Abstract
The present study investigates the influence of ferrous iron (as FeSO4) and ferric iron (as Fe2 (SO4)3), and pyrite (FeS2) on the ability of bacterial leaching of a high-grade sulfide Zn–Pb ore. In this regard, shake flask experiments were carried out at 5% (w/v) pulp density of the ore sample (having 40.7% Zn and 12.4% Pb initial metal content) using a consortium of mesophilic iron and sulfur-oxidizing acidophiles. A concentration of 0.04 mol/L of ferric iron in the leaching media was found to be optimum for zinc extraction without affecting growth of the microorganisms. Under this concentration, the dissolution of Zn, Pb, Cd, and As was found to be 57%, 0.2%, 0.03%, and 9.9% in 25 days. Using ferrous iron in the media, 0.16 mol/L of Fe2+ was found to be the optimum concentration for efficient bacterial growth and metal dissolution (54.6% Zn, 0.08% Pb, 0.03% Cd, and 10.2% As) from the sample in 25 days. On the other hand, using pyrite as the source of energy for bacterial growth, an initial 12-day lag period was observed when compared to the effect of ferrous iron in the media. Under the optimum concentration (test with 0.24 mol/L iron in the form of pyrite), the dissolution of Zn, Pb, Cd, and As was found to be 39.8%, 0.1%, 0.03%, and 10% in 25 days. The surface chemistry analysis indicated formation of a sulfur layer over the particle surface that hindered reagent diffusion and affected metal recovery through bioleaching.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
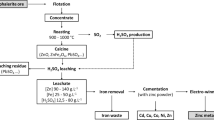
With the production of more than 85% of total world zinc [43, 44], roast–leach–electrowinning (RLE) process is the most important method for zinc extraction from sulfide ores and concentrates. However, this method is associated with several environmental and economic problems. For example, the generation of SO2 during roasting process and higher energy consumption affect the future application of RLE in metal extraction industries. It is a challenge to achieve an improved and sustainable method of zinc extraction from sulfide minerals while zinc (Zn)–lead (Pb) deposits in the form of oxide minerals are becoming increasingly depleted. Among all suggested methods, bioleaching has been accepted as an environmentally friendly process, with minimum carbon foot print [2, 3, 27, 34, 46]. In fact, the most important advantages of bioleaching are avoiding the emission of huge amount of SO2 and reducing the dependence on fossil fuels as required during the conventional roasting stage of the RLE process.
Acidophilic chemolithotrophic microbial consortia accelerate the dissolution of sulfide minerals by direct and/or indirect mechanisms [9, 31, 35, 46, 49]. Among the sulfide minerals, sphalerite (ZnS) has been seen to follow the indirect mechanism of bioleaching where the biogenic ferric iron enables dissolution of metals from the sulfide mineral [1, 4, 23, 24, 38, 39]. In this process, bacteria oxidize ferrous ions to ferric ions which in turn act as the main reagent for leaching of sulfide minerals [30, 32]. The reaction is shown in Eq. 1 [33] (MeS signifies sulfide minerals in the form of M2+S2− such as ZnS).
The Fe2+ produced during the above reactions re-oxidizes to ferric ions by bacteria (Eq. 2) and these sustainable reactions cause sulfide mineral dissolution in the presence of iron [7].
Formation of a layer of elemental sulfur in sphalerite surface has been reported [36, 42, 45, 47]. This phenomenon is responsible for low zinc recovery in many cases and to solve this problem, different methods have been studied such as two-step bioleaching [33], using l-cysteine to control the surface charge of minerals [15], using o-phenylenediamine (OPD) as a surfactant [18], and microwave as a pretreatment technique [25].
In many sulfide deposits, sphalerite (ZnS) and galena (PbS) are found together through paragenesis. Despite a low zinc recovery, the recovery of lead during bioleaching is much lower than that of zinc and other metals [5, 6, 20]. Although some quantity of galena is oxidized by the bacteria through the indirect mechanism [40], the concentration of Pb in pregnant leach solution (PLS) remains very low, because lead precipitates in the presence of sulfate in the form of PbSO4 [32]. Zn–Pb ore matrix usually contains iron minerals (such as pyrite and marmatite) and previous research have indicated that bioleaching improved with increased iron content in the ore [36, 37, 50]. Interestingly, a study by Shi et al. [38] on sphalerite, marmatite, and synthetic ZnS bioleaching indicated that an increase in iron content increased the bioleaching rate. This indicates that iron plays an important role in zinc bioleaching.
