microbiol. Biotechnol. 63 (2003) 239. https://doi.org/10.1007/s00253-003-1448-7.
Castro C, and Donati E R, Trans. Nonferrous Metals Soc. China, 26 (2016) 3004. https://doi.org/10.1016/S1003-6326(16)64431-X.
Pathak A, Morrison L, and Healy M G, Bioresour. Technol. 229 (2017) 211. https://doi.org/10.1016/j.biortech.2017.01.001.
Zhang M, Guo X, Tian B, Wang J, Qi S, Yang Y, and Xin B, Waste Manag. 87 (2019) 629. https://doi.org/10.1016/j.wasman.2019.02.047.
Yang Y, Liu W, Gao X, and Chen M, Hydrometallurgy, 186 (2019) 252. https://doi.org/10.1016/j.hydromet.2019.04.023.
Gómez C, Román E, Blázquez M L, and Ballester A, Miner. Eng. 10 (1997) 825. https://doi.org/10.1016/S0892-6875(97)00060-5.
Zhao H, Wang J, Gan X, Zheng X, Tao L, Hu M, Li Y, Qin W, and Qiu G, Bioresour. Technol. 194 (2015) 28. https://doi.org/10.1016/j.biortech.2015.07.003.
Panda S, Biswal A, Mishra S, Panda P K, Pradhan N, Mohapatra U, Sukla L B, Mishra B K, and Akcil A, Hydrometallurgy, 153 (2015) 98. https://doi.org/10.1016/j.hydromet.2015.02.006.
Fischer K, and Bipp H P, Water Air Soil Pollut. 138 (2002) 271. https://doi.org/10.1023/a:1015566207849.
Choi S C, Lee G H, and Lee H K, Korean J. Microbiol. 45 (2009) 411.
Tucker P, Douglas P, Durrant A, and Hursthouse A S, Environ. Manag. Health, 11 (2000) 47.
Deveci H, Alp I, and Uslu T, Int. Mining Congress and Exhibition of Turkey-IMCET (2003) 415.
Gómez C, Blázquez M L, and Ballester A, Miner. Eng. 12 (1999) 93. https://doi.org/10.1016/S0892-6875(98)00122-8.
Soleimani M, Petersen J, Roostaazad R, Hosseini S, Mohammad Mousavi S, Najafi A, and Vasiri A K, Miner. Eng. 24 (2011) 64. https://doi.org/10.1016/j.mineng.2010.10.003.
Ngoma E, Ojumu T V, and Harrison S T L, Miner. Eng. 75 (2015) 6. https://doi.org/10.1016/j.mineng.2015.02.007.
Acknowledgment
Saeed Shirazian acknowledged the supports by the Government of the Russian Federation (Act211, contract 02.A03.21.0011) and by the Ministry of Science and Higher Education of Russia (grant FENU-2020-0019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Foroutan, A., Bazpors Ghaziani, S., Abbas Zadeh Haji Abadi, M. et al. Intensification of Zinc Bioleaching from a Zinc–Iron Bearing Ore by Condition Optimization and Adding Catalysts. Trans Indian Inst Met 74, 1–8 (2021). https://doi.org/10.1007/s12666-020-02117-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-02117-1