Abstract
The feasibility of producing direct reduced iron from pellets made up of mill scale by utilizing coal as a reductant has been investigated. The chemical and morphological characterization studies reveal that the mill scale contains around 71% Fe and comprises of a mixture of iron oxide phases such as magnetite and hematite with a little amount of wustite. The reduction study of the mill scale pellets has been carried out using the statistical design of experimental approach employing the response surface methodology. Under optimum conditions such as a temperature of 1246 °C, a time of 1.52 h, and coal-to-mill scale ratio of 0.58, around 88–89% metallization is obtained. Similarly, around 84% of metallization can be achieved at a temperature around 1150 °C, time of about 1.5 h with coal-to-mill scale ratio of 0.59. The characterization studies of the reduced pellets using X-ray diffraction and optical microscopy show the sequential growth of the metallic phase as the reducing parameters are increased. The properties of the mill scale pellets are found to match the desired specification for the direct reduction process, and the reduction behavior of the pellets as a function of temperature, time, and coal-to-mill scale ratio suggests that iron making from mill scale through this route is a promising process.
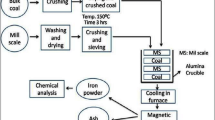
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vast amounts of solid waste are generated during the processing of steel in an integrated steel plant, which may sum to about 400–500 kg per ton of steel produced [1]. For example, the crude steel production in India was 97.42 MT in FY 17 (April 2016 to March 2017) and is expected to increase with the rising infrastructure development and growing demand for automobiles [2]. Moreover, the National Steel Policy (NSP) of 2017 by Ministry of Steel, Government of India, has set a target of producing 255 MTPA of crude steel by 2030-31 [3]. Therefore, more and more solid waste generation is on the anvil. Mill scale is one of those solid wastes which are produced from the rolling mill in steel hot rolling processes. It contains mostly iron in the range of 70–75% and traces of nonferrous metals and alkaline compounds and is contaminated with lubricants, oils, and greases from the equipment associated with rolling operations [4]. Particularly, the coarse mill scale is directly utilized in sintering processes due to its high metallic iron content [5]. However, mill scale sludge, which is much finer in size and consists of high levels of oil, cannot be recycled through the sintering process. Hence, a considerable amount of mill scale sludge containing valuable metallic content is normally treated as landfill waste [6]. Since the disposal of mill scale sludge causes serious environmental problems, its utilization, specifically in the secondary sector, is highly essential. As such, with the rise in demand for iron and steel in tandem with the gradual depletion of high-grade iron ores, the recovery of iron values from secondary resources has become very important [7,8,9].
The research on mill scale has been mostly undertaken for the preparation of reduced iron powders that have a wide range of applications in various industries, including powder metallurgy, welding, medicine, and food [10,11,12,13]. Benchiheub et al. [14] found that iron powder with a maximum metallic iron content of 98.4% could be synthesized from mill scale at 1050 °C for 180 min. Similarly, Joshi and Dhokey [15] studied the reduction kinetics of mill scale to recover iron powder using hydrogen gas as the reducing agent, particularly for powder metallurgy applications. In addition, many researchers have worked on developing a process to produce sponge iron powder from the rolling mill scale, which can be reused in electric arc furnaces [4, 16]. In fact, Saberifar et al. [17] attempted to recycle the mill scale by charging it directly to the electric arc furnace. However, the results indicated higher production time, energy, and coke consumptions, and therefore, the proposed process could not find its way to commercialization. Eissa et al. [18] reported the conversion of mill scale waste into valuable products such as high-purity iron by smelting. Recently, Sista et al. [1] explored the direct reduction recycling of mill scale by synthesizing iron powder with 97% Fe(T) for applications like nutrition supplements, body warmers, water purification, and sound insulators.
Though valuable high-purity iron powders could be recovered from mill scale, unfortunately, there has been no commercial technology to be implemented in industries so far. As a result, the mill scale wastes containing heavy metals are being dumped in landfills, thus contaminating the soil and groundwater, leading to environmental hazards. Considering the importance of upscaling the process for effective utilization of mill scale, the present study is aimed at developing a comprehensive approach of producing direct reduced iron (DRI), from mill scale, in the form of pellets with high metallic iron content. The pellets made from mill scale have been characterized with regard to compression strength, tumble index, porosity, and reduction properties, according to ISO standards. Further, an attempt has been made to study the impact of reduction process parameters such as temperature, time, and reductant-to-feed ratio on the metallization of the pellets using the statistical design of experiments and the pellets have been studied under microscope to understand the progress of metallization with respect to the variation of the process factors.