Owing to the importance of iron which affects both chemical and biological reactions in minerals bioleaching, an attempt has been made in this research work to study the effect of ferrous ion, ferric ion, and pyrite, as external iron sources, on the bacterial growth and bioleaching of typical high-grade zinc and lead ore. For this purpose, different quantities of the respective iron sources (chemical source, i.e., ferrous iron and ferric iron and mineral source, i.e., pyrite) were added to the bioleaching tests and their efficiency was compared for the dissolution of Zn, Pb, cadmium (Cd), and arsenic (As) from a high-grade Zn–Pb sulfide ore during bioleaching.
2 Materials and Methods
2.1 Ore Sample
A high-grade Zn–Pb sulfide ore, obtained from Anguoran Zinc & Lead Mine (Zanjan, Iran), was used in the present study. The ore was crushed with a roll crusher that worked with a 2-mm sieve in a closed circuit and a representative – 2-mm sample (obtained through the conventional coning and quartering method) was used for all experiments. Table 1 indicates the chemical composition of ore sample. Sphalerite (ZnS) and galena (PbS) were found to be the major minerals, and minor amounts of smithsonite (Zn(CO3)), cerussite (Pb(CO3)), calcite (Ca(CO3)), and quartz (SiO2) were seen to be present. The polished section studies under an optical microscope indicated that about 75% of galena and sphalerite particles were separated from each other at size fraction less than 2 mm.
The pyrite sample, used as an iron source in the present experiment, was prepared from Sarcheshmeh Copper Complex, Kerman, Iran. Two-step flotation, including rougher and cleaner steps, was carried out to obtain a high-grade pyrite. The XRD analysis of this concentrate showed that pyrite (FeS2) was the main mineral. The pyrite concentrate also contained few percentage of anorthite (calcium aluminum silicate) as gangue mineral. The atomic absorption spectroscopy analysis of the pyrite sample indicated that concentrates contain 43.77% iron.
2.2 Bacteria and Culture Conditions
A mesophilic bacterial consortium enriched from acid mine drainage (AMD) collected from Anguoran Mine in Iran was used for the bioleaching tests [11]. Ten milliliters of the enriched mixed bacterial culture was added to 90-mL 9K culture media [17, 41] as inoculum with 44.22 g/L FeSO4·7H2O and 5 g/L elemental sulfur [14]. The cultures were incubated at 35 °C and 120 rpm and transferred to a new media every week until the bacterial count was raised to 5.0 × 107 cells/mL. This active mixed acidophilic bacterial consortium was used for all the bioleaching experiments.
2.3 Bacterial Leaching Experiments
For the bacterial leaching tests, three independent sets of experiments were designed to notice the percentage of metal dissolution from the ore sample. The 9K medium with the microbes was used as the leach solution in the experiments. In the first study, incremental amounts of 0.08, 0.16, and 0.24 mol/L Fe2+ (added as FeSO4·7H2O) were used to examine the effect of different concentrations of Fe2+ iron that were supplied into the leaching medium. In the second set, iron in the form of Fe3+ at 0.04, 0.08, 0.16, and 0.24 mol/L Fe3+concentrations (added as Fe2 (SO4)3) was tested. Similarly, in the third test, pyrite was added (0.04, 0.08, 0.16, and 0.24 mol/L Fe) as an external iron (mineral source) to study its effect. In order to make direct comparison with the results obtained through test experiments, abiotic chemical controls (3 mL formaldehyde) were used. The experimental conditions are listed in Table 2.
Five grams of the Zn–Pb ore sample was added into shake flasks having 90-mL 9K media + 10 mL of the mixed bacterial consortium as the inoculum. The pH level was adjusted to pH 1.7 with sulfuric acid. Elemental S (1 g) was added to each flask to promote the sulfur-oxidizing bacteria growth [48]. All flasks were incubated on a shaker incubator at 35 °C and 120 rpm for 25 days. Sampling of the leach solution was periodically carried out to analyze the concentration of the respective metals. Following sampling, the volume in the respective flasks was adjusted by addition of appropriate volume of sterile 9K medium. The dissolved zinc, lead, iron, and cadmium were determined by atomic absorption spectroscopy (Varian AA 240Z) and dissolved arsenic was analyzed by atomic fluorescence spectroscopy (AF-640A).
3 Results and Discussion
3.1 Effect of Different Iron Sources on Bacterial Growth and Associated Process Conditions
3.1.1 Ferrous Iron on Bacterial Counts, pH level, and ORP
The iron sources (in the form of Fe2+ and Fe3+) affect both the dissolution process and the microbial growth [27]. In order to study the bacterial growth, cell concentration was determined by direct counting using a Neubauer chamber counter of 0.02-mm depth and 1/400 mm2 area under an optical microscope. Figure 1 shows the changes in bacterial cell counts during the 25-day treatment. In acidic bioleaching systems, the oxidation–reduction potential (ORP) predominantly indicates the Fe3+/Fe2+ ratio and thus expresses the activity of the iron-oxidizing bacteria [22]. Similarly, the activity of the bacterium Acidithiobacillus thiooxidans is seen to cause the production of sulfuric acid thereby decreasing the pH level of the bioleaching system. Therefore, ORP and pH level have been often used as an indirect index for bacterial growth and activity [28]. Sample solutions were withdrawn regularly for pH level and ORP measurements. ORP and pH level fluctuations for different tests are shown in Fig. 2.
The bacterial counts for the test experiment having no external iron resources, i.e., test 1, was seen to increase gradually until day 12, and thereafter the counts started to increase (Fig. 1). The ORP for this test started to increase sharply after day 12 (Fig. 2d) which reflected the bacterial activity. In addition, the pH level in this test experiment (Fig. 2a) has the lowest pH level which decreased from 3.97 to 0.87. The bacterial growth was very low during the first days due to the absence of Fe in the environment. However, with time and dissolution of iron minerals, the bacterial growth accelerated. This indicated that absence of iron resources decreases the bioleaching kinetics because the microorganisms especially the iron-oxidizing acidophiles should be provided with the iron source (Fe2+) from the minerals in order to meet their energy requirements for growth.
Bacterial growth progressed at a constant rate over 25 days for all tests that contained FeSO4·7H2O as iron source, i.e., test nos. 2, 3, and 4 (Fig. 1). However, the test with 0.08 mol/L Fe2+, i.e., test 2, had the highest bacterial growth and also had the highest ORP measurement compared with other Fe2+tests (Fig. 2d). The pH level increased for a period of time and then started to decrease for all tests that contained ferrous iron (Fig. 2a).
3.1.2 Ferric Iron on Bacterial Counts, pH level, and ORP
Generally, the tests that contained ferric ions had higher bacterial count in comparison to other tests. Initially, the bacterial growth curves for these tests increased sharply; however, they became smooth after 12 days. Generally, adding low concentrations of Fe2(SO4)3 as source of ferric ions can improve the bacterial growth especially during the first few days [29]. The bacterial counts indicated that increasing the ferric concentration to more than 0.04 mol/L has a negative effect on the bacterial growth. During oxidation of sulfide minerals, the Fe3+ is reduced to Fe2+ (according to Eq. 1) and the generated ferrous ions accelerate the bacterial growth [10, 16]; however, the results show that high concentrations of Fe3+ have a negative effect. Figure 2e shows ORP fluctuations for tests that contained ferric ions. As can be seen from the figure, the test experiment with 0.24 mol/L ferric ions (test 8) had the highest oxidation–reduction potential during the first 15 days compared to other tests containing ferric ions (tests 5, 6, and 7). However, after 15 days, the ORP became almost similar in all these tests and stabilized between 607 and 613 mV. As mentioned earlier, the ORP reflects the Fe3+/Fe2+ ratio. At the fixed solid content, Fe3+/Fe2+ ratio will be higher for test with higher ferric content which results in higher ORP. Like the tests with ferrous iron, the pH level for tests with ferric iron increased during the initial days and then started to decline with increase in bacterial counts.
3.1.3 Pyrite on Bacterial Counts, pH level, and ORP
For the tests containing pyrite (tests 9 to 12), the bacterial count was very low during the first 12 days. However, it increased after passing this lag period due to an increase in the ferric concentration in the solution as a result of pyrite oxidation. The ORP also decreased during the first 12 days and then further increased which can be related to pyrite bio-oxidation. The ORP started to increase sharply only after the 20th day. On the other hand, the control test containing only pyrite (test 15) had almost constant ORP during the 25-day treatment. In tests with ferrous or ferric ions, ORP started to increase slowly after almost 5 days, but for tests with pyrite, ORP started to increase sharply after 20 days. This means that replacing the iron ions with pyrite causes a delay in the time for bio-oxidation; however, after passing a lag period, a high oxidizing environment will be available in the leach solution. In tests that contained pyrite, the pH level fluctuated similar to that of test 1. The pH level for control tests (tests 13, 14, and 15) was very high and ORP was very low in comparison with those of the bioleaching tests which show the effect of bacterial activity on the leaching process.