2 Experiments
2.1 Materials
The mill scale sample used in this work was obtained from a steel plant operating in the northern region of India while the coal was procured from Australia. The as-received mill scale (Fig. 1) was comprised of very coarse and irregularly shaped platelike materials to very fine-sized powdery particles. Therefore, the platelike objects were removed from the mill scale sample, and the rest was ground in a laboratory ball mill to a particle size below 150 µm to be used for the subsequent pelletizing process. The particle size distribution of the ground mill scale particle is given in Fig. 2. It is observed that the d80 of the particles is around 95 µm. It means 80% by mass of the particles are below the size of 95 µm, which indicates that the sample is a suitable feed for pellet making [19].
The chemical composition of the mill scale, as determined using wet chemical analysis, is given in Table 1. The Hard Grove Index (HGI) of the coal sample, as determined using an instrument supplied by Hardson and Co., India, was found to be 48. The proximate analysis of the coal carried out in a thermogravimetric analyzer (Leco), the ultimate analysis conducted in a CHN analyzer (Leco) and gross calorific values (GCV) determined using a Leco make Bomb calorimeter are given in Table 2.
2.2 Pelletization
The ground mill scale, along with 1% by weight of bentonite as a binder, was thoroughly mixed in a high-intensity mixer before producing 8–10-mm-diameter pellets in a laboratory-scale disk pelletizer. The green pellets, thus prepared, were fired in a muffle furnace through the traditional hardening process, i.e., drying at 250 °C for 15 min, preheating at 1000 °C for 20 min and firing at 1300 °C for 15 min. A single layer of the pellet bed was maintained on the furnace hearth to ensure uniform oxidation of each of the pellets. The fired pellets were then subjected to cold crushing strength, tumble, and porosity tests. The compressive strength of fired pellets was determined according to ISO 4700:2007, using a universal tensile tester (provided by Shanta Engineering, Kolkata). The compressive strength for each pellet was noted in terms of the maximum load (kg/pellet) that the pellet could withstand. For a given pellet type, the average of the compressive strength values of twenty pellets of the same pellet type was recorded. The tumble test was carried out according to ISO 3271, using a modified drum of 130 mm diameter and 200 mm length at 30 rpm for 40 min, i.e., for 1200 revolutions. The tumbled pellets were then screened at 6.3 mm, and the weight percentage of + 6.3 mm was reported as a tumble index. The apparent porosity of pellets was evaluated by the hot water boiling method, which is most commonly used for burnt refractories [20].
2.3 Reduction
Reduction tests were conducted using a laboratory-scale muffle furnace. While the heating rate was kept fixed at 10 °C/min, the temperature, time, and reductant-to-feed ratio were varied as per the experimental plan. Around 100 g of pellets was placed as alternative layers with the required amount of coal as the reductant in a graphite crucible and subjected to furnace for reduction. The results of metallization, as determined through chemical analysis, under different operating conditions, were calculated as per Eq. 1.
The phase changes that occurred during reduction were investigated by X-ray diffraction (XRD) using PANalytical equipment and optical microscope supplied by Leica, Germany. All the instruments used in this study were adequately calibrated from time to time.
2.4 Design of Experiments
The reduction experiments were planned as per the central composites design (CCD) of response surface methodology. This design is basically used to develop an empirical structure–function relationship between the factors and the responses with a minimum number of experimental trials, thereby saving time and cost of testing [21, 22]. Also, it helps in identifying the important factors and interactions and optimizing the conditions for maximizing or minimizing the output variable. In this study, reduction temperature, time, and reductant-to-feed ratio were the factors that influenced the reduction behavior of the pellets in terms of metallization. The design codes, units, and maximum and minimum values for each factor and response are given in Table 3. The factors have been coded to − 1 as the lower level, + 1 as the upper level, and 0 as the middle level. The design consists of 2k number of two-level factorial points having its origin at the center; 2k axial points with all factors set to 0 but for one factor that has the value of ± α and, lastly, the replicate tests at the center. Here, k is the number of variables and α is determined as 2k/4. In the present work, twenty experiments were carried out, which constitutes six central points, eight two-level factorial points, and six axial points. The statistical studies were conducted using the software Design Expert 8.0.