3.2 Zinc Dissolution
3.2.1 Effect of Ferrous Iron
The zinc dissolution curves are presented in Fig. 3. Test 1 passed through three distinct zinc dissolution periods. During the first period (first 7 days), zinc concentration increased gradually due to the dissolution of mainly zinc carbonate mineral (smithsonite). In fact, there is no bacterially mediated dissolution in this period, due to low bacterial counts. Between days 7 and 15, the dissolved zinc concentration was almost constant and after this period, it started to increase sharply. The second period reflected a delay and the third period showed bacterial activity. In fact, in the third period, sphalerite (zinc sulfide) started to dissolve. Generally, the zinc dissolution in tests containing ferrous ions increased with almost constant slope. Test 3 (with 0.16 mol/L Fe2+) had higher zinc recovery (54.6%) after 25 days when compared to tests 2 and 4. The optimum FeSO4 concentration in 9K medium also is 44.5 g/L or 0.16 mol/L Fe2+ [41] which conformed with the result of the current research. Dissolved zinc in control test that contained ferrous ions (test 13) was only 17.7%. These show that a bacterial leaching system, in the presence of Fe2+, is better for zinc dissolution than a system operating with only Fe2+ iron.
3.2.2 Effect of Ferric Iron
Adding Fe3+ to bioleaching tests improved the zinc dissolution kinetics, as shown in Fig. 3b. In fact, Fe3+ reduced to Fe2+ during sulfide mineral dissolution (based on Eq. 1) and the generated ferrous ions have been used by bacteria as an energy resource which promoted bacterial counts. The results of the study indicated that the quantity of ferric ions beyond 0.04 mol/L has no effect on zinc dissolution. The control flask that contained ferric ions (test 14) had a very sharp increase in Zn dissolution for the first 7 days; however, it stopped after this period due to the absence of bacteria. This result obviously shows that zinc dissolution for the first days occurred due to sphalerite oxidation by ferric ions (reaction 1) and no biological reaction have occurred during this period.
3.2.3 Effect of Pyrite
Generally, tests containing pyrite had lower zinc recovery. Tests with 0.16 and 0.24 mol/L iron, in the form of pyrite, had more rapid zinc dissolution. These tests also had higher bacterial counts. This shows that increasing pyrite concentration improves the bioleaching efficiency. The control test containing pyrite (test 15) had the lowest zinc recovery (2220 mg/L) among all experiments. This test shows the intense effect of ferrous and ferric ions on chemical leaching of ZnS. Bacterial treatment of ore improved zinc dissolution efficiency as 72.28% in comparison to chemical treatment (test 15) in the presence of pyrite.
3.3 Lead Dissolution
3.3.1 Effect of Ferrous Iron
Generally, the lead dissolution was very low for all tests (between 4 and 16.2 mg/L) after 25 days. Pb concentration increased during the first 7 days for all tests mainly due to carbonate mineral leaching. For test with no external iron addition (test 1), there was a decline in total lead dissolved due to precipitation of Pb and then lead concentration increased again because of bacterial activity. In fact, with increase in bacterial counts, acidophiles produce ferric ions by oxidizing iron minerals or dissolved ferrous ions which increase the galena oxidation by providing an oxidizing environment. In the tests containing Fe2+, after passing an increasing period in lead dissolution, the Pb concentrations become almost constant.
3.3.2 Effect of Ferric Iron
The lead concentrate in tests containing ferric iron increased for 17 days and then started to decline. Generally, low concentrations of lead can dissolve in a sulfide environment. However, by increasing the \( {\mathrm{SO}}_4^{2-} \)ions in the leaching environment, Pb starts to precipitate in the form of PbSO4 (Ksp = 1.62 × 10−8) [21, 51] and makes an ash layer in the galena surface [8]. This insoluble layer prevents the leaching agent’s access to the galena surface and this results in low recovery of lead in bioleaching.
3.3.3 Effect of Pyrite
The tests containing pyrite (tests 9 and 12) followed a similar pattern. As shown in Fig. 4, the control flasks (that reflected the chemical dissolution mechanism) had almost similar lead dissolution changes. All conditions for these three tests were completely similar except iron resources. Thus, addition of the iron source has no influence in chemical lead solubilization.
A variety of methods including solvent extraction, adsorption on activated carbon, ion exchange, precipitation, cementation, and electrowinning can be readily exploited for the recovery of base metals from pregnant leach solutions (PLS) [12]. One of the most important parameters for all of these methods is PLS purity. The produced PLS, in the present method, has high quality due to very low lead concentration. In addition, zinc dissolution increases the lead grade in bioleached residue and produces a Pb concentrate that could be used for processing with other methods. Table 3 shows the chemical analysis of the solid bioleached residue for tests 3 and 13. As this table shows, the lead grade improved from 12.4 to 18.7% after 25 days of biological treatment.
3.4 Cadmium and Arsenic Dissolution
3.4.1 Effect of Ferrous Iron
The cadmium and arsenic dissolutions were monitored during treatment as well (Fig. 5). The dissolved cadmium fluctuated in the range of 50 to 83 mg/L after 25-day treatment. The cadmium dissolution graph in tests with ferrous iron had almost a constant slope. Halikia and Voudouris [13] showed that Cd dissolution from minerals is strongly dependent on pH level rather than oxidation agents. In current experiments, also the tests with ferric iron had a higher Cd recovery compared to tests with pyrite and ferrous iron, due to lower pH levels. The control tests had very low Cd recovery due to high pH levels.
Figure 5 also shows arsenic changes in the leach solution. Generally, the As concentration increased during 25-day treatment. The arsenic dissolutions in tests that contained ferrous ions passed two leaching periods and one lag phase. During the first 7 days, arsenic dissolved rapidly, and then leaching became almost constant for about 9 days and after day 16, it increased again. Arsenic (As) dissolved in the first 7 days due to the oxidizing effect of ferric ions on the arsenic minerals and it leached at a high rate after the 16th day due to increasing bacterial count with simultaneous advances in the biological reactions.
3.4.2 Effect of Ferric Iron
Adding ferric ions to tests caused increase in cadmium dissolution. After 7 days of bioleaching, the Cd concentrate for test with 0.04 mol/L ferric iron was 40.64 mg/L, while it was 62 mg/L for test with 0.24 mol/L ferric iron. On the other hand, the Cd concentrations for tests with 0.08 and 0.24 mol/L ferrous iron were 30.24 and 33.76 mg/L, after this period. As mentioned, this phenomenon can occur due to lower pH level in tests with ferric ions.
Arsenic dissolution for tests that contained ferric iron had similar pattern to the tests that contained ferrous iron.
3.4.3 Effect of Pyrite
Cd dissolution for tests with pyrite is lower than that for tests with ferrous and ferric ions due to lower ferric ion concentration in the leaching environment. The Cd dissolution for control tests with ferrous iron, ferric iron, and pyrite was 12.42, 50, and 4.8 mg/L after 25 days. On the other hand, the pH levels for these tests were around 3.4, 1.8, and 5.2 respectively which shows the effect of pH level on cadmium dissolution.
In the case of As dissolution, the tests with pyrite passed just one dissolution period, i.e., from day 16 and day 25 and there was not any arsenic dissolution before this period. In fact, absence of ferric and ferrous ions caused elimination of the first arsenic dissolution period. However, by increasing the bacterial activity and producing biological ferric ion, arsenic dissolution also increased.
3.5 Dissolution of Iron from the Sample
3.5.1 In Tests with Ferrous Iron
The iron changes were monitored in the bioleaching tests during 25 days of treatment (Fig. 6). Generally, the iron concentration decreased in tests containing ferrous ions. As shown in Fig. 6, an increase in the initial iron concentration caused iron precipitation with a steeper slope. The total dissolved iron in the bioleaching tests containing high ferrous concentration (tests 3 and 4) decreased with an almost sharp slope and then started to increase due to dissolution of iron minerals. However, the dissolved iron in the control test with ferrous ions (test 13) was lower in all 25-day treatment. The XRD analysis of bioleached residue (Fig. 7) for test that contained 0.16 mol/L ferrous ion showed that Fe precipitated in the form of iron sulfate hydrate (Fe4SO9·5H2O).
3.5.2 In Tests with Ferric Iron
The iron in tests with high ferric concentration (tests 7 and 8) decreased during the first 7 days and later stabilized in ranges of tests with lower Fe3+ concentration (tests 5 and 6). Therefore, increasing the initial ferric concentration has no effect on the bioleaching process due to precipitation of surplus iron. Figure 3b showing zinc dissolution of tests with Fe3+confirms this idea, too. According to Fig. 1, the bacterial count in these tests became fixed or decreased after about 12 days. As the bacteria have a high affinity for iron precipitation, they tend to stick in the solid particles and the bacterial population in solution tends to decrease [29].
3.5.3 In Tests with Pyrite
The dissolved iron showed no fluctuation during the first 16 days for tests containing pyrite (tests 9 to 12); however, after increasing the bacterial count, it started to increase due to pyrite bio-oxidation. The highest dissolved iron in these tests was 1.887 g/L. The control test with pyrite had no dissolved iron due to the absence of bacteria. This pyrite dissolution is very low in the absence of an oxidation agent, due to sulfide composition of this mineral.