3 Results and Discussion
3.1 Sample Characterization
The mill scale sample was studied under an optical microscope to know the constituent phases and their morphological variation. The micrographs, as displayed in Fig. 3, show the presence of phases like hematite, magnetite, and wustite. It can be observed that the hematite phases are mostly fine-grained, whereas magnetite varies from micron to millimeter scale. Hematite grains are mostly intercalated with magnetite phases while magnetite can also be found as free grains. Wustite, mostly found in lath shape, is seen to be close to magnetite. The absence of metallic iron in the sample hints toward the fact that the original metallic iron has undergone re-oxidation to form various iron oxides. The XRD pattern of the mill scale, as shown in Fig. 4, supplements the above findings.
3.2 Pellet Properties
In general, a coal-based direct reduction (DR) process is carried out using a rotary kiln as a reactor where iron ore in the form of either lump or pellets is charged along with noncoking coal as the solid reductant. Typically in India, where coking coals are not available aplenty, the coal-based DR process is emerging as a cost-effective iron-making method [23]. Therefore, in the present work, the iron ore pellets have been characterized to see whether they meet the required specifications for the coal-based DR process. The results in terms of the values of moisture content, average drop number, cold crushing strength, tumble index and apparent porosity are presented in Table 4. It is noteworthy that the cold crushing strength is an essential property for the pellets to be used in the DR process since it measures the maximum compressive load attained to cause the fracture of a pellet. For the coal-based DR process, the recommended crushing strength is 150 kg/pellet, while over 250 kg/pellet is preferred [24]. The pellets produced in this study having strength in the range of 231–273 kg/pellet can be considered reasonable. Besides, a tumble index of 94.5% indicates that the mill scale pellets offer high resistance to tumbling. Similarly, highly porous pellets with a porosity of 22–24% are usual for the DR process to provide better reducibility in the reactor [25]. Hence, the highly reducible pellets with a porosity of about 20%, which have been prepared in this work, are expected to provide maximum metallization in the coal-based DR process.
3.3 Statistical Modeling
The pellets made up of the fine ground mill scale were subjected to the reduction experiments as per the CCD. Based on the literature, the minimum and maximum values of different factors were decided. For example, the reduction temperature was chosen in the range of 1150–1250 °C considering the thermodynamics of iron oxide reduction by carbon monoxide through the equilibrium reactions, as shown in the Baur–Glaessner diagram (Fig. 5). It is the large phase field of FeO (wustite) that makes it difficult to reduce to Fe (metallic iron) as compared to other iron oxides. Therefore, the formation of metallic iron from wustite always requires high temperatures. Many researchers have reported complete metallization of mill scale in the high temperature range [4, 17, 18]. In the same way, relying on previous reports, the ranges of other factors such as time and coal-to-mill scale ratio were also decided.
Iron and iron oxide stability diagram for CO-CO2 atmospheres [26]
The details of the experiments carried out in this work, along with corresponding responses in terms of metallization, are given in Table 5. It is observed that the degree of metallization favors high temperature, high reduction time, and high coal-to-feed ratio. The data given in Table 5 are subjected to various statistical analyses and model development. A quadratic model has been found to be the best fit. The analysis of variance (ANOVA) test was carried out to screen the important factors and interactions to modify the model.
The ANOVA data presented in Table 6 show that the model has an F value of 7.11, which implies the overall significance of the model developed. There is a probability of only 0.25% for noise being responsible for this model F value. The significance of the terms as given in the ANOVA table follows the order of the F values, i.e., C2 > C > A>B2 > AB > B > A2 > AB > BC > AC. The highest F value for C2 and C (coal-to-mill scale ratio) confirms that it is the most significant term among all the factors and interactions. The terms having “Prob > F” value of more than 0.1 (with less than 90% confidence level) are not significant and hence, are removed from the final model given in Eq. 2.