3.6 SEM Characterization: Morphological Analysis of the Sample Pre and Post Bioleaching
Scanning electron microscopic (SEM) studies were carried out to investigate the surface properties and their composition pre and post bioleaching of the ore sample [26]. The sphalerite surfaces were smooth before treatment (data not shown). However, SEM images indicated that the surface became rough after both chemical and bacterial leaching. Figure 8 shows the zinc mineral surface after 25 days of bio-treatment with 0.16 mol ferric ions (test 7). As can be seen in the figure, the particle surface is covered with 5-μm crystals. The energy-dispersive X-ray (EDX) spectroscopy analysis showed that these crystals are mainly composed of S0 due to sulfur precipitation on the minerals surface, according to Eq. 1. This phenomenon caused a decrease in the contact surface between zinc particle and the bacterial lixiviant causing a reduced leaching rate ([36]; Lanet al. 2009; [37]). As shown in Fig. 3, zinc dissolution in the tests containing ferric ions (tests 5 to 8) decreased after 7 days and became constant after 16 days due to the mentioned impact. However, the surface of particles that bioleached in the presence of ferrous ions did not cover with sulfur crystals and zinc was exposed to leachate. In these tests, the zinc dissolution rate did not decrease. The iron concentration on the zinc particle surface for control test (test 14) is higher compared to that of the other tests. This iron precipitated in the form of iron sulfate hydrate as shown in Fig. 6. Previous studies suggested a log linear dependence of the ferric iron in solution to the pH level [19]. In the control test containing ferric ions (test 14), the pH level stabilized around 1.8 and in similar bioleaching test (test 7), the pH level was around 1.1. Because the pH level in chemical leaching test was above the critical acidity, the iron precipitation was more predominant in this test.
4 Conclusions
A series of laboratory scale bioleaching experiments were carried out to study the effects of ferrous iron, ferric iron, and pyrite as different iron sources on the bioleaching of high-grade zinc–lead ore. The highest zinc recoveries for tests containing ferrous iron (54.6%), ferric iron (57%), and pyrite (39.8) were achieved at the iron concentration 0.16 mol/L, 0.04 mol/L, and 0.24 mol/L, respectively. Although leaching in tests containing ferric iron was very high over the first few days, it was seen to decrease after 16 days. The scanning electron microscopy (SEM) studies showed that in these tests, sphalerite surfaces were covered with sulfur crystals and it caused to reduce the zinc dissolution efficiency due to the hindrance in contact of the leachate and sphalerite surface. Different concentrations of Fe3+ added to shaking flask tests showed that increasing the ferric concentration at 0.04 mol/L had no significant effect on the zinc recovery due to ferric iron precipitation. In the case of using pyrite, there was a 15-day lag phase in bacterial growth due to lack of iron ions and after this period, the bacterial count started to increase rapidly. Although using pyrite makes the leaching time longer, it could reduce the costs due to low price of pyrite. Finally, the arsenic concentration in the leach solution did not show any sensitivity to the presence of iron sources in the bioleaching system, while cadmium dissolution was dependent on the ferric concentration in solution.
References
Ahmadi A, Mousavi SJ (2015) The influence of physicochemical parameters on the bioleaching of zinc sulfide concentrates using a mixed culture of moderately thermophilic microorganisms. Int J Miner Process 135:32–39
Akcil A (2004) Potential bioleaching developments towards commercial reality: Turkish metal mining’s future. Miner Eng 17:477–480
Akcil A, Deveci H (2010) Mineral biotechnology of sulphides. In: Jain S, Khan A, Rai MK (eds) Geomicrobiology. Science Publishers, Enfield, pp 101–137
Baba AA, Adekola FA, Atata RF, Ahmed RN, Panda S (2011) Bioleaching of Zn(II) and Pb(II) from Nigerian sphalerite and galena ores by mixed culture of acidophilic bacteria. Trans Nonferrous Metals Soc China 21:2535–2541
Bigham JM, Algur OF, Jones FS, Tuovinen OH (2013) Solid-phase controls on lead partitioning in laboratory bioleaching solutions. Hydrometallurgy 136:27–30
Cheng Y, Guo Z, Liu X, Yin H, Qiu G, Pan F, Liu H (2009) The bioleaching feasibility for Pb/Zn smelting slag and community characteristics of indigenous moderate-thermophilic bacteria. Bioresour Technol 100:2737–2740
Deveci H, Akcil A, Alp I (2004) Bioleaching of complex zinc sulphides using mesophilic and thermophilic bacteria: comparative importance of pH and iron. Hydrometallurgy 73:293–303
Dutrizac JE, Chen TT (1994) Reaction of galena in ferric sulphate-sulphuric acid media. In: Hydrometallurgy ’94. Springer, Dordrecht. doi https://doi.org/10.1007/978-94-011-1214-7_7
Gahan CS, Srichandan H, Kim D, Akcil A (2012) Biohydrometallurgy and biomineral processing technology, a review on its past, present and future. Res J Recent Sci 1:85–99
Ghassa S, Boruomand Z, Abdollahi H, Moradian M, Akcil A (2014) Bioleaching of high-grade Zn-Pb bearing ore by mixed moderate thermophilic microorganisms. Sep Purif Technol 136:241–249
Ghassa S, Boruomand Z, Abdollahi H, Moradian M, Akcil A (2015) Microbial dissolution of Zn-Pb sulfide minerals using mesophilic iron and sulfur-oxidizing acidophiles. Miner Process Extr Metall Rev: Int J 36(2):112–122
Habashi F (1999) A textbook of hydrometallurgy, second edn. Metallurgie Extractive Quebec, Quebec City
Halikia I, Voudouris N (2013) Investigation of zinc dissolution and cadmium precipitation rates in a Cd2+/Zn cementation system. Miner Process Ext Metall 114:95–108
Hawkes RB, Franzmann PD, Plumb J (2006) Moderate thermophiles including “Ferroplasma cupricumulans” sp. nov. dominate an industrial-scale chalcocite heap bioleaching operation. Hydrometallurgy 83:229–236
He Z, Gao F, Zhong H, Hu Y (2009) Effects of L-cysteine on Ni–Cu sulfide and marmatite bioleaching by Acidithiobacillus caldus. Bioresour Technol 100:1383–1387
He Z, Yang Y, Zhou S, Hu Y, Zhong H (2014) Effect of pyrite, elemental sulfur and ferrous ions on EPS production by metal sulfide bioleaching microbes. Trans Nonferrous Metals Soc China 24(4):1171–1178
Johnson DB (1995) Selective solid media for isolating and enumerating acidophilic bacteria. J Microbiol Methods 23:205–218
Lan Z, Hu Y, Qin W (2009) Effect of surfactant OPD on the bioleaching of marmatite. Miner Eng 22:10–13
Leahy MJ, Schwarz MP (2009) Modelling jarosite precipitation in isothermal chalcopyrite bioleaching columns. Hydrometallurgy 98:181–191
Liao MX, Deng TL (2004) Zinc and lead extraction from complex raw sulfides by sequential bioleaching and acidic brine leach. Miner Eng 17:17–22
Liu YG, Zhou M, Zeng GM, Wang X, Li X, Fan T, Xu WH (2008) Bioleaching of heavy metals from mine tailings by indigenous sulfur-oxidizing bacteria: effects of substrate concentration. Bioresour Technol 99:4124–4129
Manafi Z, Abdollahi H, Tuovinen OH (2013) Shake flask and column bioleaching of pyritic porphyry copper sulphide ore. Int J Miner Process 119:16–20
Mousavi SM, Jafari A, Yaghmaei S, Vossoughi M, Roostaazad R (2006) Bioleaching of low-grade sphalerite using a column reactor. Hydrometallurgy 82:75–82
Niu Z, Huang Q, Wang J, Yang Y, Xin B, Chen S (2015) Metallic ions catalysis for improving bioleaching yield of Zn and Mn from spent Zn-Mn batteries at high pulp density of 10%. J Hazard Mater 298:170–177
Olubambi PA (2009) Influence of microwave pretreatment on the bioleaching behavior of low-grade complex sulphide ores. Hydrometallurgy 95:159–165
Panda S, Pradhan N, Mohapatra UB, Panda SK, Rath SS, Rao DS, Nayak BD, Sukla LB, Mishra BK (2013) Bioleaching of copper from pre and post thermally activated low grade chalcopyrite contained ball mill spillage. Front Environ Sci Eng 7:281–293
Panda S, Akcil A, Pradhan N, Deveci H (2015) Current scenario of chalcopyrite bioleaching: a review on the recent advances to its heap leach technology. Bioresour Technol 196:694–706
Panda S, Akcil A, Mishra S, Erust C (2017) Synergistic effect of biogenic Fe3+ coupled to S° oxidation on simultaneous bioleaching of Cu, Co, Zn and As from hazardous pyrite ash waste. J Hazard Mater 325:59–70
Pogliani C, Donati E (2000) Immobilization of Thiobacillus ferrooxidans: importance of jarosite precipitation. Process Biochem 35:997–1004
Qin W, Liu K, Diao M, Wang J, Zhang Y, Yang C, Jiao F (2013) Oxidation of arsenite (As (III)) by ferric iron in the presence of pyrite and a mixed moderately thermophilic culture. Hydrometallurgy 137:53–59