The model, as given in Eq. 2, has a model F value of 18.73, indicating only a 0.01% chance of noise being the reason for such a large F value. The coefficient of the variation (C.V.) is found to be only 6.3% suggesting a reasonably high degree of precision and reliability of the experimental data. The R2 value of 0.83 validates the significance of the model further, and the “Adeq. Precision” value of 16.055 indicates that this model can be used to navigate the design space.
Analyses in terms of the normal plot of residuals, residual versus fitted values plot, and residuals versus the order of the data plot (Fig. 6) were carried out to validate the adequacy of the model further. The residuals are known to be a measure of the quality of the data analysis, and the graphical analysis of the same is considered a useful tool to detect and explain the departures from the assumptions made during the regression. The straight-line pattern displayed in Fig. 6a reveals a normal distribution of the errors suggesting that the experimental data are supporting the constructed model. The plot of the residuals against the predicted values of the response shows a similar scattering pattern below and above the X-axis (Fig. 6b). Similarly, the residuals when plotted against the run order of the designed experiments (Fig. 6c) show a random scattering (around zero), which also implies that the model is suitable for being used for different statistical analyses.
Optimization studies were undertaken using the Nelder–Mead multidimensional pattern search technique inbuilt with the software. As many as thirty-nine sets of solutions were found suitable for maximum metallization. The surface plot, as displayed in Fig. 7, illustrates the optimum metallization as against various levels of temperature and time in the X- and Y-axis. Coal-to-mill scale ratio of 0.58 was considered as the actual factor and kept fixed. The optimum metallization of around 88.8% has been flagged at a temperature of around 1246 °C and time about 1.52 h. Three experiments were carried out at these conditions, and an average of 89% metallization was obtained confirming a good match between the model and the experiments.
The model was further subjected to analysis to find the minimum possible levels of temperature as well as time that are required to obtain a high degree of metallization. It is evident from the resulting surface plot displayed in Fig. 8 that around 84% metallization can be achieved at a temperature around 1150 °C, time of about 1.5 h with coal-to-mill scale ratio of 0.59. From the economic point of view, a drop in the metallization of about 4% is affordable since the required temperature is lesser by about 100 °C. Confirmatory experiments were conducted at these model-predicted conditions, and a reasonable agreement between the experiments and the model was noticed.
3.4 Characterization of the Reduced Mill Scale
Characterization studies, such as XRD and reflected light microscopy, were undertaken to understand the phase transformation behavior of the pellets under varying conditions of reduction. The XRD patterns of the reduced pellets with the respective reducing conditions and resulting degrees of metallization are displayed in Fig. 9. The figure reveals the dominant presence of iron metal with relatively less amount of magnetite. It is evident from the XRD studies that the intensities of the iron metal peaks increase as metallization increases indicating the fact that higher reducing conditions such as a temperature of 1250 °C, time of 2.5 h, and coal-to-pellet ratio of 0.7 are most favorable for metallization. Similarly, magnetite peaks observed under lower reducing conditions diminish gradually as metallization increases along with an increase in the operating parameters.
Optical microscopic studies were also undertaken for the same reduced pellets that were subjected to XRD analyses. It is observed that the concentration of the metallic iron increases along with the increase in the reducing parameters. For instance, the density of iron grains is comparatively less in Fig. 10a, where the metallization value is only ~ 66%. Nevertheless, with a rise in the reduction temperature, time, and reductant, the metallization increases to ~ 87%, and therefore, large metallic iron grains can be seen in Fig. 10d. Hence, it may be conferred that the reduction temperature of a minimum of 1150 °C is required for the complete metallization of the mill scale.
Likewise, the intergranular spaces between the metallic grains appear to be high in the case of Fig. 10a, while it gradually decreases with the increase in metallization. Besides, the coagulation of the metallic phases appears in Fig. 10b. In Fig. 10d, the metallic phase seems to be almost continuous because of the agglomeration of the same at high temperature, reducing period and coal-to-feed ratio.
4 Conclusion
Mill scale is one of the major waste materials generated by the ferrous industries. It has now caught the attention of the research as well as the industrial community for being exploited as a significant source of iron values. In this context, the possibilities of pelletization followed by the DR process to produce metallic iron from the mill scale were explored in this communication. A statistical model was developed and used to optimize the conditions for maximizing the metallization at different possible parametric levels. An acceptable degree of reduction to the tune of 84% could be obtained at a temperature of 1150 °C, a time of 1.5 h with coal-to-mill scale ratio of 0.59. It was also possible to obtain a metallization as high as 88% with an increase in temperature of about 100 °C. The characterization studies could provide a basic understanding of the reduction process as a function of different process conditions. It is expected that these results will provide the necessary knowledge to explore the pilot-scale trials of the process that may eventually lead to successful commercialization.