Rawlings DE, Johnson DB (2006) Mesophilic, autotrophic bioleaching bacteria: description, physiology and role, Biomining. Springer, Berlin, pp 229–245
Rehman M, Anwar MA, Iqbal M, Akhtar K, Khalid AM, Ghauri MA (2009) Bioleaching of high grade Pb –Zn ore by mesophilic and moderately thermophilic iron and sulfur oxidizers. Hydrometallurgy 97:1–7
Rodríguez Y, Ballester A, Blázquez ML, González F, Munõz JA (2002) New information on the sphalerite bioleaching mechanism at low and high temperature. Hydrometallurgy 71:57–66
Rohwerder T, Gehrke T, Kinzler K, Sand W (2003) Bioleaching review part A: progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl Microbiol Biotechnol 63:239–248
Sand W, Gehrke T, Jozsa PG, Shippers A (2001) (Bio) chemistry of bacterial leaching-direct vs. indirect bioleaching. Hydrometallurgy 59:159–175
Shi S, Fang S (2004) Bioleaching of marmatite flotation concentrate by Acidithiobacillus ferrooxidans. Hydrometallurgy 75:1–10
Shi S, Fang S, Ni J (2005) Comparative study on the bioleaching of zinc sulphides. Process Biochem 41:438–446
Shi S, Fang Z, Ni J (2006) Comparative study on the bioleaching of zinc sulphides. Process Biochem 41:438–446
Silva G (2004a) Relative importance of diffusion and reaction control during the bacterial and ferric sulphate leaching of zinc sulphide. Hydrometallurgy 73:313–324
Silva G (2004b) Kinetics and mechanism of the bacterial and ferric sulphate oxidation of galena. Hydrometallurgy 75:99–110
Silverman MP, Lundgren DG (1959) Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I An improved medium and harvesting procedure for securing high cell yields. J Bacteriol 77:642–647
Soleimani M, Hosseini S, Roostaazad R, Petersen J, Mousavi SM, Kazemi Vasiri A (2009) Microbial leaching of a low-grade sphalerite ore using a draft tube fluidized bed bioreactor. Hydrometallurgy 99:131–136
Souza AD, Pina PS, Leao VA (2007a) Bioleaching and chemical leaching as an integrated process in the zinc industry. Miner Eng 20:591–599
Souza AD, Pina PS, Leao VA, Silva CA, Siqueira PF (2007b) The leaching kinetics of a zinc sulphide concentrate in acid ferric sulphate. Hydrometallurgy 89:72–81
Wang J, Qiu G, Qin W, Zhang Y (2006) Microbial leaching of marmatite by Acidithiobacillusferrooxidans and Acidithiobacillusthiooxidans. Trans Nonferrous Metals Soc China 16:937–942
Watling HR (2006) The bioleaching of sulphide minerals with emphasis on copper sulphides - a review. Hydrometallurgy 84:81–108
Watling HR, Collinson DM, Fjastad S, Kaksonen AH, Li J, Morris C, Perrot FA, Rea SM, Shiers DW (2014) Column bioleaching of a polymetallic ore: effects of pH and temperature on metal extraction and microbial community structure. Miner Eng 58:90–99
Wei-min Z, Shi-fei GU (2007) Catalytic effect of active carbon on bioleaching of low-grade primary copper sulfide ores. Trans Nonferrous Metals Soc China 17:1123–1127
Xia L, Zeng J, Ding J, Yang Y, Zhang B, Liu J, Qiu G (2007) Comparison of three induced mutation methods for Acidithiobacillus caldus in processing sphalerite. Miner Eng 20:1323–1326
Xia L, Liu J, Xiao L, Zeng J (2008) Single and cooperative bioleaching of sphalerite by two kinds of bacteria Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Trans Nonferrous Metals Soc China 18:190–195
Ye M, Li G, Yan P, Ren J, Zheng L, Han D, Sun S, Huang S, Zhong Y (2017) Removal of metals from lead-zinc mine tailings using bioleaching and followed by sulfide precipitation. Chemosphere 185:1189–1196
Acknowledgments
We are grateful to Dr. Fariborz Gharib, Head of Applied Geological Research Center of Iran, (GRCIR) and Marzieh Moradian for providing facilities and technical assistance. Finally, we thank Prof. Olli H. Tuovinen for reviewing and comments on the manuscript.
Funding
This work was supported by the Geological Survey of Iran (GSI) under Grant No. 92-171-459.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ghassa, S., Abdollahi, H., Najafi, E. et al. A Comparative Assessment on the Effect of Different Supplemental Iron Sources on the Bio-dissolution of Zn, Pb, Cd, and As from a High-grade Zn–Pb Ore. Mining, Metallurgy & Exploration 36, 363–374 (2019). https://doi.org/10.1007/s42461-018-0001-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-018-0001-2