References
Sista K S, Dwarapudi S, and Nerune V P, ISIJ Int59 (2019) 787.
Steel-Report-Mar-2018, https://www.ibef.org/download/Steel-Report-Mar-2018.pdf (2018). Accessed 27 Jun 2019.
Mazumdar R, National Steel Policy 2017 to focus spending on infrastructure, construction, in Econ. Times (2017). Accessed 17 Apr 2018.
Martín M I, López F A, and Torralba J M, Ironmak Steelmak39 (2012) 155.
Gaballah N M, Zikry A F, Khalifa M G, Farag A B, El-Hussiny N A, and Shalabi M E H, Open J Inorg Non-Met Mater 03 (2013) 23.
Paswan D, Malathi M, Minj R K, and Bandhyopadhayay D, Mill Scale: A Potential Raw Material for Iron and Steel Making, Steelworld (2015).
Ray N, Nayak D, Dash N, and Rath S S, Clean Technol Environ Policy20 (2018) 1761.
Gao L, Liu Z, Pan Y, Ge Y, Feng C, Chu M, and Tang J, Min Metall Explor36 (2019) 375.
Sunil S R, Rayapudi V, and Dhawan N, Min Metall Explor. (2019).
Ye Q, Zhu H, Peng J, Srinivasa Kannan C, Chen J, Dai L, and Liu P, Metall Mater Trans B44 (2013) 1478.
Ye Q, Zhu H, Zhang L, Ma J, Zhou L, Liu P, Chen J, Chen G, and Peng J, J Alloys Compd613 (2014) 102.
Mechachti S, Benchiheub O, Serrai S, and Shalabi M E H, Int J Sci Eng Res4 (2013) 1467.
Cho S, Met Mater Int14 (2008) 193.
Benchiheub O, Mechachti S, Serrai S, and Khalifa M G, J Mater Environ Sci1 (2010) 267.
Joshi C, and Dhokey N B, Trans Indian Inst Met68 (2015) 31.
Sen R, Dehiya S, Pandel U, and Banerjee M K, Procedia Earth Planet Sci11 (2015) 8.
Saberifar S, Jafari F, Kardi H, Jafarzadeh M A, and Mousavi S, J Adv Mater Process2 (2014) 73.
Eissa M, Ahmed A, and El-Fawkhry M, J Metall2015 (2015) 1.
Nikai I, and Garbers-Craig A M, Miner Process Extr Metall Rev37 (2016) 42.
Chesters JH, Iron Steel Inst Lond (1973) 553.
Krishnamurthy L, Sridhara B K, and Budan D A, Mater Manuf Process22 (2007) 903.
Montgomery D C, Design and Analysis of Experiments, 8th Edition. John Wiley & Sons, Hoboken, NJ (2012).
Chatterjee A, Sponge Iron Production by Direct Reduction of Iron Oxide, PHI Learning Private Limited, Delhi (2014).
Monsen B, Thomassen E, Brakstad I, Ringdalen E, and Hoegass P H, in AISTech 2015 Proceedings, Cleveland, Ohio, USA (2015).
Gupta R C, Theory and Laboratory Experiments in Ferrous Metallurgy, Second, Asoke K. Ghosh, PHI Learning Private Limited, Delhi (2015).
Plaul F J, Böhm C, and Schenk J L, J South Afr Inst Min Metall109 (2009) 121.
Acknowledgements
The authors are thankful to the Director, CSIR-IMMT, Bhubaneswar, for his permission to publish this paper and the Ministry of Steel, Government of India (Grant No. F. No. 11(12)/GBS/2014-TW), for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nayak, D., Roy, S.K., Dash, N. et al. Investigation on the Coal-Based Direct Reduction of Mill Scale Pellets: Statistical Modeling and Characterization Studies. Trans Indian Inst Met 73, 691–701 (2020). https://doi.org/10.1007/s12666-020-01889-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-01889-w